Increasing the efficiency of zinc-phthalocyanine based solar cells through modification of the anchoring ligand†‡
Miguel
García-Iglesias
a,
Juan-José
Cid
a,
Jun-Ho
Yum
b,
Amparo
Forneli
c,
Purificación
Vázquez
a,
Mohammad K.
Nazeeruddin
*b,
Emilio
Palomares
*cd,
Michael
Grätzel
b and
Tomás
Torres
*ae
aUniversidad Autónoma de Madrid, Departamento de Química Orgánica, Cantoblanco, 28049, Madrid, Spain. E-mail: tomas.torres@uam.es; Fax: +34 9 1497 3966; Tel: +34 9 1497 4151
bLaboratory for Photonics and Interfaces, Institute of Chemical Sciences and Engineering, School of Basic Sciences, Swiss Federal Institute of Technology, CH-1015, Lausanne, Switzerland. E-mail: mdkhaja.nazeeruddin@epfl.ch; Fax: +41 21 693 4111; Tel: +41 21 693 61 24
cInstitute of Chemical Research of Catalonia (ICIQ), Lab 1. Avda. Països Calatans, 16, 43007, Tarragona, Spain. E-mail: epalomares@iciq.es; Fax: +34 977 920 223; Tel: +34 977 920 241
dICREA, Passeig Lluís Companys, 23, 08010, Barcelona, Spain
eIMDEA-Nanociencia, Facultad de Ciencias, Cantoblanco, 28049, Madrid, Spain
First published on 11th November 2010
Abstract
Several zinc-based phthalocyanines have been synthesized and used in Dye-Sensitized Solar Cells (DSSC). The results have been compared with the standard TT1 phthalocyanine, which shows good light-to-energy conversion efficiencies in comparison with other IR sensitizers used in DSSC. We show herein that the anchoring moiety is critical for both achieving high injection yields and slow back electron transfer dynamics that affect the overall device efficiency. Moreover, based on these results, we have synthesized a new phthalocyanine with a superior performance, when compared to the TT1 dye, with a subtle change on the anchoring moiety, thus leading to a higher photocurrent response.
Broader contextDevelopment of clean renewable energy sources as an alternative to fossil fuels is essential and in this respect dye-sensitized solar cell (DSSC) technology is clearly a contender. In DSSC, sensitizers with extended absorption in the near IR region of the sun emission spectra are paramount, and phthalocyanines are perfectly suited for their integration in light energy conversion systems. They exhibit very high extinction coefficients around 700 nm for efficient photon harvesting, as well as reversible redox properties and excellent photoconductivities. Thus for example, TT1 phthalocyanine is becoming a standard material which shows outstanding light-to-energy conversion efficiencies in Dye Sensitized Solar Cells. In the current paper novel zinc phthalocyanines have been designed, synthesized and used in DSSC. The results have been compared with the standard TT1 phthalocyanine. |
Introduction
Since the seminal manuscript by O'Regan and Grätzel on Dye-Sensitized Solar Cells (DSSC), ruthenium complexes have dominated the choice for efficient light harvesting and outstanding light to electrical energy conversion.1 Nonetheless, recently pure organic dyes based on polythiophene conjugated units are catching up the light-to-energy conversion records and approaching 10% under standard sun-simulated conditions at 100 mW cm−2. Yet, the main drawback of most polypyridyl ruthenium complexes and some of the organic dyes is the lack of or low absorption in the near IR region of the solar spectrum, between 650 and 750 nm.2 Needless to say that for further improvement of the device efficiency, extended red response of these sensitizers is imperative without having losses on the device photovoltage.3There have been previous attempts to use well-known sensitizers that show light absorption in the near IR region which include the use of squaraines,4–6 naphthalocyanines7 and phthalocyanines.8 In this regard, the latter has shown to be a suitable approach if zinc phthalocyanines are used. In particular, when the so-called TT1dye (9(10),16(17),23(24)-tri-tert-butyl-2-carboxy-5,28:14,19-diimino-7,12:21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16] tetraazacycloeicosinato-(2−)-N29,N30,N31,N32 zinc(II)) (mixture of regioisomers) (Fig. 1)9 was tried, efficiencies as high as up to 3.5% were achieved. Furthermore, when TT1 was modified by Mori and co-workers a 4.6% global efficiency was reached under standard conditions.8a In fact, although zinc based phthalocyanines were widely studied in the early times of DSSC,10 the breakthrough consisted in the rational choice of the peripheral surrounding substituents in order to prevent the formation of a high degree of molecular aggregates, thus allowing to enhance the electron injection and, therefore, the device photocurrent. Moreover, the TT1 molecular design has been further used recently by other groups8a and us9b to increase the efficiency of phthalocyanine based DSSC.
 | ||
| Fig. 1 Molecular structures of zinc carboxyphthalocyanines TT1, TT6, TT7, TT8, TT15 and TT16 used in this study. | ||
It is also worthy to notice the fact that the overall voltage of these devices, near IR-based DSSC, has always been a major problem to overcome since a typical range meets values between 0.35 and 0.45 V. As showed by O'Regan et al.11 using ruthenium-based phthalocyanines and later by Palomares et al.12 using ruthenium polypyridyl complexes, there is a direct relationship between the dye molecular structure and the interfacial charge recombination processes that limits the device performance. In fact, in the case of phthalocyanine-based DSSC these effects are much more pronounced than in ruthenium or some other organic donor–acceptor-based sensitizers showing catalytic recombination processes, which enhance the recombination kinetics in the device under usual working conditions.
Herein, we aim to focus on understanding the parameters that, without modifying the chromophore, can improve the device efficiency of the zinc phthalocyanine sensitizer. Previous work on molecular sensitizers used in DSSC has shown that the anchoring moiety can play a central role.13 For example, it is well known that the use of cyanoacetic acid as an anchoring motif improves the pull characteristics of the organic dyes, thus enhancing the photocurrent response of the solar cells.14,15 In this direction, we have synthesized two new zinc-based phthalocyanines TT7 and TT8 (Fig. 1) having the cyanoacetic anchoring unit conjugated to the phthalocyanine core. Moreover, as it will be explained later in the manuscript and based on the results obtained for these two new cyanoacetic modified phthalocyanines, we synthesized three more new TT1 derivatives TT6, TT15 and TT16 (Fig. 1) as well as a new zinc phthalocyanine showing a better device performance, setting a new record for TT1 based DSSC.
Results and discussion
Synthesis and characterization of TT1 derivatives
The carboxyphthalocyanine TT6 was prepared in a similar way to TT1 as previously described by our group.9a An oxidation of formylphthalocyanine 116 with NaClO2 in water and sulfamic acid gave TT6 with a 43% yield, after purification by reverse phase chromatography (Scheme 1). | ||
| Scheme 1 Synthesis of phthalocyanines TT6, TT8 and TT16. (A) (i) NaClO2, acetone, (ii) H3NO3S, H2O, 43%. (B) Piperidine, cyanoacetic acid, methanol reflux, 47%. (C) Piperidine, malonic acid, methanol reflux, 52%. | ||
On the other hand, the synthesis of phthalocyanines TT7, TT8, TT15 and TT16 was carried out by means of a Knoevenagel-type reaction (Schemes 1 and 2) in 54%, 60%, 47% and 52% yields, respectively after chromatographic purification on reverse phase. These syntheses were performed with piperidine and cyanoacetic acid in the case of TT7 and TT8, or piperidine and malonic acid for TT15 and TT16 in a similar way than that reported elsewhere for porphyrin derivatives.17 The precursor used for TT8 and TT16 was formylphthalocyanine 1 (Scheme 1), and aldehyde 2 for TT7 and TT15.
 | ||
| Scheme 2 Synthesis of phthalocyanines TT7 and TT15. (A) Piperidine, cyanoacetic acid, methanol reflux; 54%. (B) Piperidine, malonic acid, methanol reflux; 60%. | ||
All these new carboxyphthalocyanines were characterized with the use of MALDI-TOF and HR MALDI-TOF mass spectrometries, and FT-IR, UV-vis and 1H-NMR spectroscopies (see ESI†).
UV-Visible, steady-state and lifetime emission measurements
We registered all the UV-Visible absorption spectra for the new carboxyphthalocyanines either in solution or adsorbed onto nanocrystalline mesoporous metal oxide thin films. As an example the UV-Visible absorption spectra for TT7 are shown in Fig. 2. These compounds exhibit a maximum at around 675 nm (εca. 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dm3 mol−1 cm−1), and when excited within the CT absorption band at 298 K in an air-equilibrated THF solution, they display a luminescence maximum at 700 nm.
000 dm3 mol−1 cm−1), and when excited within the CT absorption band at 298 K in an air-equilibrated THF solution, they display a luminescence maximum at 700 nm.
 | ||
| Fig. 2 UV-Visible absorption spectra for TT7 in solution, 1 × 10−4 M, (THF: tetrahydrofuran) and after sensitisation onto 4 µm transparent mesoporous thin metal oxides. | ||
Based on the well-known fact that the absorption maxima (Q-band) in Pcs shift towards lower energies as a consequence of the formation of J-type molecular aggregates, we can infer in our case a low degree of aggregation of the phthalocyanines when anchored onto the nanocrystalline mesoporous thin metal oxide films (Fig. 2). It is well established that the formation of aggregates may arise from the ability of ZnPc to form π–π stacking structures in solution through the orientated carboxylic acid with the zinc core or, in addition, with the –CN group. However, in our case, from the UV-Visible spectrum in solution we could not observe the formation of the molecular aggregates. Thus, we can confirm that the presence of molecular aggregates only occurs after the adsorption process.
Moreover, we carried out steady-state luminescence measurements and time resolved emission decay kinetics using TCSPC (time-correlated single photon counting) spectroscopy. Fig. 3 shows the fluorescent emission spectra for TT7. As can be seen in good agreement with the bathochromic shift observed for the UV-Visible absorption spectra (Fig. 3) the fluorescence emission spectra of TT7/Al2O3 sample are also displaced into lower energies. Furthermore, the emission spectrum for the TT7/TiO2 film was not recorded due to the low intensity of the TT7 fluorescence emission, which is an indication of efficient electron transfer from the TT7 excited state into the TiO2 semiconductor conduction band. An indirect measurement to estimate the electron injection yield for sensitizers adsorbed onto nanocrystalline mesoporous TiO2 films is the use of fast TCSPC spectroscopy.
 | ||
| Fig. 3 Steady-state fluorescence emission spectrum for TT7 measured at room temperature under air and in solution, 1 × 10−4 M (THF: tetrahydrofuran) (grey line) and after sensitisation onto a 4 µm thin Al2O3 film (black line) upon excitation at λex = 635 nm. | ||
To analyse the electron injection yield we must prepare samples with identical dye absorbance at the excitation wavelength, λex = 635 nm, using as a blank a wide band-gap metal oxide in which the conduction band edge is well above the LUMO energy level of the sensitizers. Thus, electron injection process will be avoided and we will record the emission decay kinetics for the excited state at a fixed acquisition time. Thereafter, we will measure the emission decay kinetics for the dye excited state but when adsorbed onto the semiconductor metal oxide film, in our case TiO2, while we keep the acquisition time constant. The direct comparison between both decays and integrating the area under the decay curve will give us a fair estimation of the electron injection yield for the TiO2 sensitized film as Palomares18 and Durrant and co-workers19–21 and others have previously shown. As an example, Fig. 4 illustrates the emission decay kinetics for TT15 of the measurements carried out for the rest of the samples and Table 1 compiles the kinetic parameters and the estimated electron injection yield for all the sensitizers studied in this manuscript.
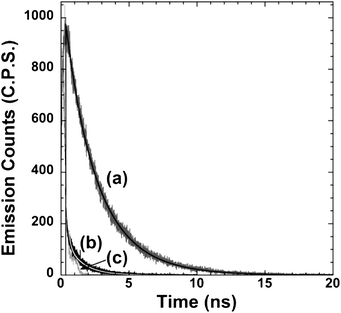 | ||
| Fig. 4 Emission decay kinetics for (a) TT15/Al2O3 and (b) TT15/TiO2 measured at room temperature under air using an acquisition time of 600 seconds. (c) corresponds to the instrument response. The solid lines correspond to the deconvolution fitting carried out using the instrument response and the recorded TT15 excited state decay kinetics. | ||
As can be seen from Fig. 4, the TT15/TiO2 emission decay dynamics results in very fast kinetics almost as fast as the instrument response of our system (350 ps measured at FWHM, Full Width at Half Maximum). Therefore, we can conclude that for TT15/TiO2 sensitised films more than 90% of the electrons are injected before 350 ps. It is worthy to notice that our measurements have been carried out in sensitized films but not in complete devices where maybe the presence of the iodine/iodide redox couple as well as the presence of electrolyte additives (tert-butylpyridine, etc.) may change not only the electron injection yield but also the excited state emission decay lifetime as has been observed in ruthenium polypyridyl complexes. Thus, a direct correlation between the estimated electron injection yield using TCSPC and the IPCE (incident photon-to-current efficiency) measurements shown in this manuscript cannot be fairly done.
Electrochemical measurements
The cyclic voltammogram of TT1 obtained using a glassy carbon disk electrode in dimethylformamide solvent with 0.1 M tetrabutylammonium tetrafluoroborate, exhibited a quasi-reversible oxidation at E1/2 = 0.82 V vs.NHE. When scanning towards negative potentials one irreversible and one quasi-reversible waves were observed. The irreversible wave at E1/2 = −0.77 is due to the reduction of the protons of the carboxylic acid groups to hydrogen and the quasi-reversible wave at E1/2 = −1.01 is assigned to the phthalocyanine based reduction. The TT1 and TT6, TT7, TT8, TT15 and TT16 exhibit similar electrochemical properties, which implicates that the subtle changes made on the core structure of the ZnPc do not have a strong influence over the LUMO and HOMO energies. The reduction and oxidation potentials confirm the possibility for fine electron injection into the TiO2 conduction band as well as the regeneration of the oxidized phthalocyanine, respectively.Device measurements
Fig. 5 (top) shows the photocurrent action spectra of the zinc phthalocyanine sensitizers obtained with a sandwich cell. It is interesting to compare the two sensitizers TT1 and TT6 in which, upon incorporation of a double bond between the anchoring carboxylic acid group and zinc phthalocyanine, the incident monochromatic photon-to-current conversion efficiency (IPCE) slightly decreased from 80 to 74% at maximum absorption wavelength. Under standard global AM 1.5 solar conditions, the TT6 sensitized cell gave a short circuit photocurrent density (JSC) of 7.37 mA cm−2, an open circuit voltage (VOC) of 609 mV and a fill factor (FF) of 0.74, corresponding to an overall conversion efficiency (η) of 3.28% (see Fig. 5(b) and Table 2). Under same condition, TT1 sensitized solar cell yielded a JSC of 7.78 mA cm−2, a VOC of 611 mV, a FF of 0.75, and a η of 3.56% (see Fig. 5(b) and Table 2). It is interesting to note that changing the anchoring group from carboxylic acid to cyanoacrylic acid in TT7 and TT8 showed relatively lower photovoltaic performance than TT1 and TT6. TT7 sensitized cell gave a lower JSC of 5.88 mA cm−2 and a VOC of 587 mV, corresponding to a η of 2.55%. And TT8 with the extended conjugation bridge between the anchoring cyanoacrylic acid group and zinc phthalocyanine showed a JSC of 6.80 mA cm−2, a VOC of 576 mV, and a η of 2.64%. The IPCE of TT7 and TT8 showed broader spectra, which could be due to the formation of a small population of aggregates. | ||
| Fig. 5 Photocurrent action spectrum (a) and current–voltage characteristics (b) of TT1 (dotted line), TT6 (solid line), TT7 (dashed line), TT8 (dashed dotted line), TT15 (dashed double-dotted line) and TT16 (line with circles) obtained with a nanocrystalline TiO2 film supported onto a conducting glass sheet and derivatized with monolayer of phthalocyanine sensitizers in the presence of chenodeoxycholic acid (CDCA). A sandwich type cell configuration was used to measure this spectrum. | ||
| Dye | J SC/mA cm−2 | V OC/mV | FF | η (%) |
|---|---|---|---|---|
| a 0.1 mM dye with 10 mM CDCA in EtOH. b 0.1 mM dye with 60 mM CDCA in EtOH. c 0.05 mM dye with 60 mM CDCA in EtOH. | ||||
| TT1 a | 7.78 | 611 | 0.75 | 3.56 |
| TT6 b | 7.37 | 609 | 0.74 | 3.28 |
| TT7 c | 5.88 | 587 | 0.75 | 2.55 |
| TT8 c | 6.80 | 576 | 0.69 | 2.64 |
| TT15 b | 9.15 | 600 | 0.72 | 3.96 |
| TT16 b | 6.86 | 584 | 0.72 | 2.87 |
It is worth noting that the molar ratio of CDCA (chenodeoxycholic acid)/dye in TT7 and TT8 for the best result should be higher than the others. CDCA is a well known molecule employed to reduce aggregates and its effect on TT1 has been studied.22 On the contrary, in the case of zinc phthalocyanine with a carboxylic acid group (see structures TT1 and TT6) the IPCE data are consistent with absorption spectra. TT15 having two carboxylic acid anchoring groups showed very comparable IPCE, in which a broader spectrum with 70% at maximum brought an increase in JSC. Indeed, the TT15 sensitized cell gave a JSC of 9.15 mA cm−2, a VOC of 600 mV and a FF of 0.72, leading to an overall conversion efficiency (η) of 3.96% (see Fig. 5(b) and Table 2). TT16, with the extended conjugation, on the other hand, showed very broad and lower IPCE compared to that of TT15 resulting in JSC and η of 6.86 mA cm−2 and 2.87%, respectively. As a consequence, the improved efficiency of the TT1, TT6, and TT15 compared to the TT7, TT8 and TT16 sensitizers clearly demonstrates the influence of anchoring group and the bridge between the sensitizer and the zinc phthalocyanine chromophore.
Conclusions
We have designed, based on our previous results on record cells made using the zinc phthalocyanine TT1, new near IR sensitizers. The dyes have been fully characterized both in terms of their spectroscopical properties and their performance on fully operative DSSC under standard working conditions. Kinetics measurements have demonstrated that the electron injection in these types of sensitizers does not represent an issue and all the zinc phthalocyanines show good or excellent electron injection yields. Moreover, the IPCE data show that for the best devices little or no dye aggregation can be observed. Finally, a new dye TT15, which shows higher performance than the longstanding near IR record dye, the phthalocyanine TT1, has been obtained. The overall results can be used for further design of new phthalocyanines with higher light harvesting properties that will lead to higher photocurrents, and their potential use, for example, for phthalocyanine-based energy relay dyes in DSSC where additional photo-response is attributed to Förster resonance energy transfer (FRET) from the energy relay dyes to the sensitizing dye.23,24 Work on this direction is currently being carried out.Acknowledgements
This work was supported by the ESF-SOHYD Program, COST Action D35, and the EU Project ROBUST DSC, FP7-Energy-2007-1-RTD, No. 212792. Financial support by the MEC, Spain (CTQ2008-00418/BQU, CONSOLIDER-INGENIO 2010 CDS 2007-00010, PLE2009-0070, PSE-120000-2009-008 FOTOMOL), CONSOLIDER-HOPE-CSD-0007-2007 and the CTQ2007-60746/BQU and CAM (MADRISOLAR2, S2009/PPQ/1533), is gratefully acknowledged. MKN thanks the World Class University (WCU) programme funded by the Ministry of Education Science and Technology (Grant No. R31-2008-000-10035-0).References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- Y. Rio, P. Vázquez and E. Palomares, J. Porphyrins Phthalocyanines, 2009, 13, 645 CrossRef CAS.
- M. Gratzel, Inorg. Chem., 2005, 44, 6841 CrossRef.
- S. Kim, K. M. Gopal, M. Paulose, O. K. Varghese, C. Baik and A. G. Craig, Langmuir, 2010, 26, 13486 CrossRef CAS.
- S. Tatay, S. A. Haque, B. O'Regan, J. R. Durrant, W. J. H. Verhees, J. M. Kroon, A. Vidal-Ferrán, P. Gaviña and E. Palomares, J. Mater. Chem., 2007, 17, 3037 RSC.
- J.-H. Yum, P. Walter, S. Huber, D. Rentsch, T. Geiger, F. Nüesch, F. De Angelis, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2007, 129, 10320 CrossRef CAS.
- L. Macor, F. Fungo, T. Tempesti, E. N. Durantini, L. Otero, E. M. Barea, F. Fabregat-Santiago and J. Bisquert, Energy Environ. Sci., 2009, 2, 529 RSC.
- (a) S. Mori, M. Nagata, Y. Nakahata, K. Yasuta, R. Goto, M. Kimura and M. Taya, J. Am. Chem. Soc., 2010, 132, 4054 CrossRef CAS; (b) For reviews see: G. de la Torre, C. G. Claessens and T. Torres, Chem. Commun., 2007, 2000 Search PubMed; (c) M. V. Martínez-Díaz and T. Torres, On the Significance of Phthalocyanines in Solar Cells, in Handbook of Porphyrin Sciences, ed. K. Kadish, K. M. Smith and R. Guilard, World Scientific Press, 2010, vol. 10 Search PubMed; (d) M. V. Martínez-Díaz, G. de la Torre and T. Torres, Chem. Commun., 2010, 46, 7090 RSC.
- (a) J.-J. Cid, J.-H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martínez-Ferrero, E. Palomares, J. Ko, M. Grätzel and T. Torres, Angew. Chem., Int. Ed., 2007, 46, 8358 CrossRef CAS; (b) J.-J. Cid, M. G. Iglesias, J. H. Yum, A. Forneli, J. Albero, E. M. Ferrero, P. Vázquez, M. Grätzel, M. K. Nazeeruddin, E. Palomares and T. Torres, Chem.–Eur. J., 2009, 15, 5130 CrossRef CAS.
- J. He, G. Benkö, F. Korodi, T. Polivka, R. Lomoth, B. Akermark, L. Sun, A. Hagfeldt and V. Sundström, J. Am. Chem. Soc., 2002, 124, 4922 CrossRef CAS.
- B. C. O'Regan, I. López-Duarte, M. V. Martínez-Díaz, A. Forneli, J. Albero, A. Morandeira, E. Palomares, T. Torres and J. R. Durrant, J. Am. Chem. Soc., 2008, 130, 2906 CrossRef CAS.
- A. Reynal, A. Forneli, E. Martínez-Ferrero, A. Sánchez-Díaz, A. Vidal-Ferrán, B. O'Regan and E. Palomares, J. Am. Chem. Soc., 2008, 130, 13558 CrossRef CAS.
- M. Miyahsita, K. Sunahara, T. Nishikawa, Y. Uemura, N. Koumura, K. Hara, A. Mori, T. Abe, E. Suzuki and S. Mori, J. Am. Chem. Soc., 2008, 130, 17874 CrossRef CAS.
- H. Qin, S. Wenger, M. Xu, F. Gao, X. Jing, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 9202 CrossRef CAS.
- K. Hara, T. Sato, R. Katoh, A. Furube, Y. Toshitada, M. Murai, M. Kurashige, S. Ito, A. Shinpo, S. Suga and H. Arakawa, Adv. Funct. Mater., 2005, 15, 246 CrossRef CAS.
- M. Quintiliani, A. Kahnt, T. Wölfle, W. Hieringer, P. Vázquez, A. Görling, D. M. Guldi and T. Torres, Chem.–Eur. J., 2008, 14, 3765 CrossRef CAS.
- (a) Q. Wang, W. M. Campbell, E. E. Bonfantani, K. W. Jolley, D. L. Officer, P. J. Walsh, K. Gordon, R. Humphry-Baker, M. K. Nazeeruddin and M. Grätzel, J. Phys. Chem. B, 2005, 109, 15397 CrossRef CAS; (b) W. M. Campbell, K. W. Jolley, P. Wagner, K. Wagner, P. J. Walsh, K. C. Gordon, L. Schmidt-Mende, M. Nazeeruddin, Q. Wang, M. Grätzel and D. L. Officer, J. Phys. Chem. C, 2007, 111, 11760 CrossRef CAS.
- A. Sánchez-Díaz, E. Martínez-Ferrero and E. Palomares, J. Mater. Chem., 2009, 19, 5381 RSC.
- S. E. Koops and J. R. Durrant, Inorg. Chim. Acta, 2008, 361, 663 CrossRef CAS.
- A. Morandeira, I. López-Duarte, M. V. Martínez-Díaz, B. O'Regan, C. Shuttle, N. A. Haji-Zaimulabidin, T. Torres, E. Palomares and J. R. Durrant, J. Am. Chem. Soc., 2007, 129, 9250 CrossRef CAS.
- A. Morandeira, I. López-Duarte, B. O'Regan, M. V. Martínez-Díaz, A. Forneli, E. Palomares and T. Torres, J. Mater. Chem., 2009, 19, 5016 RSC.
- J.-H. Yum, S.-R. Jang, R. Humphry-Baker, M. Gratzel, J.-J. Cid, T. Torres and M. K. Nazeeruddin, Langmuir, 2008, 24, 5636 CrossRef CAS.
- B. E. Hardin, E. T. Hoke, P. B. Armstrong, J.-H. Yum, P. Comte, T. Torres, J. M. J. Fréchet, Md. K. Nazeeruddin, M. Grätzel and M. D. McGehee, Nat. Photonics, 2009, 3, 406 CrossRef CAS.
- B. E. Hardin, J.-H. Yum, E. T. Hoke, T. Torres, Md. K. Nazeeruddin, M. Grätzel and M. D. McGehee, Nano Lett., 2010, 10, 3077 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and synthetic details of the new compounds. See DOI: 10.1039/c0ee00368a |
| ‡ Dedicated to the memory of Professor José Manuel Concellón Gracia. |
| This journal is © The Royal Society of Chemistry 2011 |
