Reduction of resazurin to resorufin catalyzed by gold nanoparticles: dramatic reaction acceleration by laser or LED plasmon excitation†
Carlos J. Bueno
Alejo
a,
Chiara
Fasciani
a,
Michel
Grenier
a,
José Carlos
Netto-Ferreira
*ab and
Juan C.
Scaiano
*a
aCentre for Catalysis Research and Innovation, Department of Chemistry, University of Ottawa, 10 Marie Curie, Ottawa K1N 6N5, Canada. E-mail: tito@photo.chem.uottawa.ca
bDepartamento de Química, Universidade Federal Rural do Rio de Janeiro, Seropédica, 23851-970, Rio de Janeiro, Brazil. E-mail: josecarlos@photo.chem.uottawa.ca
First published on 8th September 2011
Abstract
Plasmon excitation (532 nm) of gold nanoparticles in the presence of resazurin and hydroxylamine leads to their photocatalytic reduction to resorufin with great efficiency. In the case of laser excitation under laser-drop conditions the process is essentially complete following an ∼8 ns laser pulse at 532 nm. Excitation with LED sources at ∼530 nm proves to be a simple and cost efficient way to promote plasmon-assisted reactions. We propose that the catalytic reaction is thermally activated by the gold nanoparticle and takes advantage of the high temperatures achievable under plasmon excitation.
A. Introduction
Resazurin (1) (also known as Alamar Blue)1 is a dye widely used in biological tests due to its facile reduction to form resorufin1–3 (2) (Scheme 1). The reduction can be followed either by colorimetric methods, as the colour changes from blue (λmax = 603 nm) to pink (λmax = 570 nm), or by fluorimetric methods, given the high fluorescence emission of resorufin (λem = 582 nm) compared with the weak emission of resazurin. This reduction can be enzymatically catalyzed in cells and bacteria,3 but can also be performed by photochemical4 and electrochemical5 methods. Most of these procedures use tertiary amines as the reducing agents. Bueno et al. described a photoreduction of resazurin to resorufin using different types of amines upon irradiation at 615 nm.6 Chen et al. reported that gold nanoparticles catalyze the reduction of resazurin with hydroxylamine as the reducing agent using electrochemical methods.7,8 Chen's work used single molecule fluorescence to follow the reaction on the surface of nanoparticles. | ||
| Scheme 1 | ||
Nanoparticles are catalysts for many reactions; given their dimensions, these particles show some properties that differ from those of the bulk material. In the case of gold nanoparticles (AuNP), one of these properties is the surface plasmon resonance band (SPB) that provides the nanoparticle with a characteristic absorption band, located at ∼530 nm for spherical AuNP. The excitation of this band can affect molecules located near the AuNP in several ways. It can lead to transmitter/receiver antenna effects,9 induce electronic excitation10 and generate highly localized heat that can enhance the catalytic properties of the nanoparticles11–13 or initiate other chemical changes.11,14 Recent reviews show the great potential of nanoparticles for simultaneous molecular imaging and phototherapy15 as well as how the strong surface plasmon resonance band displayed by these nanoparticles can be used in a wide range of applications in catalysis, optics or chemical sensing.16
In this work, we carried out studies on the plasmon-mediated reduction of resazurin to resorufin by hydroxylamine, catalyzed by gold nanoparticles at room or higher temperatures. For this purpose we used LED and laser-drop excitation; the techniques were developed in our laboratory12,17 and are based on the irradiation of small drops of solution with 532 nm laser pulses that are capable of exciting the SPB of the AuNP.
B. Materials and methods
Materials
Resazurin, resorufin, hydroxylamine, and tetrachloroaurate were from Aldrich and used as received. Irgacure 2959 (I-2959) was a gift from Ciba specialty chemicals and it was recrystallized from ethyl acetate. All the solutions were prepared in Millipore water (resistivity 18.2 MΩ at 25 °C).Laser drop technique
This technique was developed in our lab and consists in irradiating a drop of solution (7–10 μL) with a pulsed laser.17 The solution is delivered to the exposure region through a Teflon tube (1/16′′ OD × 0.030′′ ID; Chromatographic Specialities) connected to a computer-controlled syringe pump. The laser excitation was performed with the frequency doubled pulses (532 nm, ∼8 ns, 1 Hz) from a Nd/YAG laser (Continuum Surelite II). The experiment was done with two different laser powers (50 and 80 mJ per pulse) and different numbers of shots per drop (1 and 10). The beam was not focused on the drop in order to avoid its disintegration. When the beam is focused, the drop is destroyed after one single shot. The drop images were captured with a Nikon D7000 DSLR camera, equipped with a Sigma 105 mm f 2.8 macro lens, controlled by the same software that controls the laser-drop system.The irradiated samples were collected in a cuvette (NaOH was added to ensure a basic pH), diluted to an absorbance of 0.1 at 532 nm (Cary-50-Bio UV-visible spectrophotometer) and their fluorescence examined in a spectrofluorimeter (Photon Technology International, PTI).
Light emitting diode (LED) irradiation
This system consists of a custom-designed irradiator using four LedEngin 10 W LZ4-40G110 emitters (nominal λexc = 530 nm) operated at 0.9 A or 0.45 A, attached to an aluminium heat sink and a fan cooling system. The actual wavelength depends on the current and temperature; in this case the dominant wavelength is ∼520 nm. In this experiment 1 mL of solution was placed in a cuvette of 0.5 × 0.5 cm path. The sample is centred in the LED irradiator and is irradiated to induce the reduction of resazurin to resorufin. To follow the reaction aliquots were taken every 2 min, diluted to 0.1 absorbance and analyzed in a spectrofluorimeter to record its emission spectrum.Microwave irradiation
These experiments were carried out using a CEM Discover Microwave. The solution was placed in a CEM 10 mL Pyrex reaction tube and sealed with a Teflon cap; each sample was irradiated for 20 min at 80 °C.C. Results
Fig. 1 shows the fluorescence emission spectra for water solutions of resazurin and resorufin (λem = 582 nm) of A530 nm = 0.1. There is a dramatic difference in the fluorescence intensity of these two compounds, which provides a reliable method to follow the reduction reaction of resazurin, in the presence of hydroxylamine, by plasmon-excitation of AuNP.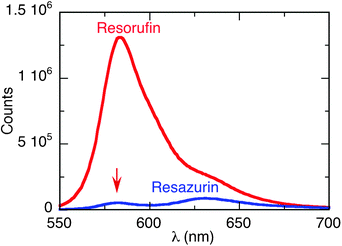 | ||
| Fig. 1 Fluorescence emission spectra for resazurin (blue) and resorufin (red). Both solutions with 0.1 absorbance at the excitation wavelength (532 nm) under basic conditions. The red arrow indicates the emission due to traces of resorufin in the resazurin sample. | ||
Laser drop technique
The reduction of resazurin to resorufin by hydroxylamine was performed at room temperature by excitation of the surface plasmon band of gold nanoparticles (AuNP) at 532 nm and employing the laser-drop technique.Solutions containing 1 mM resazurin and excess of NH2OH (2 mM) in water were added to an aqueous solution of AuNP, typically around ∼1 nM, and then irradiated (λ = 532 nm, 50 or 80 mJ per pulse) employing the laser-drop system. An instantaneous change in colour was observed upon firing the laser, with the initial reddish solution becoming bright orange and showing strong emission. The drops collected after the irradiation were diluted and their fluorescence spectrum was recorded, with the emission observed being consistent with the formation of the strongly fluorescent resorufin (see Scheme 1). Fig. 2 shows pictures of the drops photographed before and during laser excitation.
 | ||
| Fig. 2 Pictures of the drop before (resazurin) and during laser excitation (resorufin); the bright point on the left of the drops is due to a reflection of the light source illuminating the drop, and the bright orange colour of the right drop incorporates extensive product fluorescence. | ||
To evaluate the effect of changes in the number of laser shots and in the laser power, similar laser-drop experiments as described above were performed employing 1 or 10 laser shots per drop, at 50 and 80 mJ per pulse. When only one shot was delivered to the drop, the laser was concentrated and focused inside the drop, leading to its explosion as shown in the ESI.† On the other hand, for experiments in which 10 shots per drop were delivered, the laser beam was concentrated but not focused into the drop in order to avoid its explosion before reaching 10 shots of irradiation.
The plot in Fig. 3 clearly shows that independent of the conditions employed in the laser-drop experiment, resorufin formation in the presence of AuNP is very efficient, as it is shown by its highly intense fluorescence emission during the first laser shot. The emission intensity after 10 shots per drop (recorded after the completion of the experiment) is practically the same compared to that after a single shot per drop, indicating that one laser pulse achieves near-complete conversion of resazurin to resorufin. In the same way, at different laser powers the fluorescence emission intensity is very similar, from which one can conclude that 50 mJ per drop is adequate for an essentially quantitative reaction following a single laser shot. Remarkably, this means that 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 100
000 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 resazurin molecules are reduced per AuNP with a single 10 ns laser pulse!
000 resazurin molecules are reduced per AuNP with a single 10 ns laser pulse!
 | ||
| Fig. 3 Percent fluorescence emission increment relative to the maximum fluorescence emission of resorufin (λ = 582 nm) for three different experiments: (a) different laser energies (1 s/d); (b) different number of shots (50 mJ per pulse); (c) absence and presence of AuNP (50 mJ per pulse, 1 s/d); conditions for the experiments: resazurin 1 mM, NH2OH 2 mM and AuNP 1.4 nM, exception made for the control where AuNP were not employed. The individual error bars in each column show the errors within a single set of experiments, while the dashed error bar shows the reproducibility between different sets of experiments (‘a’, ‘b’ and ‘c’), frequently run on different days. | ||
To further confirm that the gold nanoparticles play a key role in the reduction of resazurin to resorufin, this reaction was performed in the absence of AuNP employing the laser-drop technique. Fig. 3 clearly shows that no conversion was obtained in the absence of the nanoparticles when this reaction is compared to that performed under the same conditions (50 mJ per pulse, 1 shot-per-drop), this time in the presence of AuNP. These results lead to the conclusion that the AuNP are required for the reaction to take place in this time scale. It is also important to note that even though a reasonable absorption at the irradiation wavelength (λ = 532 nm) is displayed by the resazurin solution due to its high concentration, no indication of resorufin formation could be observed under laser drop irradiation in the absence of AuNP.
We note that a few comparative experiments using triethylamine as a reducing agent showed only a very small fluorescence enhancement under the same laser drop exposure conditions where NH2OH causes a major fluorescence increase (see ESI†).
Light emitting diode (LED) irradiation; comparison with thermal methods
In order to establish if plasmon (or laser) excitation was required for catalysis to be effective, we performed similar experiments but using other techniques such as LED photoexcitation, a microwave oven (MW) and a thermal bath. The last two methods allow us to establish if the reaction can be simply described as a thermal process in which the AuNP acts as a catalyst for the reaction. For the MW experiment, 1 mL of solution containing resazurin, NH2OH and AuNP were added to a Pyrex tube and irradiated for 20 min at 80 °C. For the thermal bath experiment the Pyrex tube was replaced by a centrifuge tube, which was introduced into a water bath coupled to a temperature control system. In this case the experiment was performed at two different temperatures, 50 °C and 80 °C. The LED experiments confirm that upon excitation of the AuNP SPB we obtained better results than just heating up the bulk solution, but not as good as with the laser drop technique. This experiment was performed at two different LED powers (0.9 and 0.45 A) placing 1 mL of the sample into the LED irradiator and taking aliquots at different times. Fig. 4 shows the fluorescence at a given time (10 min) and the kinetic curves for all the LED and thermal bath experiments. The fact that the reaction is faster at 80 °C confirms that the reaction is temperature controlled. | ||
| Fig. 4 (Top) Kinetic traces of the experiments in the LED irradiator and the thermal bath. Solutions of resazurin (1 mM), NH2OH (2 mM) and AuNP (1.4 nM), were submitted to either the LED irradiator or a thermal bath. Samples were taken every 2 min, diluted to 0.1 absorbance and analyzed in a spectrofluorimeter to record its emission spectrum. The experiment was done at two different power settings of the LED irradiator (0.9 and 0.45 A) and at two different temperatures of the thermal bath (80 and 50 °C). (Bottom) Fluorescence emission at a given time (10 min) for the different experiments (except the LD experiment in which it corresponds to a single ∼8 ns exposure). | ||
Knowing that the AuNP and NH2OH19 are necessary for the reaction to occur, we decided to investigate the effect of varying the concentration of both, gold nanoparticles and NH2OH, on the reaction efficiency. For this purpose, solutions with different concentrations of NH2OH (from 0.5 to 2 mM) and AuNP (0.7 and 1.4 nM) were prepared and irradiated in the LED photoreactor. For these experiments the laser drop technique is not the best technique because the reaction is so fast that it was not possible to obtain a reasonable kinetic profile for the process. Fig. 5 shows the fluorescence intensity of the resorufin formed after LED irradiation for 2 minutes as a function of hydroxylamine and/or AuNP concentration.
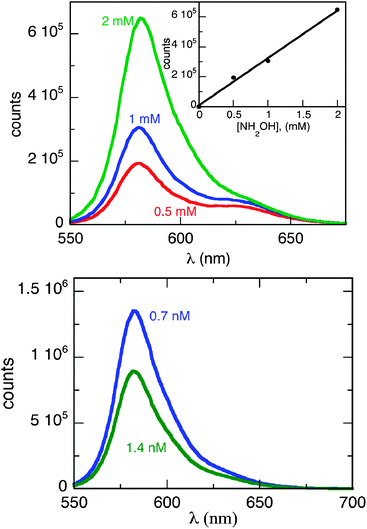 | ||
| Fig. 5 Fluorescence intensity of resorufin after LED irradiation of resazurin as a function of hydroxylamine and/or AuNP concentration. Top: different concentrations of NH2OH (0.5, 1 and 2 mM) keeping constant the concentration of the other compounds (1 mM of resazurin and 1.4 nM of AuNP); inset: fluorescence intensity vs. NH2OH concentration (0.5, 1 and 2 mM). Bottom: different concentrations of AuNP (0.7 and 1.4 nM) keeping constant the concentration of the other compounds (1 mM of resazurin and 2 mM of NH2OH). | ||
Looking at the experiments in Fig. 5 (bottom) one notes that with 0.7 nM concentration of AuNP the reaction appears to work better than with 1.4 nM, since we observe stronger fluorescence. However, it is well known that metal nanoparticles quench the fluorescence of molecules in their vicinity;9,20 quenching of resorufin fluorescence emission was performed for different concentrations of AuNP (Fig. 6) and employing the Stern–Volmer analysis of eqn (1).21,22
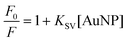 | (1) |
| KSV = kqτ | (2) |
 | ||
| Fig. 6 Top: fluorescence emission of resorufin (0.01 mM) with different concentrations of AuNP. Bottom: Stern–Volmer plots for the quenching of the emission due to the AuNP with excitation at 532 nm (red) and 560 nm (blue). | ||
From the data in Fig. 6 one estimates that for [AuNP] = 1.4 nM, over half of the resorufin molecules are associated to the AuNP. This translates to about 3570 molecules per nanoparticle; given an average AuNP size of 13 nm, this corresponds to about ∼15 Å2 per dye molecule; this area is just too small for molecules to lay flat on the AuNP surface and suggests that most molecules must bind ‘head on’ on the surface.
From these results we can assume that the differences in fluorescence intensities with different concentrations of AuNP are largely due to static quenching on the nanoparticle surface.
The study of the fluorescence growth dynamics has been summarized in Table 1; we note (see Fig. 4) that microwave irradiation (at 80 °C) is comparable to a water bath at 80 °C. For the LED at full power (0.9 A), four LEDs deliver 4 × 4.6 watt, or ∼18 watt, of this about 20% is actually absorbed by the sample; for the fastest reaction (row 2 in Table 1) about 500 J will actually be absorbed by 1 mL of solution during the half life of the reaction.
| Energy source | [AuNP]/nM | [NH2OH]/mM | t 1/2 b/seconds | Plateau reached? |
|---|---|---|---|---|
| a Half-lives (t1/2) are based on the exponential growth analysis of the resorufin fluorescence monitored every 2 minutes for 20 minutes (unless the plateau level was already achieved). b Values given in brackets when the fluorescence intensity after 20 minutes was less than 80% of the plateau value, as extrapolated from the exponential growth analysis. | ||||
| LED, 530 nm (nominal) | 0.7 | 2.0 | 180 | Yes |
| LED, 530 nm (nominal) | 1.4 | 2.0 | 132 | Yes |
| LED, 530 nm (nominal) | 1.4 | 1.0 | 186 | Yes |
| LED, 530 nm (nominal) | 1.4 | 0.5 | (700) | No |
| 80 °C water bath | 1.4 | 2.0 | 225 | Yes |
| 50 °C water bath | 1.4 | 2.0 | (>1000) | No |
D. Discussion
The reduction of resazurin to resorufin catalyzed by AuNP can be initiated in a variety of ways, from thermal methods (microwave, or a water bath) to photoexcitation of the AuNP at the plasmon transition around 530 nm. In this case, it is interesting that LED excitation under standard conditions (1.4 nM AuNP and 2 mM hydroxylamine) requires about 500 J mL−1 to achieve 50% conversion; in the case of laser excitation about 10 J mL−1 (50 mJ per pulse, ∼10 μL drop, estimated 50% light absorption) is enough to achieve over 80% conversion. Clearly, pulsed laser excitation is far more efficient, most likely because during the 8 ns laser pulse each AuNP absorbs many photons and thus the particle surface temperature can be quite high; in a recent publication we estimated this temperature to be around 500 °C.12 From a practical point of view, the photon economy of laser excitation is not enough to compensate for the convenience and cost efficiency of LED irradiation, which in fact has installation costs around 2% compared with a laboratory microwave unit. Recent studies of alcohol oxidation also illustrate the convenience of LED excitation.24In spite of the fact that the reduction of resazurin to resorufin is a very well known process, the mechanism for the catalyzed reduction of the former by hydroxylamine in the presence of AuNP is still a subject of interest. Xu et al.8,19 propose the formation of nitrite (probably from nitrous acid). At the single molecule level the concentration dependence on hydroxylamine (in excess) was not examined,8 at the ensemble level and under plasmon excitation, we observe that the reaction efficiency follows a linear dependence with hydroxylamine concentration (see Fig. 5). We believe that the AuNP behave both as a heating element through photoexcitation of their surface plasmon band, as well as a true catalyst. Given the linear dependence of the fluorescence with the concentration of NH2OH (Fig. 5), hydroxylamine should be involved in a rate-determining step of the reaction; in other words, hydroxylamine needs to either reach the resazurin molecule at the reactive site on the AuNP during the brief period12 of plasmon-induced heating, or assist in the departure of the product (resorufin) following reduction. Chen et al. proposed19 that product departure occurred by two mechanisms, substitution by fresh resazurin or ‘spontaneously’; given that NH2OH dependence was not examined, it is likely that ‘spontaneous’ product release from the surface is in fact assisted by NH2OH.
In conclusion, the laser drop technique has proven to be very useful for the reduction reaction from resazurin to resorufin using the plasmon band of the AuNP. While with other techniques this reaction can take more than 10 min to be completed, in the case of laser drop it takes just some nanoseconds, that is the duration of the laser pulse. In spite of the dramatic acceleration under laser excitation, the utility of LED plasmon excitation stands out as an inexpensive, simple and relatively fast way of promoting reactions through plasmon assisted catalysis induced by photothermal effects.
Notes and references
- J. O'Brien, I. Wilson, T. Orton and F. Pognan, Eur. J. Biochem., 2000, 267, 5421 CrossRef CAS.
- L. P. Candeias, D. P. S. MacFarlane, S. L. W. McWhinnie, N. L. Maidwell, C. A. Roeschlau, P. G. Sammes and R. Whittlesey, J. Chem. Soc., Perkin Trans. 2, 1998, 2333 RSC; N. K. Taneja and J. S. Tyagi, J. Antimicrob. Chemother., 2007, 60, 288 CrossRef CAS.
- J. D. Talbot, J. N. Barrett, E. F. Barrett and G. David, J. Neurochem., 2008, 105, 807 CrossRef CAS.
- G. V. Porcal, C. M. Previtali and S. G. Bertolotti, Dyes Pigm., 2009, 80, 206 CrossRef CAS.
- R. S. Twigg, Nature, 1945, 155, 401 CrossRef CAS; W. Xu, H. Shen, Y. J. Kim, X. Zhou, G. Liu, J. Park and P. Chen, Nano Lett., 2009, 9, 3968 CrossRef.
- C. Bueno, M. L. Villegas, S. G. Bertolotti, C. M. Previtali, M. G. Neumann and M. V. Encinas, Photochem. Photobiol., 2002, 76, 385 CrossRef CAS.
- X. Zhou, W. Xu, G. Liu, D. Panda and P. Chen, J. Am. Chem. Soc., 2009, 132, 138 CrossRef CAS; P. Chen, W. Xu, X. Zhou, D. Panda and A. Kalininskiy, Chem. Phys. Lett., 2009, 470, 151 CrossRef.
- W. Xu, J. S. Kong and P. Chen, Phys. Chem. Chem. Phys., 2009, 11, 2767 RSC.
- N. L. Pacioni, M. Gonzalez-Bejar, E. Alarcon, K. L. McGilvray and J. C. Scaiano, J. Am. Chem. Soc., 2010, 132, 6298 CrossRef CAS.
- N. Narband, M. Uppal, C. W. Dunhill, G. Hyett, M. Wilson and I. P. Parkin, Phys. Chem. Chem. Phys., 2009, 11, 10513 RSC.
- A. B. S. Bakhtiari, D. Hsiao, G. Jin, B. D. Gates and N. R. Branda, Angew. Chem., Int. Ed., 2009, 48, 4166 CrossRef CAS.
- C. Fasciani, C. J. Bueno-Alejo, M. Grenier, J. C. Netto-Ferreira and J. C. Scaiano, Org. Lett., 2011, 13, 204 CrossRef CAS.
- W. H. Hung, M. Aykol, D. Valley, W. Hou and S. B. Cronin, Nano Lett., 2010, 10, 1314 CrossRef CAS.
- K. G. Stamplecoskie, N. L. Pacioni, D. Larson and J. C. Scaiano, J. Am. Chem. Soc., 2011, 133, 9160 CrossRef CAS; L. Poon, W. Zandberg, D. Hsiao, Z. Emo, D. Sen, B. D. Gates and N. R. Branda, ACS Nano, 2010, 11, 6395 CrossRef.
- A. Ben-Yakar, D. Eversole and O. Ekici, in Non-Magnetic Metallic Nanomaterials for Life Sciences, ed. C. Kumar, John Wiley & Sons, Weinheim, 2008 Search PubMed.
- S. M. Morton, D. W. Silverstein and L. Jensen, Chem. Rev., 2011, 111, 3962 CrossRef CAS; A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096 RSC.
- J. T. Banks and J. C. Scaiano, J. Am. Chem. Soc., 1993, 115, 6409 CrossRef CAS; J. C. Scaiano and J. T. Banks, J. Braz. Chem. Soc., 1995, 6, 211 Search PubMed.
- M. L. Marin, K. L. McGilvray and J. C. Scaiano, J. Am. Chem. Soc., 2008, 130, 16572 CrossRef CAS; K. L. McGilvray, M. R. Decan, D. Wang and J. C. Scaiano, J. Am. Chem. Soc., 2006, 128, 15980 CrossRef.
- W. Xu, J. S. Kong, Y.-T. E. Yeh and P. Chen, Nat. Mater., 2008, 7, 992 CrossRef CAS.
- L. Ao, F. Gao, B. Pan, R. He and D. Cui, Anal. Chem., 2006, 78, 1104 CrossRef CAS; P. Anger, P. Bharadwaj and L. Novotny, Phys. Rev. Lett., 2006, 96, 113002 CrossRef.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Kluwer Academic-Plenum Publishers, New York, 1999 Search PubMed.
- N. J. Turro, V. Ramamurthy and J. C. Scaiano, Modern Molecular Photochemistry of Organic Molecules, University Science Publishers, New York, NY, 2010 Search PubMed.
- H. A. Montejano, M. Gervaldo and S. G. Bertolotti, Dyes Pigm., 2005, 64, 117 CrossRef CAS.
- G. L. Hallett-Tapley, M. J. Silvero, M. Gonzalez-Bejar, M. Grenier, J. C. Netto-Ferreira and J. C. Scaiano, J. Phys. Chem. C, 2011, 115, 10784 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images, drop photographs, additional fluorescence spectra and absorption spectra of dye solutions. See DOI: 10.1039/c1cy00236h |
| This journal is © The Royal Society of Chemistry 2011 |

