Enhanced catalysis under flow conditions using SiliaBond functionalized silica gels
Valerica
Pandarus
a,
Genevieve
Gingras
a,
François
Béland
*a,
Rosaria
Ciriminna
b and
Mario
Pagliaro
b
aSiliCycle Inc., 2500, Parc-Technologique Blvd, Quebec City, Quebec, Canada G1P 4S6. E-mail: fbeland@silicycle.com; Fax: +1 418 874 0355
bIstituto per lo Studio dei Materiali Nanostrutturati, CNR, Via U. La Malfa 153, 90146 Palermo, Italy
First published on 26th August 2011
Abstract
Functionalized silica gels of the SiliaBond series such as acid, base and nucleophilic catalysts can be effectively employed in solid-state syntheses under flow conditions. Comparison of reactions under batch and flow chemistry generally shows that reactions under flow result in enhanced selectivity and conversion.
Introduction
Research in heterogeneous catalysis for fine chemicals synthesis, preparative chemistry and drugs discovery is now a well established field of contemporary chemical research with plenty of practical applications.1 Many heterogenized homogeneous molecular and metal catalysts have been commercialized, including SiliaCat2 and SiliaBond3 silica-based materials made, respectively, of catalytic species and reactants entrapped in organosilica and end-capped silica sol–gel matrices. Reactions using solid, leach-proof catalysts are simpler and cleaner affording dramatic economic, technical and environmental advantages. Upon reaction completion, the precious catalyst is smoothly separated from the products by simple filtration and recycled. A further major advance enabled by these new catalytic materials is thus the possibility to carry out solid phase syntheses under flow, automated conditions; nowadays a well established technology providing with increased productivity both R&D and manufacturing in the pharmaceutical and fine chemicals industries.4 Under flow, separation of the catalyst from the products does not require any filtration (or further handling). Moreover, flow-through processes are more reliable and safer than conversions in batch. Herein we report successful application of different SiliaBond materials to several catalytic reactions widely employed in fine chemicals synthesis, including acylation, deprotection and Knoevenagel condensation. We compare reactions under batch and flow conditions showing that catalysis under flow often results in increased yields with a similar selectivity.Results and discussion
SiliaBond reagents are a class of modified silica gels for use in synthesis and purification produced via surface functionalisation of the silanol groups of sol–gel silica followed by end-capping (Fig. 1)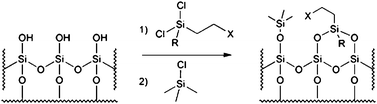 | ||
| Fig. 1 Surface functionalisation of regular silica gel, followed by end-capping. | ||
In general, the backbone of all SiliaBond products is amorphous sol–gel silica having a particle size of 40–63 μm and a pore size of 60 Å. In the following we describe application of SiliaBond DMAP (SiliCycle, R75530B), SiliaBond Tosic Acid (SiliCycle, R60530B) and SiliaBond Piperidine (SiliCycle, R71530B) reactants in catalytic amounts to different reactions. SiliaBond DMAP (Fig. 2) is the entrapped equivalent of 4-dimethylaminopyridine commonly used as a nucleophilic catalyst in a wide variety of reactions.
 | ||
| Fig. 2 Chemical structure of SiliaBond DMAP. | ||
Acylation reaction
Acylation of primary, secondary and tertiary alcohols with acetic anhydride (Ac2O) or benzoic anhydride (Bz2O) is a widely employed reaction in synthetic organic chemistry. Protection of alcoholic and phenolic hydroxyl groups is generally required. Different procedures can be applied,5 involving an acid chloride or acid anhydride with a base,6 or an anhydride in the presence of an acid catalyst. We carried out the acylation of different primary, secondary and even hindered tertiary alcohols with Ac2O and Bz2O at room temperature using a SiliaBond DMAP heterogeneous catalyst under batch and flow conditions (eqn (1)). | (1) |
Table 1 shows that primary, secondary and even hindered tertiary alcohols can be acylated with Ac2O and Bz2O at room temperature by a single passage through a simple column reactor containing a small amount of the SiliaBond DMAP catalyst. The reaction under flow is generally faster (entry 2) than in batch (entry 1) affording a complete reaction for 2-octanol; 97% yield for 1-phenyl-1-propanol (entry 6), and slightly more than 60% for hindered 1-adamantanol.
| Entry | Substrate | Reactive | Catalyst/mol% | Time/h | Batch/flow conditions | Convf (yield) (%) | Selectivityf (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Total flowd/μl min−1 | Reactor Volume/mL | Residence timee/min | |||||||
| a Reaction conditions: 4 mmol of substrate, 1.5 equiv. of acid anhydride, 1.5 equiv. of triethylamine, and 5 mol% SiliaBond DMAP stirred in 10 mL CH2Cl2 at room temperature. b Reaction conditions: 4 mmol of substrate, 1.5 equiv of acid anhydride, 1.5 equiv. of triethylamine in 10 mL CH2Cl2 and 0.45 g of SiliaBond DMAP (0.78 mmol g−1 loading) charged in Reactor R1 (3 mm ID, 0.7 mL), or 1.231 g of SiliaBond DMAP charged in Reactor R2 (6.6 mm ID, 2.4 mL). c Reactor charged with 9 mol% or 24 mol% catalyst. In each experiment the same reactor was used for all tests. d Total flow = 2 × flow rate for each pump. e Residence times in a reactor column with respect to the total flow. f Conversion and selectivity determined by GC-MS analysis. Isolated yield. | |||||||||
| 1 | 2-Octanol | Ac2O | 5 | 2 | Batch | 100 (98) | |||
| 2 | 2-Octanol | Ac2O | 4.5c (9) | 1.7 | 100.0 | R 1 | 7 | 100 | 99 |
| 0.9 | 200.0 | 3.5 | 98 | 99 | |||||
| 3 | 2-Octanol | Bz2O | 10 | 24 | Batch | 100 (91) | |||
| 4 | 2-Octanol | Bz2O | 3c (9) | 3.3 | 50.0 | R 1 | 14 | 85 | 82 |
| 6.7 | 25.0 | 28 | 93 | 95 | |||||
| 13.3 | 12.5 | 56 | 95 | 97 | |||||
| 5 | 1-Phenyl-1-propanol | Ac2O | 5 | 1.5 | Batch | 100 (98) | 99 | ||
| 6 | 1-Phenyl-1-propanol | Ac2O | 3c (9) | 3.3 | 50.0 | R 1 | 14 | 97 | 99 |
| 1.7 | 100.0 | 7 | 97 | 99 | |||||
| 0.8 | 200.0 | 3.5 | 97 | 99 | |||||
| 8 | 1-Phenyl-1-propanol | Bz2O | 5 | 24 | Batch | (88) | |||
| 9 | 1-Phenyl-1-propanol | Bz2O | 8c (24) | 1.7 | 100.0 | R2 | 24 | 88 | 98 |
| 3.3 | 50.0 | 48 | 94 | 99 | |||||
| 6.7 | 25.0 | 96 | 97 | 99 | |||||
| 10 | 1-Adamantanol | Ac2O | 6 | 24 | Batch | 67 | |||
| 11 | 1-Adamantanol | Ac2O | 8c (24) | 3.3 | 50.0 | R2 | 48 | 27 | 97 |
| 6.7 | 25.0 | 96 | 40 | 97 | |||||
| 16.7 | 10.0 | 239 | 61 | 95 | |||||
Deprotection
Protection (and deprotection) of functional groups by heterogeneous catalysis is of high practical interest.8 The methoxymethyl (MOM) group is commonly used as a hydroxyl-protecting group because it can easily be introduced and is stable under a variety of reaction conditions, including strongly basic and weakly acidic media. The cleavage of MOM ethers, furthermore, can be easily achieved using silica-supported sodium hydrogen sulfate.9 We investigated the use of SiliaBond Tosic Acid immobilized on silica for the deprotection of MOM aromatic ethers in batch and in flow chemistry using SiliaBond Tosic Acid as a catalytic mediator (eqn (2)). | (2) |
 | ||
| Fig. 3 Chemical structure of SiliaBond Tosic Acid. | ||
4-Hydroxyacetophenone and 4-methoxyphenol were chosen as models and were converted into the corresponding MOM ethers (99% conversion) by using the conventional procedure (ClOCH2OCH3, acetone, K2CO3).5
The deprotection reaction was tested in batch and in flow chemistry using dichloromethane and toluene/methanol as solvents. In batch, a mixture of 2.5 mmol of 1-(4-(MOM)phenyl)ethanone, methanol (4 equiv.) and SiliaBond Tosic Acid (0.5 equiv., 0.8 mmol g−1) in toluene (10 mL) was stirred at 65 °C for 2 h.
Under flow chemistry conditions, the flow rate and the flow reactor volume were varied to increase conversion. The reagents solution was introduced into the reactor charged with the catalyst from the sample loops to increase the volume, directly from two pumps connected to two 200 mL glass bottles. In detail, the size adjustable reactor R3 (10 mm ID, 2.1 mL) was charged with a 10 mol% SiliaBond Tosic Acid catalyst (1.56 g, 0.8 mmol g−1) and heated at 75 °C using toluene as a solvent. A solution of 12.5 mmol of 1-(4-(MOM)phenyl)ethanone in toluene (50 mL) was introduced into the 200 mL glass bottle connected to Pump 1. The second 200 mL bottle connected to Pump 2 was charged with MeOH solvent. The flow rate for the two pumps was different: 100 μl min−1 for Pump 1 and 20 μl min−1 for Pump 2. Upon completion of the reaction, the mixture was evaporated and the crude product was analysed by GC-MS. Table 2 summarizes the results of our investigation.
| Entry | Substrate | Catalyst/equiv. | Time/h | T/°C | Solvent/M | Flow conditions | Convf (yield) (%) | Selectivityf (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total flowd/μl min−1 | Reactor volume/mL | Residence timee/min | ||||||||
a Batch conditions: 1 or 2.5 mmol of substrate and 0.1–0.5 equiv. of SiliaBond Tosic Acid were stirred in 10 mL solvent at room temperature or at 65 °C. The reaction mixture was filtered and the solvent was evaporated. The crude product obtained was analysed by GC-MS.
b Flow Chemistry conditions: 2.5 mmol of substrate in 10 mL solvent were introduced from the sample loops into the flow reactor R2 (6.6 mm ID, 2.4 mL) charged with 0.35–0.5 equiv. of the SiliaBond Tosic Acid catalyst (0.8 mmol g−1 loading), at room temperature or at 65 °C. For toluene/methanol mixture add 4 equiv. methanol with respect to the substrate as additive.
c Flow Chemistry conditions: 12.5 mmol of substrate in 60 mL toluene/methanol (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were introduced directly from the pumps into the size adjustable flow reactor R3 (10 mm ID, 2.1 mL) charged with 0.1 equiv. SiliaBond Tosic Acid catalyst (0.8 mmol g−1 loading) at 65 °C.
d Total flow rate = 2 × flow for each pump.
e Residence times in a reactor column with respect to the total flow.
f Conversion and selectivity determined by GC-MS analysis. Isolated yield. 1) were introduced directly from the pumps into the size adjustable flow reactor R3 (10 mm ID, 2.1 mL) charged with 0.1 equiv. SiliaBond Tosic Acid catalyst (0.8 mmol g−1 loading) at 65 °C.
d Total flow rate = 2 × flow for each pump.
e Residence times in a reactor column with respect to the total flow.
f Conversion and selectivity determined by GC-MS analysis. Isolated yield.
|
||||||||||
| 1a |

|
0.1 | 2 | 20 | CH2Cl2 (0.1 M) | Batch | 65 | |||
| 20 | 75 | 99 | ||||||||
| 2b |
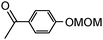
|
0.5 | 3.5 (10) | 20 | CH2Cl2 (0.1 M) | 50 | R2 | 48 | 91(55) | 98 |
| 0.5 | 1.7 (10) | 20 | CH2Cl2 (0.1 M) | 100 | R2 | 24 | 90(62) | 97 | ||
| 3a |
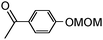
|
0.5 | 2 | 20 | Toluene/MeOH (0.24 M) | Batch | 30 | 90 | ||
| 20 | 75 | 92 | ||||||||
| 4a |

|
0.5 | 2 | 65 | Toluene/MeOH (0.24 M) | Batch | 100 | 90 | ||
| 5b |
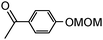
|
0.44 | 1.7 (5) | 20 | Toluene/MeOH (0.24 M) | 100 | R2 | 24 | 30 | 99 |
| 6b |

|
0.44 | 1.7 (5) | 65 | Toluene/MeOH (0.24 M) | 100 | R2 | 24 | 100 | 99 |
| 7c |

|
0.1 | 8.4 (16) | 65 | Toluene/MeOH (0.21 M) | 120 | R3 | 17.5 | 99(85) | 99 |
| 8b |

|
0.35 | 1.7 (5) | 65 | Toluene/MeOH (0.24 M) | 100 | R2 | 24 | 88 | 99 |
| 9c |

|
0.1 | 8.4 (16) | 65 | Toluene/MeOH (0.21 M) | 120 | R3 | 17.5 | 100 | 99 |
| 10c |

|
0.1 | 4.2 (10) | 65 | Toluene/MeOH (0.21 M) | 240 | R3 | 8.75 | 100(91) | 99 |
After 20 h the MOM group in the deprotection reaction of 1-(4-(MOM)phenyl)ethanone in batch at room temperature was cleaved in 75% yield (entry 1). The same conditions were tested in flow chemistry. The reagents solution was introduced into the reactor charged with the catalyst from the sample loops (10 mL solution) at 50 μl min−1 and 100 μl min−1 flow rates. Now, the MOM group was cleaved in 91% and 90% yields, respectively (entry 2). We observed a longer reaction time in dichloromethane (10 h vs. 3.5 or 1.7 h, entry 7) compared to the reaction in toluene in the presence of methanol as co-solvent.10 In this solvent system, both aromatic ethers were quantitatively cleaved with almost complete (99%) selectivity, employing modest catalytic amounts of SiliaBond Tosic Acid (0.1–0.5 equiv., entries 3–10) under mild reaction conditions.
Knoevenagel condensation
The Knoevenagel condensations between carbonyl compounds and methylene malonic esters produce several important key products that include nitriles used in anionic polymerization and unsaturated ester intermediates employed in the synthesis of several therapeutic drugs (e.g. nifedipine and nitrendipine) and other pharmacological products (e.g. calcium channel blockers and antihypertensives). Alkali metal hydroxides (e.g. NaOH and KOH), pyridine and piperidine are the traditional catalysts used in the condensation reaction. We used silica-supported piperidine SiliaBond Piperidine to catalyze the Knoevenagel condensations between benzaldehyde and ethyl cyanoacetate (eqn (3)). | (3) |
 | ||
| Fig. 4 Chemical structure of SiliaBond Piperidine. | ||
In batch, the reaction was carried out by stirring for 20 h either at room temperature or at 110 °C a solution of benzaldehyde (1.33 mmol), ethyl cyanoacetate (1.5 equiv.) and SiliaBond Piperidine (10 mol%, 1.1 mmol g−1) in toluene (10 mL).
Under flow chemistry conditions, the solution containing all reagents was introduced into the reactor directly from the pumps. The reactor (R2, 2.4 mL) was charged with 10 mol% SiliaBond Piperidine (1.364 g, 1.1 mmol g−1) and heated at 110 °C using only toluene as solvent. A mixture of 15 mmol of benzaldehyde, 1.5 equiv. of ethyl cyanoacetate in 110 mL of toluene was stirred at room temperature for 5 minutes. The mixture was divided into two equal parts and introduced in two 200 mL glass bottles connected to the pumps.
Table 3 summarizes the results of both sets of experiments. For the batch reaction (entries 1 and 2) best results were obtained at 110 °C. These conditions were thus chosen also for reactions in flow varying the flow rate and the reactor volume in order to increase the residence time in the reactor. The reagents solution was introduced into the reactor charged with the catalyst from the sample loops (10 mL solution, entry 3). Then, the volume was increased directly from the pumps (75 mL solution, entries 4–6; 110 mL solution, entry 7).
| Entry | Catalyst (mol%) | Time/h | T/°C | Batch/flow conditions | Conversion/Yieldg (%) | Selectivityg (%) | ||
|---|---|---|---|---|---|---|---|---|
| Total flowe/μl min−1 | Reactor volume/mL | Residence timef/min | ||||||
| a Reaction conditions: 1.33 mmol of benzaldehyde, 1.5 equiv. of ethyl cyanoacetate and 10 mol% SiliaBond Piperidine in 10 mL of toluene were stirred at room temperature or at 110 °C for 20 h. The reaction mixture was filtered and the solvent was evaporated. The crude product obtained was analysed by GC-MS. b Reaction conditions, entry 3: 1.33 mmol, 1 equiv. of benzaldehyde, 1.5 equiv. of ethyl cyanoacetate in 10 mL of toluene and 30 mol% SiliaBond Piperidine catalyst (0.364 g, 1.1 mmol g−1 loading) charged in Reactor 1 (3 mm ID, 0.7 mL) at 110 °C. c Reaction conditions, entries 4–6: 10 mmol, 1 equiv. of benzaldehyde, 1.5 equiv. of ethyl cyanoacetate in 75 mL of toluene and 4 mol% SiliaBond Piperidine catalyst (0.364 g, 1.1 mmol g−1 loading) charged in Reactor R1 (3 mm ID, 0.7 mL) at 110 °C or 15 mol% SiliaBond Piperidine catalyst (1.364 g, 1.1 mmol g−1 loading) charged in Reactor R2 (6.6 mm ID, 2.4 mL) at 110 °C. d Reaction conditions, entry 7: 15 mmol, 1 equiv. of benzaldehyde, 1.5 equiv. of ethyl cyanoacetate in 110 mL of toluene and 10 mol% SiliaBond Piperidine catalyst (1.364 g, 1.1 mmol g−1 loading) charged in Reactor R2 (6.6 mm ID, 2.4 mL) at 110 °C. e Total flow = 2 × flow for each pump. f Residence times in a reactor column with respect to the total flow. g Conversion and selectivity determined by GC-MS analysis. Isolated yield. | ||||||||
| 1 | 10a | 20 | R.T. | Batch | 50 | 95 | ||
| 2 | 10a | 20 | 110 | Batch | 80(77) | 98 | ||
| 3 | 30b | 3.3 | 110 | 50 | R1 | 14 | 82 | 99 |
| 4 | 4c | 25 | 110 | 50 | R1 | 14 | 75 | 99 |
| 5 | 4c | 12.5 | 110 | 100 | R1 | 7 | 72 | 99 |
| 6 | 15c | 12.5 | 110 | 100 | R2 | 24 | 89 | 99 |
| 7 | 10d | 18.5 | 110 | 100 | R2 | 24 | 90 (87) | 100 |
Clearly, the reaction under flow is greatly enhanced both in terms of yield and selectivity. Best results (entry 7 vs. entry 6) were obtained using a 10 mol% catalyst amount for a longer overall reaction time (18.5 vs. 12.5 h) pointing to an optimal amount of acidic material to ensure complete substrate conversion (probably by minimizing reactants absorption).
Conclusions
Using a commercial flow chemistry module, we have shown that silica-entrapped catalystic materials such as acid, base and nucleophilic catalysts can be effectively employed in solid-state syntheses under flow conditions. Comparison of reactions under batch and flow chemistry generally shows that flow chemistry results in enhanced selectivity and conversion. This is due to the high chemical reactivity of silica-entrapped reactants and to the intrinsic versatility of chemical processes under flow in which the time of contact between the catalyst and the reactants can be finely tuned by simply varying the reactor volume and/or the flow rate until optimal conditions are identified.Very often, functionalized silica gel materials are termed “silica-supported”. We propose, instead, to always refer to them as “silica-entrapped” or “silica-doped” materials, as the main structural feature providing these solids with their striking selective activity is the sol–gel entrapment phenomenon identified by Avnir in the early 1990s.11
As recently as in 2004, pointing to the low number of flow-through processes Kirschning and Jas remarked that “missing methods and technologies that allow rapid transfer from the research level to process development”12 required the development of effective immobilized reagents and catalysts in flow microreactors. Such reactors giving less waste, avoiding energy-consuming cryogenic cooling, and protecting-group-free synthesis to improve atom and step economy are now a scientific (and commercial) reality,13 and we believe that sol–gel silica-entrapped catalysts and reagents open the route to wide application of flow chemistry to synthetic organic chemistry, both at research and manufacturing levels. Silica-supported reagents indeed have a number of distinct advantages over their polymeric counterparts, including fast kinetics (the materials are chemical sponges that adsorb and concentrate external reactants at their surface); solvent independence (end-capped silica neither shrinks nor swells or dissolves in any solvent); ease of use (silica does not carry a static charge; it is free flowing and thus easy to weigh out and handle); and high thermal stability (withstanding temperatures of over 200 °C, suitable for use in microwave synthesizers). More continuous processes are under investigation in our laboratories.
Acknowledgements
This article is dedicated to Professor Michele Rossi on the occasion of his 70th birthday. We acknowledge to Mr. Pierre-Gilles Vaillancourt from QC Department of SiliCycle Inc. and to Mr. Maxim Drobot from Syrris (Royston, UK) for their valuable contribution.References
- See for instance the series: in Catalysts for Fine Chemical Synthesis, ed. S. M. (Main editor), Wiley-VCH, 2007, vol. 1–5 Search PubMed.
- V. Pandarus, R. Ciriminna, F. Béland and M. Pagliaro, Adv. Synth. Catal., 2011, 353, 1306 CrossRef CAS.
- For details on SiliaBond reagents and scavengers, see the URL: www.silicycle.com.
- A. E. Rubin, S. Tummala, D. A. Both, C. Wang and E. Delaney, Chem. Rev., 2006, 106, 2794 CrossRef CAS.
- P. G. M. Wuts and T. W. Green, Protecting Groups in Organic Synthesis, John Wiley & Sons, New Jersey, 4th edn, 2007 Search PubMed.
- (a) E. F. V. Scriven, Chem. Soc. Rev., 1983, 12, 129–161 RSC; (b) T. Sano, K. Ohashi and T. Oriyama, Synthesis, 1999, 1141–1144 CrossRef CAS; (c) E. Vedejs, N. S. Bennets, L. M. Conn, S. T. Diver, M. Gingras, S. Lin, P. A. Oliver and M. J. Peterson, J. Org. Chem., 1993, 58, 7286 CrossRef CAS; (d) E. Vedejs and O. Daugulis, J. Org. Chem., 1996, 61, 5702 CrossRef CAS.
- See the URL: http://www.syrris.com/flow-products/asia-flow-chemistry (last time accessed, June 13, 2011).
- G. Sartori, R. Ballini, F. Bigi, G. Bosica, R. Maggi and P. Righi, Chem. Rev., 2004, 104, 199 CrossRef CAS.
- C. Ramesh, N. Ravindranath and B. Das, J. Org. Chem., 2003, 68, 7101 CrossRef CAS.
- P. Ploypradith, P. Cheryklin, N. Niyomtham, D. R. Bertoni and S. Ruchirawat, Org. Lett., 2007, 9, 2637 CrossRef CAS.
- D. Avnir, Acc. Chem. Res., 1995, 28, 328 CrossRef CAS.
- A. Kirschning and G. Jas, Top. Curr. Chem., 2004, 242, 209 CAS.
- J.-i. Yoshida, H. Kim and A. Nagakining, ChemSusChem, 2011, 4, 331 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
