Palladium catalytic system for inhibition of O-arylation type reaction and regioselective direct arylation at C2 of phenols
Kassem
Beydoun
and
Henri
Doucet
*
Institut Sciences Chimiques de Rennes, UMR 6226 CNRS-Université de Rennes 1 “Catalyse et Organometalliques”, Campus de Beaulieu, 35042 Rennes, France. E-mail: henri.doucet@univ-rennes1.fr; Fax: +33 0223236939
First published on 4th August 2011
Abstract
The palladium-catalysed arylation of free OH phenol derivatives proceeds regioselectively either at C2 or at OH with a variety of electron-deficient aryl bromides or heteroaryl bromides. The nature of the base was found to be crucial to control the regioselectivity of the reaction. In the presence of potassium acetate, the direct arylation at C2 is favoured; whereas, under the same reaction conditions, the use of potassium carbonate gave selectively the O-arylation type products.
Introduction
Biaryl derivatives display a set of bioactive or physical properties and their preparation constitutes an active field of research in organic chemistry.1 Several methods for the preparation of those products have been described. The palladium-catalysed direct arylation of some aromatics via a C–H bond activation using aryl halides has led to successes in recent years.2,3 For such direct coupling reactions, no preparation of an organometallic derivative is required, and this is a tremendous advantage as compared to the more classical cross-coupling procedures arising from more costly Suzuki–Miyaura, Stille, or Negishi reactions. Moreover, the direct arylation reaction provides only an acid (HX) associated to a required base as a by-product and therefore presents advantages both in terms of atom-economy and relatively inert wastes. However, if this procedure has been widely applied in recent years for the synthesis of biaryls bearing alkoxy, fluoro, or amide substituents,2a on the other hand, relatively few biaryls bearing unprotected hydroxy as a substituent have been prepared by palladium-catalysed direct C–H bond functionalisation.4–7 This might be due to the possible competitive palladium-catalysed reaction of aryl halides with phenols to form ethers.8 So far, most of the examples of direct arylation of phenols employ rhodium catalysts.9 A few examples of intramolecular reactions,5 and intermolecular palladium-catalysed reactions using protected phenols have also been described.10 The arylation at C2′ of a 2-hydroxybiphenyl using Cs2CO3 as the base has also been reported.7We now report that the use of KOAc as the base controls the intermolecular direct palladium-catalysed arylation of phenols with aryl bromides at carbon C2 with inhibition of the O-arylation reaction; whereas, the use of K2CO3 as the base promotes selectively the O-arylation in high yield.
Results and discussion
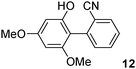
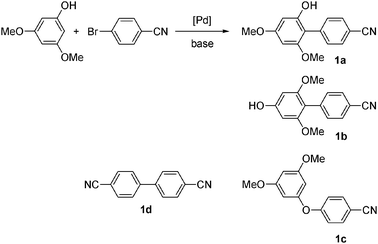
As the nature of the base has been found in recent years to be a crucial factor to promote efficiently palladium-catalysed direct arylations,11 we could expect a drastic influence of the nature of the base, associated with that of the ligand, on the selectivity for the palladium-catalysed reaction of aryl bromides with phenols.First, we have considered the reaction of 3,5-dimethoxyphenol with 4-bromobenzonitrile to reach the formation of C2 arylated phenol 1a (Scheme 1). The formation of O-arylation type product 1c was also expected.
 | ||
| Scheme 1 Arylation of 3,5-dimethoxyphenol with 4-bromobenzonitrile. | ||
We first explored the activity of several catalysts for this reaction. KOAc and DMAc were employed as the base and solvent, as we had previously observed that under these conditions the O-arylation of furfuryl alcohol11a or the N-arylation of an aminothiophene11b was inhibited to give regioselectively the direct arylation at C5 of the heteroaromatics. The assistance of carboxylate ligand on palladium for the direct arylation has been demonstrated by Fagnou.12 In the presence of 4 mol% Pd(OAc)2 as the catalyst, direct arylation product 1a was obtained in moderate yield and selectivity (Table 1, entry 1). The reaction was found to give 43% arylation at C2 and 50% homocoupling product 1d. Only traces of C4 arylated product 1b or O-arylation product 1c were detected. The use of 2 mol% Pd(OAc)2, PdCl2 or [PdCl(C3H5)]2 led to 51–60% selectivities in 1a (Table 1, entries 2–4). The addition of the phosphine ligands dppe or PPh3 was found to give low selectivities in 1a; whereas the use of dppb produced 1a in 61% selectivity, but only a partial conversion of 4-bromobenzonitrile was observed (Table 1, entries 5–7). Then, we explored the activity of PdCl(C3H5)(dppb), as we recently demonstrated it was one of the best catalysts for the direct arylation of some furans, thiophenes or thiazoles.13 Using 2 mol% of PdCl(C3H5)(dppb) as the catalyst precursor, DMAc as the solvent and KOAc as the base at 150 °C, the desired C2-arylation product 1a was obtained in 59% selectivity (Table 1, entry 8). Moreover, a complete conversion of the aryl bromide was observed. The O-arylation product 1c was also produced in 9% selectivity. We also evaluated the influence of the nature of the base. In the presence of the carbonates, K2CO3 or Cs2CO3, complete conversions of 4-bromobenzonitrile were observed, but only traces of product 1a were detected (Table 1, entries 11 and 12). With these two bases, the major product was the O-arylated phenol 1c which was obtained in 92% and 88% selectivities. Finally, it should be noted that the ratio of 3,5-dimethoxyphenol and 4-bromobenzonitrile has also an important influence on the selectivity of the reaction. A 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of these reactants gives much better results than a 1
1 ratio of these reactants gives much better results than a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Table 1, entries 8 and 16).
1 ratio (Table 1, entries 8 and 16).
| Entry | [Pd] (mol%) | Ratio ArOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ArBr ArBr |
Base | Ratio 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b 1b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d 1d |
Conv. (%) |
|---|---|---|---|---|---|
| a 4-Bromobenzonitrile (1 mmol), 3,5-dimethoxyphenol (1–4 mmol), base (2 mmol), 150 °C, 40 h, under argon, conversion of 4-bromobenzonitrile. b Isolated yield in 1a. c 130 °C. | |||||
| 1 | Pd(OAc)2 (4) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
86 |
| 2 | Pd(OAc)2 (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
78 |
| 3 | PdCl2 (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
100 |
| 4 | ½ [PdCl(C3H5)]2 (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
100 |
| 5 | Pd(OAc)2/dppe (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
45 |
| 6 | Pd(OAc)2/dppb (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
74 |
| 7 | Pd(OAc)2/2 PPh3 (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
54 |
| 8 | PdCl(C3H5)(dppb) (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
100 (39)b |
| 9 | PdCl(C3H5)(dppb) (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NaOAc | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 58 |
90 |
| 10 | PdCl(C3H5)(dppb) (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOAc | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
90 |
| 11 | PdCl(C3H5)(dppb) (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
100 |
| 12 | PdCl(C3H5)(dppb) (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Cs2CO3 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 |
| 13 | PdCl(C3H5)(dppb) (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KF | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 87 |
60 |
| 14 | PdCl(C3H5)(dppb) (2) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
40c |
| 15 | PdCl(C3H5)(dppb) (2) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57 |
92 |
| 16 | PdCl(C3H5)(dppb) (2) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOAc | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
65 |
The scope of the coupling of phenols at C2 or O using other aryl bromides was then investigated using PdCl(C3H5)(dppb) catalyst and either KOAc (Table 2) or K2CO3 (Table 3) as the base (Scheme 2).
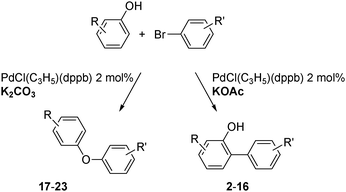 | ||
| Scheme 2 Palladium-catalysed C- or O-arylation of phenols. | ||
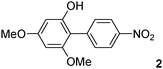
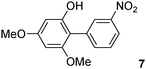
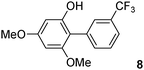
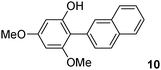
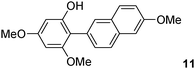
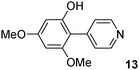
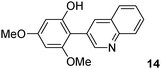
The C2 arylation reactions were performed using DMAc, KOAc, 150 °C and 2 mol% catalyst as the reaction conditions. First, we studied the reactivity of para-substituted aryl bromides. In the presence of electron-deficient aryl bromides such as 4-bromonitrobenzene, 4-trifluoromethylbromobenzene, 4-bromobenzophenone or 4-bromopropiophenone, the products 2–5 were obtained in 32–38% yields (Table 2, entries 1–4). Similar yields were obtained in the presence of the meta- or ortho-substituted aryl bromides, 2- or 3-bromobenzonitriles, 3-bromonitrobenzene, 3-trifluoromethylbromobenzene and also with 2-bromonaphthalene (Table 2, entries 5–7, 9 and 10). An higher yield of 54% was obtained with 3,5-bis(trifluoromethyl)bromobenzene (Table 2, entry 8). N-containing heterocycles are very common motifs in pharmaceutically active compounds. Therefore, preparative methods of biheteroaryl derivatives containing pyridines or quinolines remain an essential research topic in organic synthesis. We observed that 4-bromopyridine or 3-bromoquinoline also gave regioselectively the desired coupling products 13 and 14 in 43% and 44% yields (Table 2, entries 12 and 13). For all these reactions, complete conversions of the aryl bromides were observed. However, in all cases, quite large amounts of the homocoupling products of the aryl bromides were formed as side-products lowering the yields.
With a phenol bearing a dimethylamino substituent at C3, the direct arylation proceeds regioselectively at C6 (Scheme 3). No arylation at C2 was detected. This regioselectivity might come from steric factors. Using 4-bromobenzonitrile or 3,5-bis(trifluoromethyl)bromobenzene 15 and 16 were obtained in 41% and 44% yields, respectively.
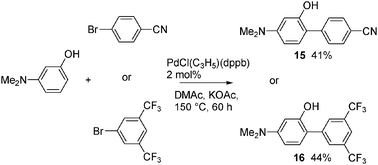 | ||
| Scheme 3 Palladium-catalysed direct arylation of 3-dimethylaminophenol. | ||
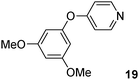
Then, we also extended the scope of the O-arylation of phenols using K2CO3 as the base and PdCl(C3H5)(dppb) catalyst (Table 3). Such simple coupling reaction conditions employing a cheaper base and a ligand than those usually employed (Cs2CO3 and electron-rich phosphanes) might be useful. Four aryl bromides, 2- or 4-bromobenzonitriles, 4-bromonitrobenzene or 4-bromopyridine, have been reacted with 3,5-dimethoxyphenol. In all cases, the desired arylation products 1c and 17–19 were obtained very selectively and in good yields of 74–91% (Table 3, entries 1–4). Four other phenol derivatives were also employed successfully. The coupling of 3-dimethylaminophenol, 4-methoxyphenol, 3,5-dimethylphenol or 2,6-dimethoxyphenol with 4-bromonitrobenzene gave 20–23 in 83–93% yields (Table 3, entries 5–8).
Conclusion
In summary, we have demonstrated that when appropriate reaction conditions are employed, the palladium-catalysed direct arylation of free OH phenol derivatives proceeds regioselectively either at C2 or at OH with a variety of electron-deficient aryl bromides and heteroaryl bromides. The regioselectivity of the coupling depends on the nature of the base. In the presence of K2CO3 selective O-arylations were observed. On the other hand, the use of KOAc as the base inhibits the O-arylation and promotes the arylation at C2. These regioselectivities are consistent with a concerted metallation deprotonation mechanism (CMD) demonstrated by Lapointe and Fagnou.12b To our knowledge, this is the first method for palladium-catalysed direct mono-arylation at C2 of unprotected phenols. This procedure generally gives lower yields than the rhodium-catalysed direct arylation of phenols;9 on the other hand, lower catalyst and ligand loadings were employed, and a wider functional group tolerance on the aryl bromide was observed.Experimental section
DMAc (N,N-dimethylacetamide) (99%) was purchased from Acros. KOAc (99%), K2CO3 (99%), [Pd(C3H5)Cl]2 (56.5%) and dppb [1,4-bis(diphenylphosphino)butane] (98%) were purchased from Alfa Aesar. These compounds were not purified before use.Preparation of the PdCl(C3H5)(dppb) catalyst14
An oven-dried 40 mL Schlenk tube equipped with a magnetic stirring bar under an argon atmosphere was charged with [Pd(C3H5)Cl]2 (182 mg, 0.5 mmol) and dppb (426 mg, 1 mmol). 10 mL of anhydrous dichloromethane were added, then, the solution was stirred at room temperature for twenty minutes. The solvent was removed in vacuum. The yellow powder was used without purification. 31P NMR (81 MHz, CDCl3) δ = 19.3 (s).General procedure for the direct ortho-arylation of phenols 1a and 2–16
In a typical experiment, the aryl bromide (1 mmol), phenol derivative (4 mmol), KOAc (196 mg, 2 mmol) and PdCl(C3H5)(dppb) (13.6 mg, 0.02 mmol) were dissolved in DMAc (10 mL) under an argon atmosphere. The reaction mixture was stirred at 150 °C for 24–60 h (see Table 2). Then, the solvent was evaporated and the product was purified by silica gel column chromatography.General procedure for the formation of ethers 1c and 17–23
In a typical experiment, the aryl bromide (1.2 mmol), phenol derivative (1 mmol), K2CO3 (276 mg, 2 mmol) and PdCl(C3H5)(dppb) (13.6 mg, 0.02 mmol) were dissolved in DMAc (10 mL) under an argon atmosphere. The reaction mixture was stirred at 150 °C for 24–40 h (see Table 3). Then, the solvent was evaporated and the product was purified by silica gel column chromatography.Acknowledgements
K.B. is grateful to CNRS and “Conseil regional de Bretagne” for a grant. We thank the CNRS and “Rennes Metropole” for providing financial support.Notes and references
- J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS.
- (a) K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS; (b) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (c) T. Satoh and M. Miura, Chem. Lett., 2007, 200 CrossRef CAS; (d) L.-C. Campeau, D. R. Stuart and K. Fagnou, Aldrichimica Acta, 2007, 40, 35 CAS; (e) I. V. Seregin and V. Gevoryan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (f) B.-J. Li, S.-D. Yang and Z.-J. Shi, Synlett, 2008, 949 CAS; (g) F. Bellina and R. Rossi, Tetrahedron, 2009, 65, 10269 CrossRef CAS; (h) L. Ackermann, R. Vicente and A. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS; (i) J. Roger, A. L. Gottumukkala and H. Doucet, ChemCatChem, 2010, 2, 20 CrossRef CAS; (j) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Commun., 2010, 46, 677 RSC; (k) C. Fischmeister and H. Doucet, Green Chem., 2011, 13, 741 RSC.
- For selected recent examples of palladium-catalysed direct arylations of heteroaromatics from our laboratory: (a) A. L. Gottumukkala and H. Doucet, Adv. Synth. Catal., 2008, 350, 2183 CrossRef CAS; (b) Y. Fall, H. Doucet and M. Santelli, ChemSusChem, 2009, 2, 153 CrossRef CAS; (c) J. J. Dong, J. Roger, F. Pozgan and H. Doucet, Green Chem., 2009, 11, 1832 RSC; (d) J. J. Dong, J. Roger and H. Doucet, Tetrahedron Lett., 2009, 50, 2778 CrossRef CAS; (e) J. Roger, F. Požgan and H. Doucet, Green Chem., 2009, 11, 425 RSC; (f) M. Ionita, J. Roger and H. Doucet, ChemSusChem, 2010, 3, 367 CAS; (g) J. J. Dong and H. Doucet, Eur. J. Org. Chem., 2010, 611 CrossRef CAS; (h) J. Roger, S. Mom, M. Beaupérin, S. Royer, P. Meunier, V. V. Ivanov, H. Doucet and J.-C. Hierso, ChemCatChem, 2010, 2, 296 CrossRef CAS; (i) L. Chen, J. Roger, C. Bruneau, P. H. Dixneuf and H. Doucet, Chem. Commun., 2011, 47, 1872 RSC.
- For examples of palladium-catalysed intermolecular direct polyarylation of phenols: Y. Kawamura, T. Satoh, M. Miura and M. Nomura, Chem. Lett., 1999, 961 CrossRef CAS.
- For examples of palladium-catalysed intramolecular direct arylation of phenols: (a) S. Wiegand and H. J. Schaefer, Tetrahedron, 1995, 51, 5341 CrossRef CAS; (b) D. D. Hennings, S. Iwasa and V. H. Rawal, J. Org. Chem., 1997, 62, 2 CrossRef CAS; (c) D. D. Hennings, S. Iwasa and V. H. Rawal, Tetrahedron Lett., 1997, 38, 6379 CrossRef CAS; (d) T. Harayama, Y. Kawata, C. Nagura, T. Sato, T. Miyagoe, H. Abe and Y. Takeuchi, Tetrahedron Lett., 2005, 46, 6091 CrossRef CAS.
- For examples of palladium-catalysed intermolecular direct arylation of protected phenols: (a) N. Guerbuez, I. Ozdemir and B. Cetinkaya, Tetrahedron Lett., 2005, 46, 2273 CrossRef CAS; (b) M. Lafrance and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 16496 CrossRef CAS; (c) C. Qin and W. Lu, J. Org. Chem., 2008, 73, 7424 CrossRef CAS; (d) J. Kim, M. Jo, W. So and Z. No, Tetrahedron Lett., 2009, 50, 1229 CrossRef CAS; (e) B. Xiao, Y. Fu, J. Xu, T.-J. Gong, J.-J. Dai, J. Yi and L. Liu, J. Am. Chem. Soc., 2010, 132, 468 CrossRef CAS; (f) R. B. Bedford, C. J. Mitchell and R. L. Webster, Chem. Commun., 2010, 46, 3095 RSC; (g) F. Shibahara, E. Yamaguchi and T. Murai, Chem. Commun., 2010, 46, 2471 RSC.
- For selected examples of direct arylation of 2-hydroxybiphenyls: (a) T. Satoh, Y. Kawamura, M. Miura and M. Nomura, Angew. Chem., Int. Ed. Engl., 1997, 36, 1740 CrossRef CAS; (b) T. Satoh, J.-I. Inoh, Y. Kawamura, M. Miura and M. Nomura, Bull. Chem. Soc. Jpn., 1998, 71, 2239 CrossRef CAS.
- For examples of palladium-catalysed synthesis of ethers from alcohols and aryl halides: (a) M. Palucki, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1996, 118, 10333 CrossRef CAS; (b) N. Kataoka, Q. Shelby, J. P. Stambuli and J. F. Hartwig, J. Org. Chem., 2002, 67, 5553 CrossRef CAS; (c) C. H. Burgos, T. E. Barder, X. Huang and S. L. Buchwald, Angew. Chem., Int. Ed., 2006, 45, 4321 CrossRef CAS; (d) T. Hu, T. Schulz, C. Torborg, X. Chen, J. Wang, M. Beller and J. Huang, Chem. Commun., 2009, 47, 7330 RSC.
- For examples of rhodium-catalysed direct arylation at C2 of phenols: (a) R. B. Bedford, S. J. Coles, M. B. Hursthouse and M. E. Limmert, Angew. Chem., Int. Ed., 2003, 42, 112 CrossRef CAS; (b) R. B. Bedford and M. E. Limmert, J. Org. Chem., 2003, 68, 8669 CrossRef CAS; (c) S. Oi, S. Watanabe, S. Fukita and Y. Inoue, Tetrahedron Lett., 2003, 44, 8665 CrossRef CAS; (d) R. B. Bedford, M. Betham, A. J. M. Caffyn, J. P. H. Charmant, L. C. Lewis-Alleyne, P. D. Long, D. Polo-Ceron and S. Prashar, Chem. Commun., 2008, 990 RSC; (e) R. B. Bedford, M. F. Haddow, R. L. Webster and C. J. Mitchell, Org. Biomol. Chem., 2009, 7, 3119 RSC; (f) R. M. Denton, J. T. Scragg, A. M. Galofre, X. Gui and W. Lewis, Tetrahedron, 2010, 66, 8029 CrossRef CAS; (g) P. D. MacLeod, A. M. Reckling and C.-J. Li, Heterocycles, 2010, 80, 1319 CrossRef CAS.
- For selected examples of palladium-catalysed intramolecular direct arylation of protected phenols: (a) D. E. Ames and A. Opalko, Synthesis, 1983, 234 CrossRef CAS; (b) T. Harayama, T. Sato, A. Hori, H. Abe and Y. Takeuchi, Synthesis, 2004, 1446 CrossRef CAS; (c) H. Abe, S. Takeda, T. Fujita, K. Nishioka, Y. Takeuchi and T. Harayama, Tetrahedron Lett., 2004, 45, 2327 CrossRef CAS; (d) L.-C. Campeau, P. Thansandote and K. Fagnou, Org. Lett., 2005, 7, 1857 CrossRef CAS; (e) M. Parisien, D. Valette and K. Fagnou, J. Org. Chem., 2005, 70, 7578 CrossRef CAS; (f) L.-C. Campeau, M. Parisien, A. Jean and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 581 CrossRef CAS; (g) C. Huang and V. Gevorgyan, J. Am. Chem. Soc., 2009, 131, 10844 CrossRef CAS; (h) C. Huang and V. Gevorgyan, Org. Lett., 2010, 12, 2442 CrossRef CAS; (i) H. Lusic, R. Uprety and A. Deiters, Org. Lett., 2010, 12, 916 CrossRef CAS.
- (a) J. Roger, F. Pozgan and H. Doucet, Adv. Synth. Catal., 2010, 352, 696 CrossRef CAS; (b) F. Derridj, J. Roger, S. Djebbar and H. Doucet, Org. Lett., 2010, 12, 4320 CrossRef CAS.
- (a) D. L. Davies, S. M. A. Donald and S. A. Macgregor, J. Am. Chem. Soc., 2005, 127, 13754 CrossRef CAS; (b) D. Lapointe and K. Fagnou, Chem. Lett., 2010, 39, 1118 CrossRef.
- (a) F. Derridj, J. Roger, S. Djebbar and H. Doucet, J. Organomet. Chem., 2009, 694, 455 CrossRef CAS; (b) J. Roger, C. Verrier, R. Le Goff, C. Hoarau and H. Doucet, ChemSusChem, 2009, 2, 951 CrossRef CAS; (c) J. J. Dong, J. Roger, C. Verrier, T. Martin, R. Le Goff, C. Hoarau and H. Doucet, Green Chem., 2010, 12, 2053 RSC.
- T. Cantat, E. Génin, C. Giroud, G. Meyer and A. Jutand, J. Organomet. Chem., 2003, 687, 365 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |