Solvent free synthesis of nanocrystalline hexaaluminate-type mixed oxides with high specific surface areas for CO oxidation reaction†
Said
Laassiri
ab,
Daniel
Duprez
b,
Sébastien
Royer
ab and
Houshang
Alamdari
*a
aDepartment of Mining, Metallurgical and Materials Engineering, University Laval, Québec (QC), Canada G1V 0A6. E-mail: houshang.alamdari@gmn.ulaval.ca; Fax: +1-418-656-5343; Tel: +1-418-656-7666
bUniversité de Poitiers, LACCO UMR 6503 CNRS, 40 Avenue du Recteur Pineau, 86022 Poitiers Cedex, France
First published on 23rd August 2011
Abstract
Nanocrystalline hexaaluminate-type mixed oxides having crystallite sizes of around 20 nm and high surface areas (>77 m2 g−1) were synthesized using an original mechanosynthesis technique. These nanocrystalline materials were evidenced to be highly active heterogeneous catalysts compared to the conventional parent materials.
Nanoparticles have attracted great interests in many fields of research including physics, chemistry, biology, etc.1,2 In heterogeneous catalysis, synthesis of nanostructured metals or oxides/mixed-oxides is of crucial importance, due to the large surface areas and high proportions of accessible surface atoms generated over nanoparticles, leading to largely improved reactivity.2,3 In addition, the chemical or surface properties below a critical size could show a non-linear evolution with crystallite size (“nano effect”).4 Mixed-oxides, amongst perovskites and spinels, containing at least one reducible cation inserted in a defined crystalline structure, are evidenced to exhibit high activity for redox heterogeneous reactions (VOC and NOx remediation, reforming, selective oxidationetc.).5,6 Indeed, mixed-oxides can be produced over a wide range of compositions offering different surface and chemical properties which make them suitable for various catalytic applications. However, high temperature treatments are usually required for crystallization. These treatments result in crystal growth and inevitable surface reduction that provides a fine limited accessible surface for catalytic reactions. The syntheses of perovskites and hexaaluminates are representative examples of the difficulty to maintain limited crystal size and high surface area, due to the high calcination temperature required to complete their crystallization (>600 °C for perovskite, >1100 °C for hexaaluminate). Consequently, surface areas rarely exceed 20 m2 g−1 after thermal treatment. During the past 20 years, numerous procedures have been proposed to achieve nanocrystalline mixed-oxide structure crystallization. Among several processes, only the microemulsion process was efficient to achieve very high surface areas for hexaaluminate structures (∼100–160 m2 g−1, compared to <20 m2 g−1 classically obtained), leading to high catalytic activities.7–9 Such high surface areas are obtained only when freeze-drying or supercritical drying procedures are used, while lower surface areas are obtained using conventional drying processes. In addition, the control of the synthesis emulsion media is quite difficult, and the amount of material produced by this route remains limited.10 On these surfaces, high catalytic activities can be obtained over hexaaluminate type materials, as evidenced by Zazur and Ying in the case of methane combustion.9 Nevertheless, the use of hexaaluminate in heterogeneous catalysis remains really limited compared to other mixed-oxide structures such as perovskites and spinels. In fact, the limited use of hexaaluminates (mainly for methane combustion,7,11methane reforming,12 and recently for space propulsion13) may be related to their low surface areas.
In this work, a new and simple route for the synthesis of nanocrystalline hexaaluminates (∼20 nm crystal size) with high surface areas is presented. The synthesis route (activated reactive synthesis, ARS) is a combination of two grinding processes, high energy and low energy ball milling. The high energy ball milling causes a significant crystallite size reduction. Although the mean crystallite size is reduced down to 20 nm, the resulting material is highly agglomerated with very high amount of grain boundaries. A second milling process with lower energy is therefore performed to deagglomerate the nanostructured material and to develop high specific surface areas. This route has been previously reported to be efficient for the synthesis of nanocrystalline perovskite-type materials with improved catalytic and gas sensing properties.14,15 Nevertheless, it is considerably easier to crystallize perovskite than hexaaluminate, and here we demonstrate the feasibility of nanostructuring a high temperature crystalline phase. In addition, the scale-up of the proposed process is easy, and a large amount of material could be synthesized without any requirement for expensive waste treatments and sophisticated process control as in the case of applying both microemulsion and freeze drying processes. Indeed, materials presented in this work are prepared in 60 g batches.
Barium hexaaluminate (BHa) nanomaterials were synthesized using the activated reactive synthesis (ARS) process. Micron-sized BHa, crystallized by solid state reaction at high temperature, was milled in a tungsten carbide (WC) crucible, sealed with a Viton O-ring under air and fixed on a laboratory high energy shaker (SPEX). Different milling times from 30 to 300 min, at an agitation speed of 1100 cycles per minute, were applied to study the evolution of the crystallite size during milling. The resulting powder was subjected to a second milling step at low energy, on a laboratory attritor operated at a rotation speed of 250 rpm, to deagglomerate nanocrystals and increase the surface area. In addition, other formulations were prepared using the same process in which an aluminium cation is substituted by Co or Mn–Pd. The efficiency of the synthesis process is validated over a hexaferrite structure (characterization results given in ESI†). The synthesized materials were characterized using XRD, TEM and N2-sorption to evaluate the efficiency of the process to provide materials with high surface areas while keeping their crystallinity. Catalytic activity of selected nanomaterials (containing Pd) was evaluated using the probe surface reaction of CO oxidation since it allows finely characterizing the surface state of materials.6 This point is especially important since it allows concluding on the grinding effect on surface reactivity which can be altered by possible poisoning. All details concerning synthesis and characterization are given in ESI.†
Structural evolution of the material during the applied milling process is followed by XRD, exemplified in Fig. 1 for the BaAl12O19 material, while the effect of milling time on the structure and crystal size is presented in Fig. S1 and S2, ESI.† The SSR material exhibits only reflections of the Ba-β-Al2O3 as classically reported in the literature.9,11 Intense and sharp peaks are then observed [Fig. 1(a)]. After the HEBM step, the material keeps its Ba-β-Al2O3 structure [Fig. 1(b)], and no phase segregation or impurity formation can be observed. Nevertheless, the reflections are broadened, indicating a significant crystallite size reduction. The Scherrer equation applied to the (114) plan leads to the formation of crystals at a size below 27 nm no matter what the synthesized material will be (Table 1). This size of the crystals is considerably smaller than the initial crystal size (>1 μm, as evaluated by TEM in our case, but classically obtained by SSR11a). A supplementary LEBM step is evidenced to induce no modification of the structure, and as it is shown in Table 1, the same crystal sizes were obtained. Similar evolutions are also observed during the ball milling of the substituted HBa materials (Fig. S3 and S4, ESI†) and the hexaferrite material (Fig. S5, ESI†).
 | ||
| Fig. 1 XRD patterns of BaAl12O19 at each step of the synthesis. BaAl12O19 after (a) SSR, (b) HEBM (180 min) and (c) HEBM (180 min) followed by LEBM (15 min). | ||
| Solid state reaction (SSR) | High energy ball mill (HEBM) | Low energy ball mill (LEBM) | T 10% c/°C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SSA a/m2 g−1 | D cryst b/nm | SSA/m2 g−1 | D cryst/nm | SSA/m2 g−1 | D cryst/nm | SSR | HEBM | LEBM | |
| a SSA is the specific surface area evaluated using the BET model. b D crystal is the crystal domain size evaluated from the X-ray line broadening using the Scherrer equation. c T 10% is the temperature at 10% conversion of CO into CO2. | |||||||||
| BaAl12O19 | 2 | >1 μm | 4 | 19 | 100 | 19 | — | — | — |
| BaCoAl11O19 | 1 | >1 μm | 4 | 23 | 79 | 26 | — | — | — |
| BaMnPd0.07Al10.93O19 | 1 | >1 μm | 4 | 26 | 77 | 27 | 205 | 210 | 130 |
Due to the large crystal size of the SSR materials, very low surface areas are measured for the three presented materials (always ≤2 m2 g−1, Table 1). These low surface areas are in agreement with those reported for materials synthesized at a similar temperature (4–6 m2 g−1 for BaAl12O19 synthesized at 1400–1600 °C11a). Despite a considerable crystallite size reduction after the HEBM step, the specific surface area remains low (∼4 m2 g−1 whatever the solid, Table 1). These surface areas are then lower than those obtained using conventional sol–gel or precipitation routes (generally around 10–20 m2 g−1).11b,16
Nevertheless, materials subjected to a supplementary LEBM step display very high surface areas (between 77 m2 g−1 for BaMnPd0.07Al10.93O19 and 100 m2 g−1 for BaAl12O19, Table 1). Such high surface areas to our knowledge were only reported over similar solids using reverse microemulsion synthesis followed by a non-conventional drying process.7–9 Reverse microemulsion is however more difficult to proceed than the simple procedure proposed in this work.
The evolution of physical properties described below easily correlates to the morphological evolution, as observed by microscopy. While large micrometric crystals are easily observed by SEM and TEM for the SSR material [Fig. 2(a) and Fig. S6, ESI†], completely modified morphology is observed after the HEBM step [Fig. 2(b) for low magnification TEM and Fig. S7 (ESI†) for TEM and SEM]. Indeed, dense aggregates of nanometric crystals are clearly evidenced. Then, the strong agglomeration of the nanoparticles explains why limited surface areas are measured. After the LEBM step, the crystal size remains unchanged (Table 1), but particles seem to deagglomerate [Fig. 2(c) and Fig. S8, ESI†], which can explain the development of higher surface areas that were measured (related to the limited crystal sizes). Then, morphological evolution at the micrometric (aggregates) and nanometric (crystal) scales can explain the large differences in physical properties.
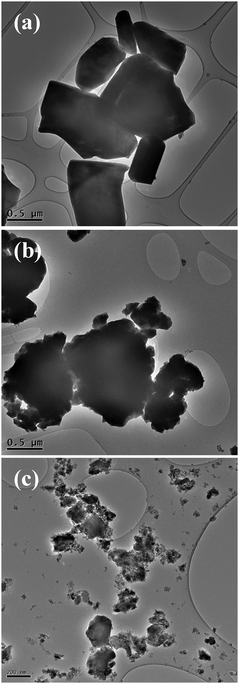 | ||
| Fig. 2 TEM micrographs of BaAl12O19 at each step of the synthesis. BaAl12O19 after (a) SSR, (b) HEBM (180 min) and (c) HEBM (180 min) followed by LEBM (15 min). | ||
Activity of materials containing palladium was evaluated for the CO oxidation reaction, a probe molecule allowing a fine characterization of the solid surfaces.6 Materials having only a transition metal in the structure present limited activity at low temperature, as verified comparing BaMnPd0.07Al10.93O19 with BaMnAl11O19 after the SSR step. Adding palladium only has a limited effect on the activity, with higher activity at low temperature while complete conversion is obtained for the two materials at temperatures higher than 260 °C (Fig. 3). No important improvement in catalytic activity can be observed after the first milling step (HEBM), which is consistent with the limited increase in surface area, suggesting that the crystal size decrease does not induce any drastic decrease in surface site specific activity. However, an important increase in CO activity is clearly observed after the second milling step (LEBM) with a decrease of 10% CO conversion temperature of ∼80 °C (Table 1). Therefore, complete conversion over LEBM material is achieved only at 175 °C (then 85 °C lower than the low surface area materials, Fig. 3). This increase in catalytic activity is consistent with the increase in surface area measured over the different materials, and is consistent with the suprafacial oxidation mechanism proposed for the CO oxidation reaction.6 Indeed, the low reaction temperatures for CO oxidation suggest only the participation of the catalyst surface, without participation of the oxygen from the bulk materials,5 that renders this reaction suitable for surface characterisation. Nevertheless, the participation of bulk oxygen in the reaction cannot be completely excluded over these systems on the basis of the results presented in this work. The activity evolution however confirms that the grinding process has no negative effect on the surface reactivity of the final materials. This is a crucial point since grinding could result in surface contamination originated from the grinder, a phenomenon which can result in slight inhibition of catalytic activity.15,17 Nevertheless, contamination can be limited by using a tungsten carbide crucible for grinding, as in this study.
 | ||
| Fig. 3 Catalytic activity for CO oxidation of BaMnPd0.07Al10.93O19: (■) SSR; (▲) HEBM (180 min); (◆) LEBM. (●) BaMnAl11O19 SSR reference. | ||
Conclusions
Nano-unsubstituted and -substituted Ba-hexaaluminates with high surface areas (77–100 m2 g−1) were successfully prepared by the simple activated reactive synthesis process. This method provides a waste free and scaling up process to prepare nanomaterials that are difficult to obtain using classical soft chemical routes. The nanomaterials synthesized, presenting high surface areas, exhibit high catalytic activities. This simple method will allow the synthesis of new catalysts for a wide variety of redox reactions.Notes and references
- A. Figuerola, R. Di Corato, L. Manna and T. Pellegrino, Pharmacol. Res., 2010, 62, 126 CrossRef CAS.
- T. Trindade, P. O'Brien and N. L. Pickett, Chem. Mater., 2001, 13, 3843 CrossRef CAS.
- R. Nagarajan and T. Alan Hatton, in Nanoparticles: Synthesis, Stabilization, Passivation, and Functionalization, American Chemical Society, 2008 Search PubMed; G. A. Ozin, A. C. Arsenault and L. Cademartiri, in Nanochemistry: A Chemical Approach to Nanomaterials, The Royal Society of Chemistry, 2nd edn, 2009 Search PubMed.
- N. Lopez, T. V. W. Janssens, B. S. Clausen, Y. Xu, M. Mavrikakis, T. Bligaard and J. K. Nørskov, J. Catal., 2004, 223, 232 CrossRef CAS.
- L. G. Tejuca and J. L. G. Fierro, in Properties and Applications of Perovskite-Type Oxides, M. Dekker, Inc., NY, 1993 Search PubMed; Y. Nishihata, J. Mizuki, T. Akao, H. Tanaka, M. Uenishi, M. Kimura, T. Okamoto and N. Hamada, Nature, 2002, 418, 164 Search PubMed; C. H. Kim, G. Qi, K. Dahlberg and W. Li, Science, 2010, 327, 1624 CrossRef CAS.
- S. Royer and D. Duprez, ChemCatChem, 2011, 3, 24 Search PubMed.
- Z. Jiang, Z. Hao, J. Su, T. Xiao and P. P. Edwards, Chem. Commun., 2009, 3225 RSC.
- F. Teng, J. Xu, Z. Tian, J. Wang, Y. Xu, Z. Xu, G. Xiong and L. Lin, Chem. Commun., 2004, 1858 RSC.
- A. J. Zazur and J. Y. Ying, Nature, 2000, 403, 65 CrossRef CAS.
- S. Eriksson, U. Nylén, S. Rojas and M. Boutonnet, Appl. Catal., A, 2004, 265, 207 CrossRef.
- (a) M. Machida, K. Eguchi and H. Arai, J. Am. Ceram. Soc., 1988, 71, 1142 CAS; (b) G. Groppi, M. Bellotto, C. Cristiam, P. Forzatti and P. L. Villa, Appl. Catal., A, 1993, 104, 101 CrossRef CAS; (c) A. Baylet, S. Royer, C. Labrugère, H. Valencia and P. Marécot, Phys. Chem. Chem. Phys., 2008, 10, 5983 RSC.
- Z. Xu, M. Zhen, Y. Bi and K. Zhen, Appl. Catal., A, 2000, 198, 267 CrossRef CAS.
- S. Zhu, X. Wang, A. Wang, Y. Cong and T. Zhang, Chem. Commun., 2007, 1695 RSC.
- M. Ghasdi and H. Alamdari, Sens. Actuators, B, 2010, 148, 478 CrossRef.
- S. Royer, F. Bérubé and S. Kaliaguine, Appl. Catal., A, 2005, 282, 273 CrossRef.
- P. Artizzu-Duart, J. M. Millet, N. Guilhaume, E. Garbowski and M. Primet, Catal. Today, 2000, 59, 163 CrossRef CAS; M. Machida, K. Eguchi and H. Arai, J. Catal., 1989, 120, 377 CAS.
- S. Royer, D. Duprez and S. Kaliaguine, J. Catal., 2005, 236, 364 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1cy00211b |
| This journal is © The Royal Society of Chemistry 2011 |
