Convenient synthesis and applications of gram scale boron nitride nanosheets†
Liancheng
Wang
ab,
Changhui
Sun
a,
Liqiang
Xu
*a and
Yitai
Qian
a
aKey Laboratory of Colloid and Interface Chemistry (Shandong University), Ministry of Education, Jinan 250100, PR China. E-mail: xulq@sdu.edu.cn; Fax: +86-531-8836-6280; Tel: +86-531-8836-6280
bHefei National Laboratory for Physical Science at Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, People's Republic of China
First published on 25th July 2011
Abstract
In this study, gram scale ultrathin (4 nm on average) boron nitride nanosheets (BNNSs) have been prepared through a simple route by using boron oxide, zinc powders and N2H4·2HCl in a stainless steel autoclave at 500 °C. The high thermal stability and high specific surface area (226 m2 g−1) of these BNNSs enable them to be candidates for the high efficiency catalyst support. For example, monodispersed Pt and Au nanoparticles with mean diameters of ∼4.0 and 3.3 nm were successfully loaded on the surfaces of BNNSs, respectively. The Au/BNNSs and Pt/BNNSs would be efficient catalysts in various reactions. It is found that the Pt/BNNSs catalysts towards CO conversion have shown lower full conversion temperature and higher stability. Besides, the gram scale BNNSs might have many other potential applications, such as in polymer composites with high thermal conductivity, electron field emission and the absorbent in gas/water purification.
1. Introduction
Boron nitride (BN) is a structural analog of carbon. Though h-BN and graphite have the same layered crystal structure with nearly the same cell parameters, the electronic properties of them are distinct. In contrast to the semimetallic graphite, h-BN is typically a wide band semiconductor. The near band cathodoluminescence (CL) emission of h-BN could make it an ultraviolet-light emitter.1,2 Moreover, BN materials exhibit higher chemical and thermal stability than carbon materials. Those properties as well as high thermal conductivity make BN a promising catalyst support under relatively harsh conditions, as it could avoid the sintering of the supported catalyst on hot spots.3 For example, the BN supported barium-promoted ruthenium catalyst exhibited unprecedented activity and stability in ammonia synthesis. Boron nitride materials with high specific surface areas could be of high research interest for catalytic supports.4,5Owing to the attractive properties mentioned above, the ultrathin boron nitride nanosheets (BNNSs) could be excellent complementary materials to carbon nanosheets and graphene, which are of intense research interest, recently.6 Similar to that of few layered boron nitride nanotubes, few layered BNNSs with an exposed (002) crystal surface are considered valuable to exploit many interesting properties and innovative applications,7 such as in electrically insulating polymer composites,8 electron field emission, gas absorption and ultraviolet optoelectronic devices.9,10 It is found that the ultrathin BNNSs (<20 layers) filler can improve the thermal and mechanical properties of the polymeric composites.11 The vertically aligned BNNSs (below 20 nm) show superhydrophobicity which might be valuable in self-cleaning coating.7
Exfoliated in organic solvents,11–13 micromechanical cleavage14–17 and the chemical vapor deposition7 were employed to fabricate monolayer or few layers of BN materials.18 Though the 10 mg scale few layered BNNSs were obtained,11,13 further improved yields of these will meet the increasing demand of catalytic supports, polymer fillers or other usage, and facilitate the further studies. Recently, few layered BN has been synthesized through boric acid and urea at 900 °C.19 Besides, solvothermal synthesis combined with sonication was applied to prepare gram scale graphene,20 however, to the best of our knowledge, there have been few reports about the synthesis of gram scale BNNSs at mild temperatures, especially at temperatures below 500 °C.
In this study, a convenient solid state route for the synthesis of gram scale ultrathin aligned BNNSs with a thinness of 2–6 nm (4 nm on average) was reported. The BNNSs were prepared at a mild temperature (500 °C) in a sealed autoclave at first. The high thermal stability and the high specific surface area (226 m2 g−1) of these BNNSs enable them to be candidates for the high efficiency catalyst support. The monodispersed Pt and Au nanoparticles with mean diameters of ∼4.0 and 3.3 nm were loaded on BNNSs, respectively. The Pt/BNNSs catalyst towards CO conversion was taken as an example, which shows lower full conversion temperature and higher stability compared with the previous reports and the effects of an Au/BNNSs catalyst towards CO conversion have also been investigated.
2. Experimental section
2.1 Materials
The B2O3 powder was purchased from Liaobin Chemical Co. Ltd. (Yingkou, China, >98.5%). The zinc powder was obtained from Shuangshuang Chemical Co. Ltd. (Yantai, China). All the other reagents used in the experiments were purchased from Shanghai Chemical Reagents Co. As the zinc powder is sensitive to water, it was pretreated at 70 °C for several hours.2.2 Sample preparation
In a typical preparation process (S1), 20 mmol B2O3, 130 mmol zinc powder and 50 mmol N2H4·2HCl were mixed and placed in a stainless steel autoclave of 20 mL capacity. The sealed autoclaves were heated in an electric stove from room temperature with an increasing rate of 10 °C min−1 to 500 °C and then maintained at 500 °C for 12 h. After that, it was cooled to room temperature naturally. The crude samples were treated with dilute hydrochloric acid overnight. Then the as-obtained products were filtered and dried at 80 °C, and finally about 0.33 g of BNNSs were obtained. (Fig. S1a, ESI†).2.3 Preparation of M/BNNSs materials (M = Au, Pt)
Pt/BNNSs: The Pt/BNNSs material was prepared by an impregnation method described elsewhere.21a The BNNSs were impregnated with an ethanol solution of H2PtCl6 firstly. Then they were dried at 110 °C overnight, and calcined at 500 °C for 3 h in a flowing N2 atmosphere. Finally, the samples were reduced at 300 °C for 1 h in the flowing hydrogen (40 mL min−1). Au/BNNSs: the Au/BNNSs material was prepared by a modified DP urea method.21b The as-obtained BNNSs were hydrothermally treated by H2O2 in a 20 mL teflon lined autoclave at 120 °C for 6 h. Then, the HAuCl4 solution in an appropriate amount as well as urea (100 times as much as HAuCl4) was added to 60 mg of BNNSs. After being maintained at 80 °C for 20 h, the solid powder was separated by centrifugation and washed several times with deionized water. Finally, it was dried at 80 °C overnight, and calcined at 350 °C for 3 h.2.4 Sample characterization
X-Ray powder diffraction (XRD) measurements were determined by a Bruker D8 advanced X-ray diffractometer. The morphology and structure of the products was investigated by field emission scanning electron microscopy (FE-SEM, Hitachi, SU-70), transmission electron microscopy (TEM, Hitachi, H-600) and high-resolution TEM (HRTEM, JEOL 2100). A CL spectrophotometer attached to the SU-70 FE-SEM was used to investigate optical properties of the synthesized BNNSs. The Fourier transform infrared spectroscopy (FTIR) spectrum instrument is a Bruker VERTEX 70 with a resolution of 4 cm−1. The optical absorption spectrum was recorded by a UV-Vis-NIR spectrometer (Shimadzu, UV-3700). TG analysis was carried out using a TA SDT Q600 simultaneous thermogravimetric analyzer in the ambient atmosphere. The specific surface area was estimated by the Brunauer–Emmet–Teller (BET) equation based on the nitrogen adsorption isotherm (77 K) using a NAVA 2000e surface area and pore size analyzer.2.5 Catalytic activity test
Catalytic performance was measured by a continuous flow fixed-bed microreactor at atmospheric pressure. The system was first purged by N2, then the catalytic activity of 40 mg Pt/BNNSs toward CO oxidation was studied in the mixed gases (1% CO, 10% O2 and 89% N2). The outlet gas samples were analyzed by an online infrared gas analyzer (Gasaboard-3121, cubic Co., Wuhan, China) which simultaneously detects CO and CO2 with a resolution of 10 ppm.3. Results and discussion
Fig. 1a shows the typical XRD pattern of the as-obtained S1. Two obvious broad peaks centered at 26.24° (3.394 Å) and 42.08° (2.145 Å) are consisted with (002) and (10) diffractions of boron nitride (JCPDS card no. 34-0421). No characteristic peaks associated with other crystalline byproducts were detected. It is noticed that as illustrated by the dashed line, the (002) diffraction is shifted to the lower angle direction, indicating an increase in interplanar distances. The FTIR spectrum of S1 is shown in Fig. 1b. Two strong absorption bands located at 1383 and 799 cm−1 can be assigned to the in-plane B–N stretching vibrations and out-of-plane B–N–B bending vibrations of BN, respectively.22a The increased interlayer spacing of S1 could induce the red shift from crystalline h-BN 815–819 cm−1 to lower frequency 799 cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22b,23 Besides, the peaks centered at 3395 and 3166 cm−1 could be attributed to the stretching modes of the H–N–H or O–H groups. The presence of the groups or defect sites would facilitate the covalent functionalization of the product by stearoyl or other organic groups.24 The as-prepared S1 was further examined by Raman spectroscopy (Fig. S2, ESI†), and the single peak located at 1356 cm−1 corresponds to an in-plane vibration (E2g). High yield BNNSs (>80%) can be observed in S1 by TEM. Similar to that of BN (or carbon) nanosheets (or nanoribbon), bending and scrolling characters might be the nature of suspended ultrathin nanostructures.15,16 In this study, the TEM image indicate that the BNNSs were curved and aligned (Fig. 1c). HRTEM image show that the wall thickness of the nanosheets is mainly in the range of 2–6 nm (∼4 nm on average, the particle size distribution is shown in Fig. S3c, ESI†), which is composed of 6–20 layers (Fig. 1c and d). The interplane spacing of (002) is mainly in the range of 0.33–0.34 nm.
22b,23 Besides, the peaks centered at 3395 and 3166 cm−1 could be attributed to the stretching modes of the H–N–H or O–H groups. The presence of the groups or defect sites would facilitate the covalent functionalization of the product by stearoyl or other organic groups.24 The as-prepared S1 was further examined by Raman spectroscopy (Fig. S2, ESI†), and the single peak located at 1356 cm−1 corresponds to an in-plane vibration (E2g). High yield BNNSs (>80%) can be observed in S1 by TEM. Similar to that of BN (or carbon) nanosheets (or nanoribbon), bending and scrolling characters might be the nature of suspended ultrathin nanostructures.15,16 In this study, the TEM image indicate that the BNNSs were curved and aligned (Fig. 1c). HRTEM image show that the wall thickness of the nanosheets is mainly in the range of 2–6 nm (∼4 nm on average, the particle size distribution is shown in Fig. S3c, ESI†), which is composed of 6–20 layers (Fig. 1c and d). The interplane spacing of (002) is mainly in the range of 0.33–0.34 nm.
 | ||
| Fig. 1 The typical XRD pattern (a), FT-IR spectrum (b), TEM and HRTEM images (c) and (d) of S1. | ||
The nitrogen adsorption–desorption curve of the as-prepared BNNSs is shown in Fig. 2a. The isotherm corresponds to a type IV in Brunauer’s classification and the hysteresis loop is of type H2. The desorption branch of the nitrogen isotherms of S1 reveals a step at a relative pressure of 0.46, which can be attributed to capillary evaporation in a mesoporous structure. Additionally, the isotherm still rises above the relative pressure of 0.9, indicating the presence of a macroporous structure.25 The specific surface area of S1 was 226 m2 g−1 (the total pore volume is 0.405 cm3 g−1) according to the BET method, which is larger than that of the activated BN (168 m2 g−1),26 hollow BN particles (80 m2 g−1),27 and porous BN (50 m2 g−1).28 For many uses and potential applications, the thermal stability of the material is very important. In order to investigate the thermal stability of the as-obtained S1, the TGA analysis was carried out in an ambient atmosphere. It is found that the S1 is stable even at 850 °C. The obvious weight gain thereafter can be ascribed to the oxidation of BNNSs (Fig. 2b).
 | ||
| Fig. 2 (a) Nitrogen adsorption–desorption isotherms, (b) TGA curve of as-prepared S1 carried out in an ambient atmosphere. | ||
With the increasing of temperature, the N2H4·2HCl would be decomposed into gaseous N2, H2 and HCl at around 240 °C (eqn (1)). The molten B2O3 could be reduced by the zinc powder. As the newly formed species reacted with the fresh N2, BN was produced (eqn (2)). The reaction between Zn powder and HCl leads to the formation of by-product ZnCl2 (eqn (3)). The in situ formed ZnCl2 plays an active agent role, which has been widely applied to the preparation of the structural analog of graphite with high specific surface areas.29
| N2H4·2HCl → N2 + 2H2 + 2HCl | (1) |
| B2O3 + 3Zn + N2 → 2BN + 3ZnO | (2) |
| Zn + 2HCl → ZnCl2 + H2 | (3) |
The overall reaction involved can be tentatively described below:
| 2B2O3 + 11 Zn + 5N2H4·2HCl → 4BN + 6ZnO + 5ZnCl2 + 3N2 + 15H2 | (4) |
It is observed from Fig. 3a that aligned solid particles coated with sheets were the final products under TEM observation after the crude products were treated with absolute alcohol. The corresponding HRTEM image indicates the presence of boron nitride and a ZnO composite (Fig. 3b). After ZnO was removed by hydrochloric acid, BNNSs were finally obtained.
 | ||
| Fig. 3 (a)The TEM image of the crude sample was treated by absolute alcohol. (b) The HRTEM image of the area framed in (a). (c) TEM image of the BN obtained in the case of Mg. | ||
In this experiment, zinc powder plays a critical role in the synthesis of BNNSs. No BN could be obtained in the absence of zinc. When Fe was used instead of Zn, the yield of the final products was reduced dramatically. If zinc was replaced by a proper amount of Mg, larger scale BNNSs could also be obtained, but with an average thickness of ∼10 nm (Fig. 3c).
The removal of CO is of fundamental importance in air purification, fuel cells and automobile exhaust systems. In Lin's work,21aBN with a specific surface area of 125 m2 g−1 was used as a catalytic support. The CO conversion vs. temperature profile of 1% Pt/BN was conducted in the He balanced system. However, (1) the stability of the Pt/BN catalyst needs to be estimated; (2) the BN support with a higher specific surface area should also be considered. As the O2 in an ambient atmosphere was partially consumed, the mixed gas composed of 1% CO, 10% O2 and 89% N2 was adopted in the present experiment. Fig. 4a shows the activity profiles of 40 mg Pt/BNNSs with different Pt contents toward CO oxidation. Obviously, nearly full conversion can be detected in all samples. For 16% Pt/BNNSs, nearly full CO conversion gets around 135 °C. In the case of 2%, 1% and 0.04% Pt/BNNSs, nearly full conversion temperatures were around 165, 210 and 310 °C, respectively. Though the catalyst used was only 40 mg, the full conversion temperature of 1% Pt/BNNSs is lower than that of 1% Pt/BN,21a 2% Pt/Al2O3,30 2.5 mg Pt nanoparticles, and it is also comparable to 1% Pt/CeO2.31 The 1% Pt/BNNSs catalyst after the use was investigated by TEM (Fig. 4b). It is found that Pt nanoparticles with an average size of ∼3 nm dispersed well on the BNNSs. Its stability was further investigated through using 1% Pt/BNNSs at 250 °C under different CO concentrations (0.5–5.9%) at a flow rate of 66.7 mL min−1 in the presence of 10% O2. It is found that the CO2 concentration measured at the reactor outlet increased in a stepwise fashion, which follows the CO concentration adjustment while the nearly full conversion without deactivation can be observed after 4.5 hours conversion (Fig. 4c).
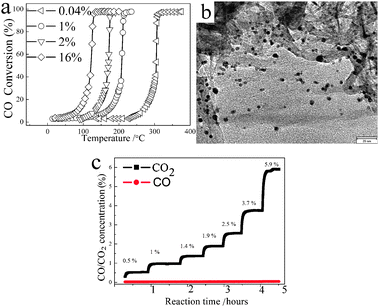 | ||
| Fig. 4 (a) The conversion of CO over temperatures by 40 mg Pt/BNNSs catalyst with different loadings (0.04%, 1%, 2% and 16%) in the gas flow (1% CO, 10% O2 and 89% N2). (b) TEM image of 1% Pt/BNNSs. (c) The catalytic performance of 1% Pt/BNNSs with different CO concentrations from 0.5% to 5.9% at a flow rate of 66.7 mL min−1 at 250 °C. The O2 concentration was fixed at 10%. | ||
Different from the strong interaction between oxides and noble metal nanoparticles, the interaction between Au nanoparticles and pristine boron nitride was generally weak.3 To obtain highly Au functionalized BN material, magnetron sputting32 and assembly at the surface of both amine- and thiol-functionalized BNNTs (boron nitride nanotubes) via a covalent bond33 were adopted. In this study, the loading of the ultrathin Au nanoparticles was followed by the H2O2 hydrothermal treatment in order to create functional groups on BNNSs and enhance the interaction between BN and Au nanoparticles.34 It is found that ultrathin Au nanoparticles (3.3 nm on average, Fig. S3b, ESI†) dispersed well on the treated BNNSs (Fig. 5a and b). The H2O2 treatment was crucial for the formation of ultrathin Au nanoparticles. In the absence of H2O2 treatment, the amount of loaded Au particles was lower and the size was larger (7.7 nm on average, Fig. 5c and Fig. S3c, ESI†). Now that the Au nanoparticles are ultrathin, the Au/BNNSs materials could be considered, in principle, as catalysts for CO oxidation at low temperature (Fig. 5d). In addition, the Au/BNNSs materials might have potential applications in the areas such as optical materials, sensors and photocatalysis (see Fig. S4, ESI†).
 | ||
| Fig. 5 TEM images of 2% Au/BNNSs with (a, b) and without (c) the H2O2 treatment. (c) Inset gives the HRTEM image of the doped nanoparticles, the interplane spacing of 0.24 nm can be attributed to the (111) plane of Au. (d) The conversion of CO over temperatures by 40 mg 2% Au/BNNSs catalyst in the gas flow (1% CO, 10% O2 and 89% N2). | ||
4. Conclusions
In this study, gram scale ultrathin BNNSs (4 nm on average) were prepared by boron oxide, zinc powders and N2H4·2HCl in an autoclave at 500 °C. The high thermal stability and high specific surface area (226 m2 g−1) of these BNNSs enable them to be candidates for the high efficiency catalyst support in various reactions. The Pt/BNNSs catalyst towards CO conversion shows lower full conversion temperature as well as higher stability. The gram scale BNNSs could be considered in the applications such as in polymer composites with high thermal conductivity, electron field emission and the absorbent in gas/water purification.Acknowledgements
This work was supported by National Natural Science Found of China (No. 20871075, 20971079), the 973 Project of China (No. 2011CB935901) and the Independent Innovation Foundation of Shandong University (Grant No. 2009TS017, 2009JC019). The authors thank Prof. Ding Yi for the catalytic measurement.Notes and references
- Q. Li, J. Yu, M. Y. Li, F. Liu and X. D. Bai, Nanotechnology, 2011, 22, 215602 CrossRef.
- Y. Kubota, K. Watanabe, O. Tsuda and T. Taniguchi, Science, 2007, 317, 932–934 CrossRef CAS.
- C. A. Lin, J. C. S. Wu, J. W. Pan and C. T. Yeh, J. Catal., 2002, 210, 39–45 CrossRef CAS.
- C. J. H. Jacobsen, J. Catal., 2001, 200, 1–3 CrossRef CAS.
- G. Postole, M. Caldararu, N. I. Ionescu, B. Bonnetot, A. Auroux and C. Guimon, Thermochim. Acta, 2005, 434, 150–157 CrossRef CAS.
- M. Topsakal, E. Aktürk and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 115442 CrossRef.
- J. Yu, L. Qin, Y. Hao, S. Kuang, X. Bai, Y. M. Chong, W. Zhang and E. Wang, ACS Nano, 2010, 4, 414–422 CrossRef CAS.
- C. Y. Zhi, Y. Bando, T. Terao, C. C. Tang, H. Kuwahara and D. Golberg, Adv. Funct. Mater., 2009, 19, 1857–1862 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang, D. Golberg, R. Xie and T. Sekigushi, Appl. Phys. Lett., 2005, 86, 213110–213113 CrossRef.
- D. Golberg, Y. Bando, C. C. Tang and C. Y. Zhi, Adv. Mater., 2007, 19, 2413–2432 CrossRef CAS.
- C. Y. Zhi. Y. Bando, C. C. Tang, H. Kuwahara and D. Golberg, Adv. Mater., 2009, 21, 2889–2893 CrossRef.
- G. Lian, X. Zhang, M. Tan, S. Zhang, D. Cui and Q. Wang, J. Mater. Chem., 2011, 21, 9201–9207 RSC.
- Y. Lin, T. V. Williams and J. W. Connell, J. Phys. Chem. Lett., 2010, 1, 277–283 CrossRef CAS.
- D. Pacile, J. C. Meyer, C. O. Girit and A. Zettl, Appl. Phys. Lett., 2008, 92, 133107 CrossRef.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS.
- T. J. Booth, P. Blake, R. R. Nair, D. Jiang, E. W. Hill, U. Bangert, A. Bleloch, M. Gass, K. S. Novoselov, M. I. Katsnelson and A. K. Geim, Nano Lett., 2008, 8, 2442–2446 CrossRef CAS.
- N. Alem, R. Erni, C. Kisielowski, M. D. Rossell, W. Gannett and A. Zettl, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 155425 CrossRef.
- M. Corso, W. Auwarter, M. Muntwiler, A. Tamai, T. Greber and J. Osterwalder, Science, 2004, 303, 217–220 CrossRef CAS.
- A. Nag, K. Raidongia, K. P. S. S. Hembram, R. Datta, U. V. Waghmare and C. N. R. Rao, ACS Nano, 2010, 4, 1539–1544 CrossRef CAS.
- M. Choucair, P. Thordarson and J. A. Stride, Nat. Nanotechnol., 2008, 4, 30–33 CrossRef.
- (a) L. X. Lin, Z. H. Li, Y. Zheng and K. M. Wei, J. Am. Ceram. Soc., 2009, 92, 1347–1349 CrossRef CAS; (b) L. Delannoy, N. E. Hassan, A. Musi, N. N. LeTo, J. M. Krafft and C. Louis, J. Phys. Chem. B, 2006, 110, 22471–22478 CrossRef CAS.
- (a) P. Dibandjo, L. Bois, F. Chassagneux and P. Miele, J. Eur. Ceram. Soc., 2007, 27, 313–317 CrossRef CAS; (b) A. S. Rozenberg, Y. A. Sinenko and N. V. Chukanov, J. Mater. Sci., 1993, 28, 5675–5678 CrossRef CAS.
- L. C. Wang, L. Q. Xu, C. H. Sun and Y. T. Qian, J. Mater. Chem., 2009, 19, 1989–1994 RSC.
- C. Y. Zhi, Y. Bando, C. C. Tang, Q. Huang and D. Golberg, J. Mater. Chem., 2008, 18, 3900–3908 RSC.
- L. X. Lin, Y. Zheng, Z. H. Li and K. M. Wei, Scr. Mater., 2008, 59, 1151–1154 CrossRef CAS.
- W. Q. Han, R. Brutchey, T. D. Tilley and A. Zettl, Nano Lett., 2004, 4, 173–176 CrossRef CAS.
- C. H. Sun, C. L. Guo, X. J. Ma, L. Q. Xu and Y. T. Qian, J. Cryst. Growth, 2009, 311, 3682–3686 CrossRef CAS.
- J. A. Perdigon-Melon, A. Auroux, D. Cornu, P. Miele, B. Toury and B. Bonnetot, J. Organomet. Chem., 2002, 657, 98–106 CrossRef CAS.
- A. Ahmadpour and D. D. Do, Carbon, 1996, 34, 471–479 CrossRef CAS.
- O. P. Yadav, A. Palmqvist, N. Cruise and K. Holmberg, Colloids Surf., A, 2003, 221, 131–134 CrossRef CAS.
- P. Bera, A. Gayen, M. S. Hegde, N. P. Lalla, L. Spadaro, F. Frusteri and F. Arena, J. Phys. Chem. B, 2003, 107, 6122–6130 CrossRef CAS.
- T. Sainsbury, T. Ikuno, D. Okawa, D. Pacile, J. M. J. Frechet and A. Zettl, J. Phys. Chem. C, 2007, 111, 12992–12999 CAS.
- H. Chen, H. Z. Zhang, L. Fu, Y. Chen, J. S. Williams, C. Yu and D. P. Yu, Appl. Phys. Lett., 2008, 92, 243105 CrossRef.
- C. Y. Zhi, Y. Bando, T. Terao, C. C. Tang, H. Kuwahara and D. Golberg, Chem.–Asian J., 2009, 4, 1536–1540 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: optical images of BNNSs products, Raman spectra, thickness distribution as well as optical properties are shown. See DOI: 10.1039/c1cy00191d |
| This journal is © The Royal Society of Chemistry 2011 |
