IBX in aqueous medium: a green protocol for the Biginelli reaction
Santosh
Takale
a,
Sanket
Parab
a,
Kiran
Phatangare
a,
Rajaram
Pisal
a and
Atul
Chaskar
*b
aC. K. Thakur Research Centre, Navi Mumbai-410206, India. Tel: +91-22-27464193 Tel: +91-22-27455760
bDepartment of Chemistry, National Taiwan University, Taipei, Taiwan. E-mail: achaskar@rediffmail.com; Tel: +886-917352249
First published on 8th July 2011
Abstract
Iodoxy benzoic acid (IBX) used as an effective catalyst for the synthesis 3,4-dihydropyrimidine-2(1H)-ones (DHPMs) via the Biginelli reaction in water. The immobilized catalyst could be easily recovered by simple filtration and recycled without significant decrease of the catalytic activity.
Introduction
Multicomponent reactions (MCR) have been gaining notable attention and interest since their discovery1 for their outstanding capacity to form numerous new bonds from a variety of starting materials in one pot, in a single operation.2 Owing to their bond forming efficiency, multicomponent reactions are the preferred approach in the drug discovery process.3,4 When compared with traditional approaches multicomponent reactions present several advantages in terms of synthesis procedure, work-up, product yield, cost involvement, time requirement, labour expenditure and waste production. Nowadays efforts are being made to discover and design novel multicomponent reactions using three to four components to produce valuable heterocyclic scaffolds which have extensive and diverse applications in different areas of applied chemistry.5Dihydropyrimidinones (DHPMs) and their derivatives, outcomes of the Biginelli reaction,1 fall under the major class of heterocycles that possess a broad range of important biological and pharmacological activities. They have been observed to act as calcium channel blockers, antihypertensive agents, antagonists, and neuropeptide Y (NPY) antagonists,6–8 besides this many of the derivatives have antifungal, antiviral, anticancer, antibacterial, antitumor and anti-inflammatory activities.9,10Alkaloidse.g. Batzalladine found in marine sources containing a dihydropyrimidinone core, exhibit significant HIVgp-120-CD4 inhibitor activity.11 Therefore the synthesis of these privileged scaffolds by using convenient, green chemistry procedure is of prime concern.
The most straightforward synthesis of this heterocyclic system involves a three-component coupling of aromatic aldehyde, urea or thiourea and β-keto esters. Remarkable modifications in the synthetic protocol were also carried out to produce DHMPs in good yields. Most of these procedures made use of diverse catalysts like heteropolyacids,12 CuCl2·H2O,13L-proline,14praseodymium methanesulfonate,15chloroacetic acid,16H2SO4,17 BF3·EtOH–CuCl,18 LaCl3·7H2O with catalytic concentrated HCl,19 CeCl3·7H2O,20InCl3,21BiCl3,22Cu(OTf)2,23 TMSCl,24LiClO4,25LiBr,26InBr3,27phenyl pyruvic acid,28 FeCl3·6H2O–HCl,29TMSI30 and CdCl231. Very recently it has also been shown that DHPMs can be synthesized by using thiamine hydrochloride under ultrasound conditions.32 Hitherto, all these methodologies come up with drawbacks such as prolonged reaction time, tedious catalyst preparation and work-up, formation of inevitable side or sticky products, and exhaustive usage of energy sources and solvents which result in a lower yield of the desired product. So, considering all these facts and with an emphasis on human health and environmental protection, the development of an eco-friendly and environment friendly reagent system is highly desirable.
The novel reagents which could provide chemoselectivity with efficiency are being constantly sought for contemporary organic synthesis. In this regard the hypervalent iodine reagent IBX is amply demonstrated in organic synthesis owing to its mild, highly specific and eco-friendly nature.33–36 To date reported data about IBX reveals that it is used in alcohol oxidation,37 conversion of primary alcohols/aldehydes to carboxylic acids,38oxidation of 1,2 amino alcohols to 1,2-diols without oxidative cleavage of the C–C bond39 and in the conversion of 1,4-diols to γ-lactols.40 With the background of global warming, disposal of organic solvent is a major problem. The idea of replacing them with water has gained interest in last few years due to its non-toxic and non-flammable properties. Besides, the physical and chemical properties of water encourage good reactivity and selectivity in reactions in comparison to organic solvents.41
In the context of our ongoing research project on the development of green methodologies for the synthesis of heterocyclic compounds of biological importance and the use of heterogeneous catalysts in organic reactions,42 here we would like to report our investigation concerning the IBX-mediated synthesis of 3,4-dihydropyrimidine-2-(1H)-ones in water (Scheme 1).
 | ||
| Scheme 1 Synthesis of 3,4-dihydropyrimidine-2-(1H)-one. | ||
Results and discussion
The development of the IBX-mediated Biginelli reaction commenced with the screening of a variety of catalysts. To investigate the need for a catalyst in the synthesis of 3,4-dihydropyrimidine-2-(1H)-one we had run two sets of reactions, (1) a control reaction—where we reacted benzaldehyde, ethylactoacetate and urea in water in absence of any catalyst, (2) a test reaction—in this set we reacted the previously mentioned substrates in the presence of various catalystsviz. UHP, TBHS, β-CD, CuCl, CAS, iodine, DABCO and IBX. This set was used for the purpose of screening of most efficient catalyst by observing the influence of the catalyst on the reaction parameters. After the completion of reactions we observed that the control reaction yielded 20% of the product after 45 h at 100 °C and the effects of the catalysts on the reaction parameters are summarized in Table 1.| Entry | Catalyst | Temp. (°C) | Time (h) | Yieldb |
|---|---|---|---|---|
| a Reaction condition: benzaldehyde (1 mmol), ethylacetoacetate (1 mmol), urea (1.2 mmol), catalyst (5 mol%), solvent: water (5 mL), time: 2.5 h, temp.: 60–100 °C. b Isolated yield. | ||||
| 1 | None | 100 | 45 | 20 |
| 2 | UHP | 80 | 6 | 76 |
| 3 | TBHS | 100 | 6 | 65 |
| 4 | β-CD | 100 | 6 | 69 |
| 5 | CuCl | 100 | 6 | 79 |
| 6 | CAS | 100 | 7 | 71 |
| 7 | Iodine | 80 | 4 | 81 |
| 8 | DABCO | 80 | 3.5 | 80 |
| 9 | IBX | 60 | 2.5 | 90 |
The observations reveal that IBX offers the best results owing to its ability to activate the carbonyl group of aldehydes and ketones which speeds up the iminium formation and subsequently nucleophilic addition of the enolised β-keto ester. The same reaction was carried out separately in the presence of IBA and 2-iodobenzoic acid, and it was observed that the reaction needed more time for completion and produced a low yield, presumably due to their low reactivity.43
Further to probe the effect of solvents, reactions were carried out in the presence of IBX as the catalyst, using various solvents and keeping the reaction time constant while the control reaction was set in a solvent free system. The success of the reaction was judged on the criteria of the product yield and reaction temperature (Table 2). Water accelerates the rate of reaction owing to its polar and protic nature.
| Entry | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|
| a Reaction condition: benzaldehyde (1 mmol), ethylacetoacetate (1 mmol), urea (1.2 mmol) IBX (5 mol%), solvent: 5 mL, time: 2.5 h, temp.: 60 °C. b Isolated yield. | |||
| 1 | Water | 60 | 90 |
| 2 | Methanol | 50 | 78 |
| 3 | Ethanol | 60 | 72 |
| 4 | Chloroform | 55 | 63 |
| 5 | Dichloromethane | 35 | 59 |
| 6 | DMF | 120 | 63 |
| 7 | Acetonitrile | 75 | 47 |
| 8 | THF | 55 | 78 |
| 9 | DMSO | 120 | 67 |
| 10 | Solvent free | 100 | 20 |
According to the results in Table 1 and 2, the most efficient reaction system is IBX in water. With respect to the appealing results obtained from the catalyst and solvent screening, we framed the general protocol for the synthesis of 3,4-dihydropyrimidine-2(1H)-one by treating several aromatic aldehydes and β-keto esters with urea or thiourea using IBX in water (Table 3). The reaction conditions were compatible with various functional groups, ranging from the electron-withdrawing cyano and nitro groups to the halides. Indeed, it has been observed that the oxidation prone groups (–OH, –OMe) are tolerated in this reaction. In general the yields are high, regardless of the structural variations. Upon completion of reaction, IBX has been recovered along with some amount of IBA, the reduced analog of IBX. This IBA then on its oxidation collectively contributes to overall recovery of IBX. Thus about 60% of IBX was recovered and reused without loss of its activity for next few reaction cycles.
| Entry | Compound | R | R1 | X | Time (h) | % Yield |
|---|---|---|---|---|---|---|
| a All products were well characterized using 1H NMR, and IR spectra. b Yields refer to isolated pure products. | ||||||
| 1 | 4a |

|
CH3 | O | 1.5 | 90 |
| 2 | 4b |
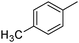
|
CH3 | S | 2.5 | 92 |
| 3 | 4c |
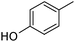
|
C2H5 | O | 3.0 | 87 |
| 4 | 4d |

|
CH3 | O | 3.0 | 83 |
| 5 | 4e |

|
CH3 | O | 3.0 | 82 |
| 6 | 4f |
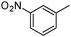
|
CH3 | S | 2.5 | 72 |
| 7 | 4g |

|
C2H5 | O | 3.0 | 85 |
| 8 | 4h |

|
C2H5 | S | 3.5 | 71 |
| 9 | 4i |

|
CH3 | O | 3.5 | 82 |
| 10 | 4j |

|
C2H5 | S | 3.0 | 86 |
| 11 | 4k |
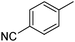
|
C2H5 | O | 2.0 | 90 |
| 12 | 4l |

|
C2H5 | S | 2.0 | 93 |
| 13 | 4m |

|
C2H5 | O | 2.5 | 90 |
| 14 | 4n |
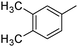
|
CH3 | S | 3.5 | 88 |
| 15 | 4o |

|
C2H5 | O | 3.0 | 77 |
| 16 | 4p |

|
C2H5 | S | 3.0 | 80 |
| 17 | 4q |

|
C2H5 | O | 2.5 | 72 |
| 18 | 4r |

|
C2H5 | S | 3.0 | 86 |
| 19 | 4s |
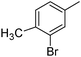
|
C2H5 | O | 2.5 | 79 |
| 20 | 4t |

|
C2H5 | S | 3.0 | 82 |
Experimental
General procedures
All commercial reagents were used as received without purification and all solvents were reagent grade. The reaction was monitored by TLC using 0.25 mm E-Merck silica gel 60 F254 precoated plates, which were visualized with UV light. Melting points were taken in open capillaries on a Veego apparatus and are uncorrected. Infrared spectra were recorded on a Simadzu spectrophotometer in a KBr disc, and the absorption bands are expressed in cm−1. 1H NMR spectra were recorded on a Varian AS 400 MHz spectrometer in DMSO-d6, chemical shifts (δ) are in parts per million (ppm) relative to TMS.General procedure
A mixture of benzaldehyde (1 mmol), ethyl acetoacetate (1 mmol) and a urea (1.2 mmol) in water (5 mL) was stirred at 60 °C in the presence of a catalytic amount of IBX (5 mol%). The progress of the reaction was monitored by TLC. After completion of the reaction IBX was recovered by filtration. The product was extracted with diethyl ether (2 × 5 mL) and the combined organic layer was washed with brine (2 × 5 mL), dried over MgSO4 and evaporated under reduced pressure. The crude product obtained was purified by column chromatography and further recrystallized from ethanol to obtain the pure product.Physical and spectral data of the products
Conclusion
In summary, we have developed an expedient, elegant and clean protocol for the synthesis of 3,4-dihydropyrimidine-2(1H)-ones (DHPMs) from aldehyde, urea/thiourea and β-keto esters. The remarkable features of this procedure are atom efficiency, high conversions, operational simplicity and ready availability of reagents at low cost. We anticipate that the recyclability of the catalyst and use of aqueous reaction medium will make this protocol more appealing and commercially viable.Acknowledgements
We are thankful to Dr S.T. Gadade, Principal, C. K. Thakur College, for his generous support.References
- (a) A. Streker, Liebigs Ann. Chem., 1850, 75, 27 CrossRef; (b) P. Biginelli, Ber., 1891, 24, 1317 CrossRef; (c) P. Biginelli, Ber., 1891, 24, 2962 CrossRef; (d) P. Biginelli, Ber., 1893, 26, 447 CrossRef.
- C. O. Kappe, Acc. Chem. Res., 2000, 33, 879 CrossRef CAS.
- A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef CAS.
- (a) I. Ugi, A. Domling and B. Werner, J. Heterocycl. Chem., 2000, 37, 647 CrossRef CAS; (b) H. Bienayme, C. Hulme, G. Oddon and P. Schmitt, Chem.–Eur. J., 2000, 6, 3321 CrossRef CAS.
- (a) A. M. Shestopalov, Y. M. Emeliyanova, A. A. Shestopalov, L. A. Rodinovskaya, Z. I. Niazimbetova and D. H. Evans, Org. Lett., 2002, 4, 423 CrossRef CAS; (b) M. C. Bagley, J. W. Dale and J. Bower, Chem. Commun., 2002, 1682 RSC; (c) U. Bora, A. Saikia and R. C. Boruah, Org. Lett., 2003, 5, 435 CrossRef CAS; (d) D. Dallinger, N. Y. Gorobets and C. O. Kappe, Org. Lett., 2003, 5, 1205 CrossRef CAS.
- K. S. Atwal, B. N. Swanson, S. E. Unger, D. M. Floyd, S. Moreland, A. Hedberg and B. C. O'Reilly, J. Med. Chem., 1991, 34, 806 CrossRef CAS.
- G. C. Rovnyak, K. S. Atwal, A. Hedberg, S. D. Kimball, S. Moreland, J. Z. Gougoutas, B. C. O'Reilly, J. Schwartz and M. F. Malley, J. Med. Chem., 1992, 35, 3254 CrossRef CAS.
- G. J. Grover, S. Dzwonczyk, D. M. McMullen, D. E. Normandin, C. S. Parham, P. G. Sleph and S. Moreland, J. Cardiovasc. Pharmacol., 1995, 26, 289 CrossRef CAS.
- M. M. Ghorab, S. M. Abdel-Gawad and M. S. A. El-Gaby, Farmaco, 2000, 55, 249 CrossRef CAS.
- B. Shivarama Holla, B. Sooryanarayana Rao, B. K. Sarojini and P. M. Akberali, Eur. J. Med. Chem., 2004, 39, 777 CrossRef.
- (a) A. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. De Brosse, S. Mai, A. Truneh, D. J. Faulkner, B. Carte, A. L. Breen, R. P. Hertzberg, R. K. Johnson, J. W. Westley and B. C. M. Potts, J. Org. Chem., 1995, 60, 1182 CrossRef CAS; (b) A. V. R. Rao, M. K. Gurjar and J. Vasudevan, J. Chem. Soc., Chem. Commun., 1995,(13), 1369 RSC; (c) B. B. Snider, J. Chen, A. D. Patil and A. J. Freyer, Tetrahedron Lett., 1996, 37, 6977 CrossRef CAS.
- G. P. Romanelli, A. G. Sathicq, J. C. Autino, G. Baronetti and H. J. Thomas, Synth. Commun., 2007, 37, 3907 CrossRef CAS.
- F. Xu, J.-J. Wang and Y.-P. Tian, Synth. Commun., 2008, 38, 1299 CrossRef CAS.
- J. Mabry and B. Ganem, Tetrahedron Lett., 2006, 47, 55 CrossRef CAS.
- M. Wang, Z. Song and H. Gong, Prep. Biochem. Biotechnol., 2008, 38, 105 CrossRef CAS.
- Y. Yu, D. Liu and G. Luo, Bioorg. Med. Chem. Lett., 2007, 17, 3508 CrossRef CAS.
- K. Folkers and T. B. Johnson, J. Am. Chem. Soc., 1933, 55, 2886 CrossRef CAS.
- E. H. Hu, D. R. Sidler and U.-H. Dolling, J. Org. Chem., 1998, 63, 3454 CrossRef CAS.
- J. Lu, Y. Bai, Z. Wang, B. Yang and H. Ma, Tetrahedron Lett., 2000, 41, 9075 CrossRef CAS.
- D. S. Bose, L. Fatima and H. B. Mereyala, J. Org. Chem., 2003, 68, 587 CrossRef CAS.
- B. C. Ranu, A. Hajara and U. Jana, J. Org. Chem., 2000, 65, 6270 CrossRef CAS.
- K. Ramalinga, P. Vijayalakshmi and T. N. B. Kaimal, Synlett, 2001, 863 CrossRef CAS.
- A. S. Paraskar, G. K. Dewkar and A. Sudalai, Tetrahedron Lett., 2003, 44, 3305 CrossRef CAS.
- Y.-L. Zhu, S.-L. Huang and Y.-J. Pan, Eur. J. Org. Chem., 2005, 2354 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, R. Srinivas, C. Venugopal and T. Ramalingam, Synthesis, 2001,(09), 1341 CrossRef CAS.
- G. Maiti, P. Kundu and C. Guin, Tetrahedron Lett., 2003, 44, 2757 CrossRef CAS.
- N.-Y. Fu, Y.-F. Yuan, Z. Cao, S.-W. Wang, J.-T. Wang and C. Peppe, Tetrahedron, 2002, 58, 4801 CrossRef CAS.
- M. M. Abelman, S. C. Smith and D. R. James, Tetrahedron Lett., 2003, 44, 4559 CrossRef CAS.
- J. Lu and H. Ma, Synlett., 2000, 63 CAS.
- G. Sabitha, G. S. K. Reddy, Ch. S. Reddy and J. S. Yadav, Synlett, 2003, 858 CrossRef CAS.
- (a) A. V. Narsaiah, A. K. Basak and K. Nagaiah, Synthesis, 2004, 1253 CAS; (b) J. C. Rodriguez-Dominguez, D. Bernardi and G. Kirsch, Tetrahedron Lett., 2007, 48, 5777 CrossRef CAS.
- P. G. Mandhane, R. S. Joshi, D. R. Nagargoge and C. H. Gill, Tetrahedron Lett., 2010, 51, 3138 CrossRef CAS.
- (a) A. Varvoglis, Hypervalent Iodine in Organic Synthesis, Academic Press, London, 1997 Search PubMed; (b) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523 CrossRef CAS; (c) U. Ladziata and V. V. Zhdankin, Arkivoc, 2006, ix, 26 Search PubMed.
- M. M. Heravi, M. Tajbakhsh and M. Ghassemzadeh, Naturforschung, 1999, 54, 394 CAS.
- K. Surendra, N. Srilakshmi Krishnaveni, M. Arjun Reddy, Y. V. D. Nageswae and K. Rama Rao, J. Org. Chem., 2003, 68, 2058 CrossRef CAS.
- K. C. Nicolaou, T. Montagnon, P. S. Baran and Y.-L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245 CrossRef CAS.
- M. Frigerio, M. Santagostino, S. Sputore and G. Palmisano, J. Org. Chem., 1995, 60, 7272 CrossRef CAS.
- R. Mazitschek, M. Mülbaier and A. Giannis, Angew. Chem., Int. Ed., 2002, 41, 4059 CrossRef CAS.
- M. Friegerio and M. Santagostino, Tetrahedron Lett., 1994, 35, 8019 Search PubMed.
- E. J. Corey and A. Palani, Tetrahedron Lett., 1995, 36, 7945 CrossRef CAS.
- (a) R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef CAS; (b) R. Breslow, Acc. Chem. Res., 2004, 37, 471 CrossRef CAS; (c) N. Azizi and M. R. Saidi, Org. Lett., 2005, 7, 3649 CrossRef CAS; (d) S. Tiwari and A. Kumar, Angew. Chem., 2006, 118, 4942 ( Angew. Chem., Int. Ed. , 2006 , 45 , 4824 ) CrossRef.
- (a) A. Chaskar, V. Padalkar, K. Phatangare, K. Patil, B. Langi and A. Bodkhe, Appl. Catal., A, 2009, 359, 84 CrossRef CAS; (b) K. Phatangare, V. Padalkar, D. Mhatre, K. Patil and A. Chaskar, Synth. Commun., 2009, 39, 4117 CrossRef CAS; (c) P. Gawand, H. Deokar, B. Langi, A. Yadav and A. Chaskar, Synth. Commun., 2009, 39, 4171 CrossRef CAS.
- D. M. Browne, O. Niyomura and T. Wirth, Org. Lett., 2007, 9, 3169 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
