Spectroscopic and structural characterization of the formation of olefin metathesis initiating sites on unsupported β-Mo2C
I.
Temprano
a,
G.
Goubert
a,
G.
Behan
b,
H.
Zhang
b and
P. H.
McBreen
*a
aDépartement de chimie, Faculté des sciences et de génie, Université Laval, Québec, Canada. E-mail: peter.mcbreen@chm.ulaval.ca; Fax: (001) 418 6567916; Tel: (001) 418 6567867
bCentre for Research on Adaptive Nanostructures and Nanodevices (CRANN) and CRANN Advanced Microscopy Laboratory, School of Physics, Trinity College, Dublin, Ireland. E-mail: hozhang@tcd.ie; Fax: 353 1 8963037; Tel: 353 1 8964655
First published on 22nd August 2011
Abstract
Reflection absorption infrared spectroscopy (RAIRS), X-ray photoelectron spectroscopy (XPS) and thermal desorption spectrometry (TDS) measurements were used to follow the preparation of surface alkylidenes on β-Mo2C. Treating the molybdenum carbide surface with acrolein and acetone respectively formed propylidiene and 2-propylidene groups. The propylidiene functionalised surface was found to be active for a ring opening insertion reaction with 1,3,5,7-cyclooctatetraene (COT). Helium ion microscopy (HIM) was used to determine the topological features of the surface. The surface region giving the XPS and RAIRS data is composed of large grains of several microns in diameter. In turn, the surface of these grains shows finer structure in the form of small grains suggesting that the olefin metathesis reactivity is related to the morphology of the sample.
1. Introduction
An increased industrial demand for propene,1 and potential applications in the processing of renewable raw materials has lead to a strong renewal of interest in the development of heterogeneous olefin metathesis catalysis.2–9 The catalysts are typically prepared by dispersing Re, W or Mo oxides on high surface area supports.10 For example, tungsten oxide on silica is used to produce propene from a mixture of ethene and 2-butene in the olefin conversion technology licensed by ABB Lummus Inc.4 Recently, Bao et al.3,5–8 reported a series of measurements on Mo aluminosilicate metathesis catalysts. In this paper, we present surface spectroscopy and HIM data for olefin metathesis related chemistry on molybdenum carbide. The first observation of olefin-metathesis on pure metal carbides was made in a surface science study by Oudghiri-Hassani and co-workers.11 In subsequent studies Siaj et al. used RAIRS, TPD and XPS measurements to explore olefin-metathesis surface processes on bulk β-Mo2C.12,13As described by the Chauvin mechanism,14olefin metathesis proceeds through the formation of metal carbene initiating and propagating species. On contact with the reactant, catalyst precursors generate metal carbene initiating sites. Direct evidence of metathesis active sites formed on metallic surfaces are limited to β-Mo2C,11–13MoAl15 and Ru.16–18 Interestingly, these systems involve Mo or Ru, the metals most associated with the development of homogeneous olefin metathesis.14 Active Ru nanoparticles and polycrystalline films may be prepared by using diazo compounds to generate surface alkylidene groups. Active polycrystalline molybdenum carbide foils may be prepared through carbonyl bond breaking in chemisorbed aldehydes and ketones to yield surface alkylidene and oxo groups.19–22 To date, this type of surface chemistry has not been reported for single crystal metal carbide samples. In this study, HIM imaging is used to characterize the foil as a first step towards determining if the carbide structure plays an important role in the olefin metathesis related surface chemistry.
2. Experimental
The molybdenum carbide sample was prepared by carburizing molybdenum foil in CH4/H2 using the temperature programmed technique.23,24 The formation of bulk β-Mo2C was verified using X-ray diffraction. The sample was mounted on the cold finger of an ultrahigh vacuum manipulator by wrapping a Ta foil against the back of the carbide and around the front edges. This method of holding the sample left approximately 90% of the surface free for analysis using surface science techniques. The tantalum foil was shaped so as to join it to current feedthroughs via two narrow strips. The copper feedthroughs served to cool the sample to approximately 100 K, using liquid nitrogen in the cold finger, and to resistively heat the sample to approximately 1400 K. Traces of sulfur contamination were removed using argon ion sputtering as verified by XPS measurements. XPS was also used to establish the stoichiometry of the surface region as Mo2C. Holding the sample at 600 K and exposing it to propene minimized oxygen contamination. Excess carbon at the surface was removed by annealing to 1385 K. The sample was heated to 1300 K as a final cleaning step prior to each surface science experiment. Helium ion microscopy studies were performed to determine the morphology of the surface. The Carl Zeiss Orion Plus HIM was operated at an accelerating voltage of 30.8 kV and an imaging current of 13 pA. These settings provided a sufficiently strong backscattered ion signal for imaging.All of the surface spectroscopy measurements were performed in a UHV chamber (base pressure approximately 2 × 10−11 Torr). Dosing of gases via leak valves was performed in the 10−8–10−7 Torr range, using uncorrected gauge readings, and is expressed as Langmuirs (1 L = 1 × 10−6 Torr.s). Acetone, acrolein and 1,3,5,7-cyclooctatetraene were purified by successive freeze-pump-thaw cycles prior to dosing and their purity was checked in situ using the quadrupole mass spectrometer. RAIRS spectra were measured with the sample held at 100 K by averaging 800 sample scans and using the clean surface as a reference. Data recorded using InSb and narrow band MCT detectors are presented separately. All of the experiments were repeated multiple times to confirm results. TPD spectra were measured by placing the sample face at approximately 1 mm from the entrance of the shielded quadrupole mass spectrometer. The temperature was controlled electronically and set to 0.5 K/s.
3. Results and discussion
3.1 Surface 2-propylidene
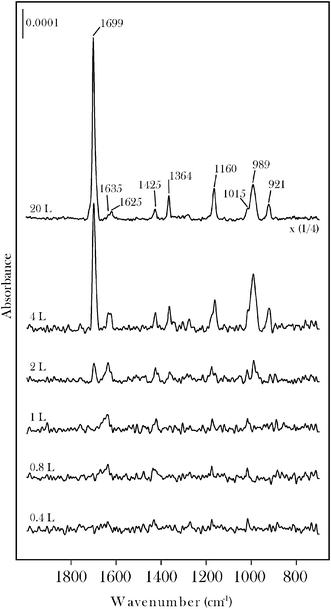
Two routes to form surface carbene groups on molybdenum carbide were explored using acetone and acrolein. Data for acetone will be presented first. Reference RAIRS spectra (Fig. 1) were obtained for molecular adsorption at 100 K. Monolayer saturation is observed at approximately 3 L after which the carbonyl band characteristic of condensed acetone grows in at 1716 cm−1.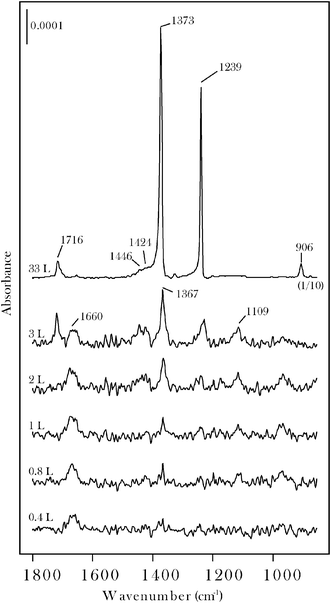 | ||
| Fig. 1 RAIRS data for acetone on β-Mo2C at 100 K. Saturation of the chemisorbed monolayer occurs at an exposure of ∼3 L as shown by onset of intense bands due to condensed acetone. The 33 L spectrum is characteristic of condensed acetone. | ||
The chemisorption layer ν(CO) band, at 1660 cm−1, is characteristic of an η1(O) state, as also reported25–27 for acetone on Pt(111). The molecularly chemisorbed layer also displays bands at 1424, 1367, 1239 and 1109 cm−1 that are assigned to δa(CH3), δs(CH3), νa(C–C–C) and ρ(CH3) modes, respectively.25–28 As shown in Fig. 2, the η1(O) state can be isolated by heating the sample to 200 K while annealing to 300 K leads to the loss of the ν(CO) band. When compared to acetone on Pt-group metals25–28 the molybdenum carbide sample displays a distinct surface chemistry in that carbonyl bond scission occurs to form surface 2-propylidene and oxo groups on annealing to the 300–500 K range. This may be seen in Fig. 2 by noting that while the ν(CO) band is eliminated, bands characteristic of the CH3CCH3 moiety are retained. By reference to data for other carbonyl molecules on β-Mo2C19–22 the two new bands which grow in at 982, 1120 cm−1 are assigned to the oxo (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibration and a vibration with Mo
O) vibration and a vibration with Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching character, respectively.
C stretching character, respectively.
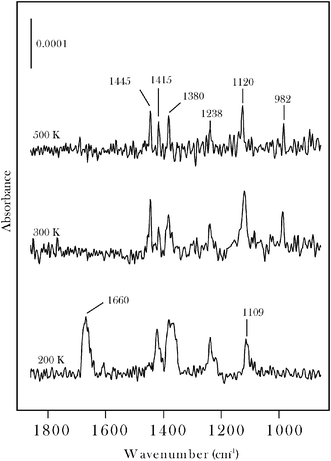 | ||
| Fig. 2 RAIRS spectra for 30 L acetone on β-Mo2C measured at 100 K following annealing to the indicated temperatures. | ||
XPS C(1s) data for acetone are presented in Fig. 3 where they are compared with reference surface sensitive synchrotron-XPS spectra29 for clean β-Mo2C and for the surface following decomposition of 10 L ethene at 600 K (spectra 3 a, b). While the clean surface displays a single peak at 282.8 eV the ethene treated surface is characterized by a peak at 283.4 eV corresponding to excess surface carbon.29 Multilayer acetone (spectrum c) displays methyl and carbonyl C(1s) peaks at 285.3 and 288.2 eV, respectively. Annealing the multilayer to 400 K leads to two new peaks at 284.8 and 285.8 eV also with an intensity ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The latter peaks are attributed to the methyl groups and tentatively to the sp2carbon of 2-propylidene, respectively. Although the binding energy attributed to the sp2carbon is surprisingly high, the assignment is supported by previous XPS data for other alkylidenes on β-Mo2C.19
1. The latter peaks are attributed to the methyl groups and tentatively to the sp2carbon of 2-propylidene, respectively. Although the binding energy attributed to the sp2carbon is surprisingly high, the assignment is supported by previous XPS data for other alkylidenes on β-Mo2C.19
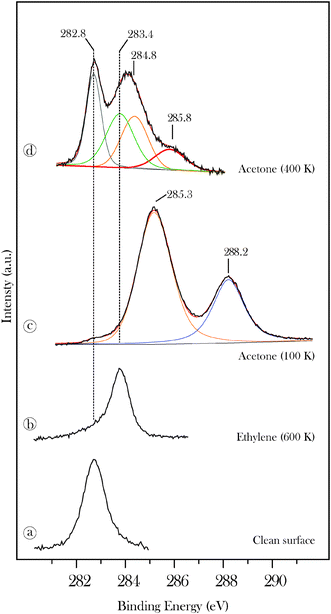 | ||
| Fig. 3 High-resolution C(1s) data recorded for 20 L acetone on β-Mo2C (c) and for acetone annealed to 400 K (d). Reference spectra, discussed in ref. 29, are shown for the clean carbide (a) and for the surface treated with 10 L ethene at 600 K (b). | ||
3.2 Surface propylidiene
RAIRS data for acrolein on β-Mo2C are displayed in Fig. 4 and 5. The chemisorbed monolayer is saturated at an exposure of 2 L (Fig. 4). Condensed acrolein is characterized by a carbonyl band at 1699 cm−1. Molecularly chemisorbed acrolein, isolated at 200 K (Fig. 5), displays bands at 1635, 1425, 1175, 1015 and 887 cm−1 in good agreement with the HREELS spectrum for acrolein on Pt(111).30,31 The feature at 1635 cm−1 is due to either the ν(CO) stretching vibration of η1(O) chemisorbed acrolein or to the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching vibration, or to an overlap of both. The bands at 887, 1175 and 1425 cm−1 may be assigned to ρ(CH2), ν(C–C) and δ(CH2) modes, respectively.30,31Annealing to 500 K leads to a number of new bands that we attribute to the formation of surface propylidiene and oxo groups. The Mo
C) stretching vibration, or to an overlap of both. The bands at 887, 1175 and 1425 cm−1 may be assigned to ρ(CH2), ν(C–C) and δ(CH2) modes, respectively.30,31Annealing to 500 K leads to a number of new bands that we attribute to the formation of surface propylidiene and oxo groups. The Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band is observed at 990 cm−1. The band at 1625 cm−1 is assigned to the ν(C
O band is observed at 990 cm−1. The band at 1625 cm−1 is assigned to the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) vibration of the vinyl alkylidene. The band at 1517 cm−1 is tentatively assigned to a mixed ν(C
C) vibration of the vinyl alkylidene. The band at 1517 cm−1 is tentatively assigned to a mixed ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) + ν(Mo
C) + ν(Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) mode. The ν(C–C) mode is observed at 1155 cm−1. These peaks are also present at 300 K, but with different relative intensities, consistent with dissociation through an η2(C,O) intermediate. CH stretching region spectra for propylidiene formed from acrolein on β-Mo2C are published elsewhere.12
C) mode. The ν(C–C) mode is observed at 1155 cm−1. These peaks are also present at 300 K, but with different relative intensities, consistent with dissociation through an η2(C,O) intermediate. CH stretching region spectra for propylidiene formed from acrolein on β-Mo2C are published elsewhere.12
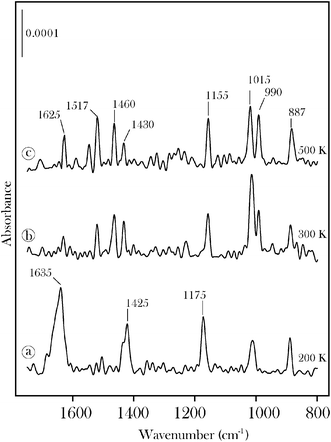 | ||
| Fig. 5 RAIRS data for (a) molecular acrolein on β-Mo2C at 200 K and for the surface groups formed on annealing the sample to (b) 300 K and (c) 500 K. | ||
The thermal stability of the propylidiene surface species was studied by XPS and TPD. Surface propylidiene is characterized by a peak at 284.3 eV and a smaller peak at 285.8 eV (Fig. 6d). As described above for acetone, the latter peak is tentatively attributed to the carbon atom that forms a double bond to the metal. Annealing to higher temperatures leads to a transfer of C(1s) intensity to the peak at 283.4 eV. This is consistent with the deposition of carbon and CxHy fragments on the carbide surface through the progressive decomposition of the alkylidene species.19,32 However, a fraction of the propylidiene surface groups remain stable to 900 K consistent with previous reports of the anomalously high thermal stability of alkylidenes on the molybdenum carbide surface.11,19,32 Complete removal of the alkylidenes from the surface occurs at 920 K as shown by the recombinative desorption peak shown in Fig. 7. The low temperature peak is due to desorption from the condensed layer. The structure in the H2 desorption spectrum in the 800–1200 K region is attributed to competition between recombinative desorption of acrolein and decomposition of propylidiene to progressively more dehydrogenated CxHy surface species.32
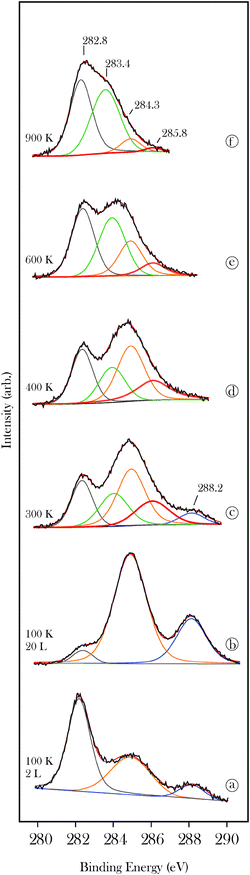 | ||
| Fig. 6 X-ray photoelectron C(1s) spectra for acrolein (a, b) on β-Mo2C as a function of exposure at 100 K and (c–f) as a function of annealing temperature for the 20 L exposed sample. | ||
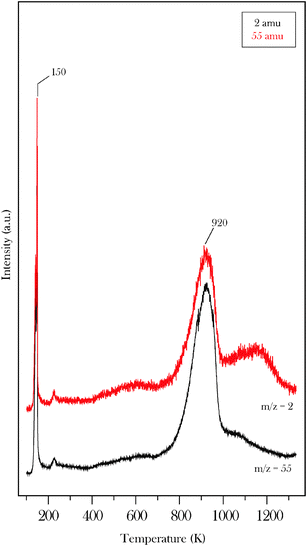 | ||
| Fig. 7 Temperature programmed desorption data obtained following 20 L acrolein exposure to β-Mo2C at 100 K. The spectrum shows data for H2 and molecular acrolein. | ||
3.3 Interaction with 1,3,5,7-cyclooctatetraene
The propylidiene functionalized surface, prepared at 400 K, was exposed to 1,3,5,7-cyclooctatetraene (COT) in order to probe for ring opening metathesis polymerization (ROMP) activity. Reference RAIRS spectra (not shown) taken at 100 K show only two peaks, a CH stretching vibration at 3009 cm−1 and a ring distortion mode at 802 cm−1 in rough agreement with previous low resolution HREELS measurements on different metal surfaces.33,34 C(1s) measurements for the adsorption of COT on β-Mo2C at temperatures above 200 K show a single peak at 283.8 eV due to molecular cracking (spectrum not shown). In contrast, difference spectra show that exposure of a propylidiene-modified surface to COT at 300 K leads to the growth of a C(1s) feature at 284.3 eV (Fig. 8). The difference spectra are relative to the propylidiene functionalized surface (spectrum 6d). The feature at 284.3 eV is attributed to a ring opening insertion of COT at surface propylidiene sites.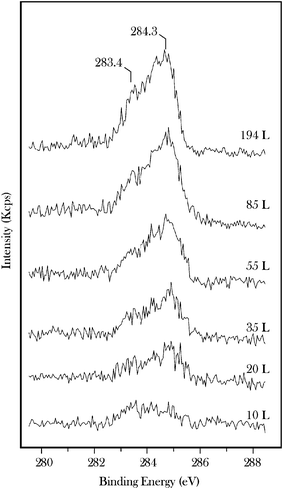 | ||
| Fig. 8 Difference C(1s) spectra taken as a function of exposure of propylidiene functionalized β-Mo2C to 1,3,5,7-cyclooctatetraene (COT) at 300 K. The difference spectra are with respect to a propylidiene functionalized surface (spectrum 6d). | ||
This conclusion is supported by RAIRS measurements (Fig. 9) showing that the interaction of COT with the propylidiene functionalized surface leads to a new band at 3033 cm−1. The solution phase infrared spectra of both trans, trans-1,3,5,7-octatetraene and all-trans-1,3,5,7,9-decapentaene35 display strong bands at ∼3030 and ∼3009 cm−1. The increase in C(1s) intensity at 283.4 eV (Fig. 8) shows that COT also undergoes decomposition on the functionalized surface. Neither the XPS nor the RAIRS results show evidence for multiple insertions of COT, and it is likely that an optimal surface coverage of initiating akylidenes is required for chain growth. The surface coverage of propylidiene used in the present experiments was estimated as 0.32 of a monolayer using established XPS methods.36,37 The monolayer is defined by modelling the entire surface as the (0001) face and assuming full occupancy of two Mo sites per unit cell. This approximate surface coverage is in very good agreement with a previous study of cyclopentylidene on the same β-Mo2C sample.19
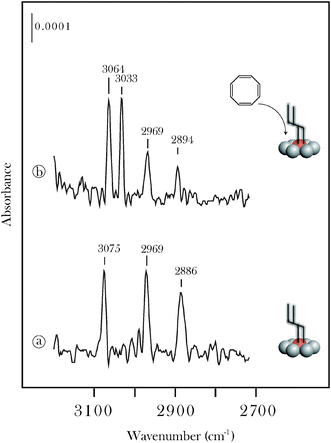 | ||
| Fig. 9 RAIRS data for the interaction of 1,3,5,7-cyclooctatetraene (COT) with a propylidiene functionalized β-Mo2C surface. The alkylidene functionalized surface (a) was prepared by chemisorbing (20 L) acrolein and annealing to 400 K. The interaction with COT was studied at 300 K. | ||
3.4 Surface morphology
As described above, the sample used in the study was prepared by completely transforming a polycrystalline molybdenum foil into β-Mo2C. The RAIRS and XPS measurements provide an averaged signal over the central region of the front of the sample. Neither of these two techniques is extremely sensitive. For example, it is unlikely that alkylidenes located only at a small fraction of active sites would furnish the RAIRS signal observed for the 500 K annealed samples (Fig. 2 and 5). Under the assumption that the alkylidene species are distributed over the surface, helium ion microscopy (HIM) measurements were performed to probe the morphology of the region giving the XPS and RAIRS data.Fig. 10 presents two typical images of the sample showing both the topological features (Fig. 10a) and contrast due to channelling (Fig. 10b). It is apparent that the carbide surface is composed of large grains typically 5–10 microns in diameter. The channelling contrast in particular is important as it is dependent on the orientation of the grain with the beam. When the beam is oriented along a direction parallel to a crystallographic direction the ions travel further into the sample. As a consequence of enhanced channelling, less backscattered ions are able to be collected by the detector and the grain appears dark.38 Selecting an area on the surface, Fig. 11a is a HIM image of the area at 0 degrees while Fig. 11b shows that tilting the sample causes the the contrast to vary. Local minima of the intensity values indicate when a channelling condition has been achieved. Comparing the curves in Fig. 11b we observe that while all of the large grains exhibit signs of channelling the minima appear at different intensities. This implies that the region labelled ‘B’, for example, is a surface of low areal density while the region labelled ‘C’, displaying the highest backscattered yield, is indicative of a densely packed plane.
 | ||
| Fig. 10 Helium ion microscopy (HIM) images of a region of the β-Mo2C surface representative of the area giving the RAIRS and XPS data shown above. (a) SE2 image of the active carbide surface showing the topological features of the sample. (b) Backscattered ion image of the same area showing channelling contrast from the grains. (c) High magnification SE2 image of the surface showing an example of the surface nanostructure. | ||
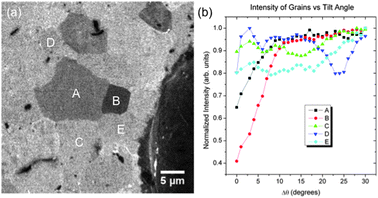 | ||
| Fig. 11 Helium ion microscopy data. (a) Backscattered ion image of the active carbide surface showing the channelling contrast in the sample. (b) Normalized intensity from the regions labelled A–E. The local minima indicate a channelling condition. The large particle on the right of the image was used for image registration. | ||
On closer inspection, the surfaces of the large grains show additional structure (Fig. 10c) in the form of much smaller grains. The overall morphology of the surface is akin to a compacted powder where the smallest particles are nano-sized. The surface morphology of the sample may play a role in the demanding carbonyl bond dissociation step inherent to alkylidene formation. We assume that this step only requires isolated metal surface atoms, as single tungsten atom complexes can form oxo-W-alkylidene products through carbonyl bond scission in direct analogy to the surface reaction.39 Careful studies of α-Mo2C(0001) single crystals40 show that it is possible to prepare Mo- and C-terminated surfaces. Studies on carbide surface layers on Mo single crystals show that an annealing step at 1200 K is required to drive carbon from the surface into interstitial sites so as to generate a surface with a reactivity characteristic of the bulk carbide.41,42 We propose that the activity of the highly structured carbide foil arises from the presence of unsaturated Mo atoms in the outermost surface layer. On the basis of these results, the targeted design of nanostructured films is expected to play a key role in exploring the impressive catalytic and surface reactivity properties of molybdenum carbide.23,42–46
4. Conclusions
Surface vibrational spectroscopy and XPS data show the dissociative adsorption of acetone and acrolein on β-Mo2C to yield 2-propylidene and propylidiene surface alkylidene groups. The propylidiene functionalized surface displays activity towards ring opening insertion of 1,3,5,7-cyclooctatetraene (COT). Helium ion microscopy characterization of the area of the sample probed by the spectroscopic techniques shows that the surface is composed primarily of large grains, and that the surface of these grains show additional structure in the form of smaller grains typically less than 200 nm in width. The combination of spectroscopy and microscopy data suggests that the observed olefin metathesis related reactivity of the carbide sample is related to its surface morphology.Notes and references
- X. Zhu, X. Li, S. Xie, S. Liu, G. Xu, W. Xin, S. Huang and L. Xu, Catal. Surv. Asia, 2009, 13, 1–8 CrossRef CAS.
- D. P. Debecker, B. Schimmoeller, M. Stoyanova, C. Poleunis, P. Bertrand, U. Rodemerck and E. M. Gaigneaux, J. Catal., 2011, 277, 154–163 CrossRef CAS.
- J. Guan, G. Yang, D. Zhou, W. Zhang, X. Liu, X. Han and X. Bao, Catal. Commun., 2008, 9, 2213–2216 CrossRef CAS.
- L. Harmse, C. van Schalkwyk and E. van Steen, Catal. Lett., 2010, 137, 123–131 CrossRef CAS.
- X. Li, J. Guan, D. Zhou, G. Li, X. Han, W. Zhang and X. Bao, THEOCHEM, 2009, 913, 167–172 CrossRef CAS.
- X. Li, W. Zhang, X. Li, S. Liu, H. Huang, X. Han, L. Xu and X. Bao, J. Phys. Chem. C, 2009, 113, 8228–8233 CAS.
- X. Li, W. Zhang, S. Liu, S. Xie, X. Zhu, X. Bao and L. Xu, J. Mol. Catal. A: Chem., 2009, 313, 38–43 CrossRef CAS.
- X. Li, W. Zhang, S. Liu, L. Xu, X. Han and X. Bao, J. Phys. Chem. C, 2008, 112, 5955–5960 CAS.
- H. Liu, S. Huang, L. Zhang, S. Liu, W. Xin and L. Xu, Catal. Commun., 2009, 10, 544–548 CrossRef CAS.
- J. C. Mol, Top. Catal., 2004, 27, 97–104 CrossRef CAS.
- E. M. Zahidi, H. Oudghiri-Hassani and P. H. McBreen, Nature, 2001, 409, 1023 CrossRef CAS.
- M. Siaj, N. Dubuc and P. H. McBreen, J. Phys. Chem. C, 2009, 113, 12331–12339 CAS.
- M. Siaj and P. H. McBreen, Science, 2005, 309, 588 CrossRef CAS.
- A. H. Hoveyda and A. R. Zhugralin, Nature, 2007, 450, 243 CrossRef CAS.
- F. Gao, Y. Wang and W. T. Tysoe, J. Am. Chem. Soc., 2006, 128, 7091–7096 CrossRef CAS.
- W. Chen, S. Chen, F. Ding, H. Wang, L. E. Brown and J. P. Konopelski, J. Am. Chem. Soc., 2008, 130, 12156–12162 CrossRef CAS.
- F. Ren, A. K. Feldman, M. Carnes, M. Steigerwald and C. Nuckolls, Macromolecules, 2007, 40, 8151 CrossRef CAS.
- G. S. Tulevsky, M. B. Myers, M. S. Hybertsen, M. L. Steigerwald and C. Nuckolls, Science, 2005, 309, 591 CrossRef.
- M. Siaj, H. Oudghiri-Hassani, C. Maltais and P. H. McBreen, J. Phys. Chem. C, 2007, 111, 1725 CAS.
- M. Siaj, H. Oudghiri-Hassani, E. M. Zahidi and P. H. McBreen, Surf. Sci., 2005, 579, 1 CrossRef CAS.
- M. Siaj, C. Reed, T. Oyama, S. L. Scott and P. H. McBreen, J. Am. Chem. Soc., 2004, 126, 9514 CrossRef CAS.
- M. Siaj, I. Temprano, N. Dubuc and P. H. McBreen, J. Organomet. Chem., 2006, 691, 5497 CrossRef CAS.
- S. T. Oyama, Transition Metal Carbides, Nitrides, and Phosphides, Wiley-VCH Verlag GmbH & Co. KGaA, 2008 Search PubMed.
- Monograph of The Chemistry of Transition Metal Nitrides and Carbides, ed. J. Wang, M. Castonguay, P. H. McBreen, S. Ramanathan and S. T. Oyama, Chapman & Hall, London, 1996 Search PubMed.
- M. A. Vannice, W. Erley and H. Ibach, Surf. Sci., 1991, 254, 1 CrossRef CAS.
- N. R. Avery, Surf. Sci., 1983, 125, 771–786 CrossRef CAS.
- E. L. Jeffery, R. K. Mann, G. J. Hutchings, S. H. Taylor and D. J. Willock, Catal. Today, 2005, 105, 85 CrossRef CAS.
- A. B. Anton, N. R. Avery, B. H. Toby and W. H. Weinberg, J. Am. Chem. Soc., 1986, 108, 684 CrossRef CAS.
- M. Siaj, C. Maltais, E. M. Zahidi, H. Oudghiri-Hassani, J. Wang, F. Rosei and P. H. McBreen, J. Phys. Chem. B, 2005, 109, 15376 CrossRef CAS.
- D. Loffreda, Y. Jugnet, F. Delbecq, J. C. Bertolini and P. Sautet, J. Phys. Chem. B, 2004, 108, 9085 CrossRef CAS.
- L. E. Murillo and J. G. Chen, Surf. Sci., 2008, 602, 919 CrossRef CAS.
- H. Oudghiri-Hassani, M. Siaj and P. H. McBreen, J. Phys. Chem. C, 2007, 111, 5954 CAS.
- M. J. Hostetler, R. G. Nuzzo, G. S. Girolami and L. H. Dubois, J. Phys. Chem., 1994, 98, 2952 CrossRef CAS.
- A. F. Lee, C. J. Baddeley, C. Hardacre and R. M. Lambert, J. Am. Chem. Soc., 1995, 117, 7719 CrossRef CAS.
- S. Hirata, H. Yoshida, H. Torii and M. Tasumi, J. Chem. Phys., 1995, 103, 8955 CrossRef CAS.
- D. C. Bell, Microsc. Microanal., 2009, 15, 147 CrossRef CAS.
- A. L. Bramblett, M. S. Boeckl, K. D. Hauch, B. D. Ratner, T. Sasaki and J. W. Rogers Jr., Surf. Interface Anal., 2002, 13, 506–515 CrossRef.
- A. Gulino, F. Lupo, M. E. Fragalà and S. Lo Schiavo, J. Phys. Chem. C, 2009, 113, 13558–13564 CAS.
- J. C. Bryan and J. M. Mayer, J. Am. Chem. Soc., 1990, 112, 2298–2308 CrossRef CAS.
- T. P. St. Clair, S. T. Oyama and D. F. Cox, Surf. Sci., 2000, 468, 62–76 CrossRef CAS.
- B. Fruhberger, J. G. Chen, J. J. Eng and B. E. Bent, J. Vac. Sci. Technol., A, 1996, 14, 1475–1481 Search PubMed.
- H. H. Hwu and J. G. Chen, Chem. Rev., 2004, 105, 185–212 CrossRef.
- A.-M. Alexander and J. S. J. Hargreaves, Chem. Soc. Rev., 2010, 39, 4388–4401 RSC.
- N. Schweitzer, T. Schaidle, O. Ezekoye, X. Pan, S. Linic and L. T. Thompson, J. Am. Chem. Soc., 2011, 133, 2378–2381 CrossRef CAS.
- A. Hanif, T. Xiao, A. P. E. York, J. Sloan and M. L. H. Green, Chem. Mater., 2002, 14, 1009–1015 CrossRef CAS.
- C. A. Wolden, A. Pickerell, T. Gawai, S. Parks, J. Hensley and J. D. Way, ACS Appl. Mater. Interfaces, 2011, 3, 517–521 CAS.
| This journal is © The Royal Society of Chemistry 2011 |