Selective hydrogenation of functionalized nitroarenes under mild conditions
Valerica
Pandarus
a,
Rosaria
Ciriminna
b,
François
Béland
*a and
Mario
Pagliaro
*b
aSiliCycle Inc., 2500, Parc-Technologique Blvd, Quebec City, Quebec, Canada G1P 4S6. E-mail: fbeland@silicycle.com; Fax: +1 418 874 0355
bIstituto per lo Studio dei Materiali Nanostrutturati, CNR, via U. La Malfa 153, 90146 Palermo, Italy. E-mail: mario.pagliaro@cnr.it; Fax: +39 091 680 92 47
First published on 8th June 2011
Abstract
Functionalized anilines are selectively synthesized from the corresponding nitroarenes by simple heterogeneous catalytic hydrogenation carried out under mild conditions over a very low amount (0.5 mol%) of a new sol–gel entrapped Pd(0) catalyst. Only halo-nitroarenes could not be selectively converted. The catalyst is leach-proof and can be reused in many consecutive reaction cycles.
Introduction
Functionalized anilines are important intermediates for pharmaceuticals, polymers, dyes, urethanes and other industrially important chemical products.1 These aromatic amines are generally obtained by catalytic hydrogenation of nitroarenes with various heterogeneous metal catalysts (supported nickel, copper, cobalt) including Pd/C.2 Yet, the selective reduction of a nitro group with H2 when other reducible groups are present in the same molecule is generally not feasible with the abovementioned catalysts, and requires either homogeneous catalysis,3 or delicate strategies involving multi-component catalytic materials.4The use of supported Pd(0) nanoparticles for the hydrogenation of nitro groups in the presence of “moderately” reducible functional groups under mild reaction conditions has been demonstrated, for example using Pd nanoparticles supported over carbon nanofibers;5 in aluminium oxy-hydroxide;6 and over magnesium oxide.7 However, when more challenging substrates containing sensitive functionalities (such as double, and triple bonds, carbonyls, etc.) are involved, different strategies need to be undertaken to transform non-selective supported Pd or Pt catalysts into more selective species.8 Yet, most of these strategies require the employment of relatively harsh conditions and typically require high amount of solid catalyst (for example 0.1 g catalyst for 0.5 g substrate at 70–140 °C under 10–40 bar H2).4 It is thus of relevant interest the discovery reported herein of the chemoselective catalytic activity of new Pd(0) heterogeneous catalysts for the hydrogenation of a wide series of functionalized nitroarenes dissolved in methanol in a simple hydrogen balloon under remarkable mild conditions of room temperature and 1 bar H2.
Results and discussion
Experimental section
Trademarked SiliaCat Pd0: this new series of patent-protected9 sol–gel entrapped Pd nanocatalysts is made of highly dispersed Pd nanoparticles (uniformly in the range 4.0–6.0 nm) encapsulated within an organosilica matrix via an alcohol-free sol–gel process typical of enzyme sol–gel encapsulation.10 Specifically the entrapment process starts with controlled distillation in a rotovapor of the ethanol (a reductant) released with ongoing hydrolysis and condensation of the MeSi(OEt)3 and Si(OEt)4 precursors (eqn (1)): | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na(AcO)3BH = 1
Na(AcO)3BH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 molar ratio) in 80 mL THF, washed with THF and H2O and dried in air to afford the SiliaCat Pd0–4 catalyst used in the catalytic experiments described below.
6 molar ratio) in 80 mL THF, washed with THF and H2O and dried in air to afford the SiliaCat Pd0–4 catalyst used in the catalytic experiments described below.
The dopant metallite particles are made of nanostructured crystalline palladium(0) as clearly shown by the XRD pattern of the powder catalyst in which the diffraction peaks of a face centered cubic structure of metallic Pd are evident (Fig. 1), and for which a typical 5.7 nm metallite particle size was calculated using the Scherrer formula from the line broadening of (111) reflection (Table 1).
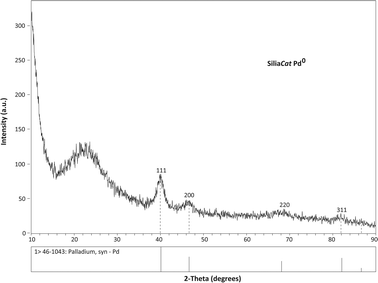 | ||
| Fig. 1 The crystalline nature of the active Pd(0) nano-phase in a SiliaCat Pd(0) catalysts is evident from the succession of X-ray diffraction peaks. | ||
| Catalyst | Diffraction angle 2θ | Mean crystallite size/nm | |||
|---|---|---|---|---|---|
| 111 | 200 | 220 | 311 | ||
| a The Powder Diffraction File of The International Centre for Diffraction Data is used to identify the diffraction peaks characteristic of crystalline Pd(0) with a face centered cubic (fcc) lattice identified Pd(0). | |||||
| Pd(0)a | 40.12 | 46.66 | 68.12 | 82.10 | N.A. |
| SiliaCat Pd0–4 | 39.96 | 46.66 | 68.11 | 81.90 | 5.7 |
For each hydrogenation experiment, the substrate (2 mmol, 1 equiv.) and the catalyst SiliaCat Pd0 (0.5 mol%; catalyst load = 0.063 mmol g−1 measured using the CAMECA SX100 instrument equipped with an EPMA analyzer) were combined in MeOH (HPLC grade) and stirred in a hydrogen balloon at room temperature. Methanol was found to be the optimal reaction solvent after a screening that included other solvents (tetrahydrofuran, toluene and acetonitrile). Increasing the loading of the catalyst in reactions performed under same conditions did not afford significant benefits in terms of reaction rate whereas decreasing its content below 0.5 mol% resulted in drastic reduction of the reaction rate. The conversion with respect to the final product was determined by GC/MS analysis. The preliminary test with substrates displayed in Table 2 shows that by using 0.5–1 mol% of catalyst, reaction proceeds at completion with a minimum 97% selectivity, and most often with complete selectivity.
| Entry | Substrate | Time/h | Product | Conv. (%)c | Selectivity (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 0.5 mol% SiliaCat Pd0; MeOH solvent HPLC grade 0.1 M, molar concentration with respect to the substrate; room temperature under 1 bar H2. b 1 mol% SiliaCat Pd0; MeOH solvent HPLC grade 0.05 M, molar concentration with respect to the substrate. c The conversion with respect to the hydrogenated product was evaluated by GC/MS analysis. d The hydrogenolysis of one chlorine is observed. | |||||
| 1 |
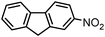
|
1 |
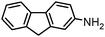
|
100b | 100 |
| 2 |
|
1 |
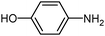
|
80 | 100 |
| 2 | 100 | ||||
| 3 |
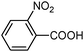
|
1 |
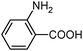
|
100 | 98 |
| 4 |
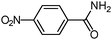
|
1 |
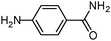
|
100b | 100 |
| 5 |

|
0.5 |
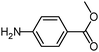
|
100 | 99 |
| 6 |

|
1 |
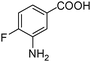
|
100 | 100 |
| 7 |
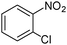
|
1 |
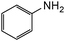
|
100 | 100d |
| 8 |
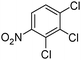
|
1 |

|
100 | 97 |
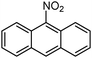
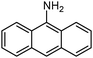
Based on these promising results a wide variety of nitroarenes bearing different functionalities were tested in the catalytic hydrogenation in methanol over 0.5–1 mol% SiliaCat Pd0 under the same mild conditions mentioned above. Table 2 shows that complete conversion was obtained typically after 1 hour, with very fast conversion (0.5 h) in the case of the important pharmaceutical intermediate 4-nitrobenzonitrile; and requiring 4 h only in the case of 9-nitroanthracene, a nitro-polycyclic aromatic hydrocarbon also employed as intermediate in organic synthesis.
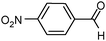
In detail, results in Table 3 show that the hydrogenation proceeds either at completion or with minimum 80% chemoselectivity independently of the substrate's molecular size (entries 1–5). Most importantly, the nitro groups can be reduced selectively with SiliaCat Pd0 under mild conditions when other different functionalities are present in the same molecule, including easily reducible groups such as amide, nitrile, carboxylic acid and ester residues (entries 6–20). Only when such functionalities are carbonyl or nitrile groups, the nitro groups are not reduced selectively. For example, 4-nitrobenzaldehyde (entry 14 in Table 3) is reduced quantitatively to 4-aminobenzyl alcohol.
| Entry | Substrate | Catalyst amount/mol% | Solventb/M | Time/h | Product | Conv.a (%) | Selectivity (%) |
|---|---|---|---|---|---|---|---|
| a Reaction at room temperature under 1 bar H2. Conversion with respect to the desired product was evaluated by GC/MS analysis. b Molar concentration of MeOH with respect to the substrate. c 20% anthracene. d 5% fluorentene. e 8% aminotoluene. f 4-Nitrobenzaldehyde is reduced to 4-aminobenzyl alcohol. | |||||||
| 1 |
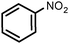
|
SiliaCat Pd0 | MeOH | 1 |

|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
| 2 |
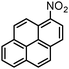
|
SiliaCat Pd0 | MeOH | 1 |

|
100 | 100 |
| 1 | (0.05 M) | ||||||
| 3 |
|
SiliaCat Pd0 | MeOH | 4 |
|
100 | 80c |
| 1 | (0.05 M) | ||||||
| 4 |
|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 95d |
| 1 | (0.05 M) | ||||||
| 5 |
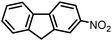
|
SiliaCat Pd0 | MeOH | 1 |
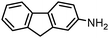
|
100 | 100 |
| 1 | (0.05 M) | ||||||
| 6 |
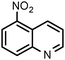
|
SiliaCat Pd0 | MeOH | 1 |
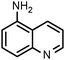
|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
| 7 |
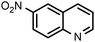
|
SiliaCat Pd0 | MeOH | 1 |
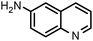
|
80 | 100 |
| 0.5 | (0.07 M) | 2 | 100 | ||||
| 8 |
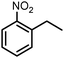
|
SiliaCat Pd0 | MeOH | 1 |
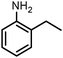
|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
| 9 |
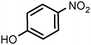
|
SiliaCat Pd0 | MeOH | 1 |

|
80 | 100 |
| 0.5 | (0.1 M) | 2 | 100 | ||||
| 10 |

|
SiliaCat Pd0 | MeOH | 1 |
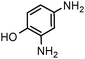
|
100% | 100 |
| 1 | (0.1 M) | ||||||
| 11 |
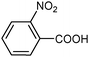
|
SiliaCat Pd0 | MeOH | 1 |
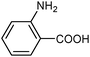
|
100 | 98 |
| 0.5 | (0.1 M) | ||||||
| 12 |
|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
| 13 |
|
SiliaCat Pd0 | MeOH | 1 |
|
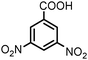
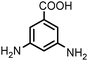
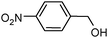
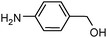
100 | 92e |
| 0.5 | (0.1 M) | ||||||
| 14 |
|
SiliaCat Pd0 | MeOH | 1 |

|
100 | 0f |
| 0.5 | (0.1 M) | ||||||
| 15 |

|
SiliaCat Pd0 | MeOH | 0.5 |
|
90 | 10 |
| 0.5 | (0.1 M) | ||||||
| 16 |

|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 100 |
| 1 | (0.07 M) | ||||||
| 17 |
|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
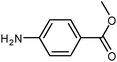
| 18 |
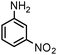
|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 100 |
| 1 | (0.1 M) | ||||||
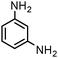
| 19 |
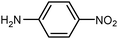
|
SiliaCat Pd0 | MeOH | 1 |

|
100 | 99 |
| 0.5 | (0.1 M) | ||||||
| 20 |
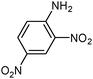
|
SiliaCat Pd0 | MeOH | 1 |
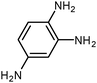
|
100 | 100 |
| 1 | (0.07 M) |
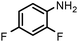
Finally, the SiliaCat Pd0 catalyst was tested in the selective catalytic hydrogenation of different halo-nitroarenes. Again, rapid (0.5–2 h) and complete substrate conversion was observed for each molecule tested (Table 4). Now, however, dehalogenation generally takes place concomitant to reduction of the nitro group. Selective reduction of the –NO2 functionality is observed only when strongly deactivating fluoride is bound to the aryl ring (entries 1–5 in Table 4).
| Entry | Substrates | Catalyst/mol% | Solventb/Mc | Time/h | Product | Conv.a (%) | Selectivity (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: room temperature under 1 bar H2. The conversion with respect to the desired product was evaluated by GC/MS analysis. b Mc, molar concentartion of the solvent with respect to the substrate. c Hydrogenolysis of halide is favored. d Hydrogenolysis of chloride is observed. e Hydrogenolysis of one chloride group is observed. f Hydrogenolysis of both Br residues is favored. | |||||||
| 1 |
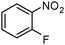
|
SiliaCat Pd0 | MeOH | 1 |
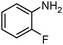
|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
| 2 |
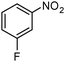
|
SiliaCat Pd0 | MeOH | 1 |
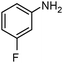
|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
| 3 |

|
SiliaCat Pd0 | MeOH | 1 |
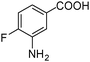
|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
| 4 |
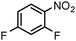
|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 100 |
| 0.5 | (0.1 M) | ||||||
| 5 |
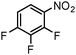
|
SiliaCat Pd0 | MeOH | 1 |
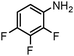
|
100 | 99 |
| 0.5 | (0.1 M) | ||||||
| 6 |
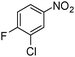
|
SiliaCat Pd0 | MeOH | 2 |

|
90 | 30c |
| 0.5 | (0.1 M) | 4 | 100 | 0 | |||
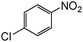
| 7 |
|
SiliaCat Pd0 | MeOH | 1 |
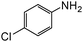
|
100 | 20 |
| 0.5 | (0.1 M) | 2 | 100 | 0 | |||
| 8 |
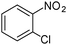
|
SiliaCat Pd0 | MeOH | 1 |
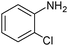
|
100 | 0 |
| 0.5 | (0.1 M) | ||||||
| 9 |
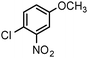
|
SiliaCat Pd0 | MeOH | 2 |
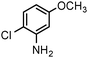
|
100 | 65c |
| 0.5 | (0.1 M) | ||||||
| 10 |
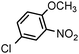
|
SiliaCat Pd0 | MeOH | 4 |
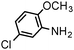
|
99 | 14c |
| 0.5 | (0.1 M) | ||||||
| 11 |

|
SiliaCat Pd0 | MeOH | 2 |
|
99 | 5c |
| 0.5 | (0.1 M) | ||||||
| 12 |
|
SiliaCat Pd0 | MeOH | 2 |
|
99 | 28c |
| 0.5 | (0.1 M) | ||||||
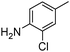
| 13 |
|
SiliaCat Pd0 | MeOH | 2 |
|
99 | 67c |
| 0.5 | (0.1 M) | ||||||
| 14 |
|
SiliaCat Pd0 | MeOH | 1 |

|
93 | 66d |
| 0.5 | (0.1 M) | ||||||
| 15 |
|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 97e |
| 0.5 | (0.1 M) | ||||||
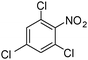
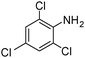
| 16 |
|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 90e |
| 0.5 | (0.1 M) | ||||||
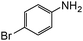
| 17 |

|
SiliaCat Pd0 | MeOH | 1 |
|
100 | 10c |
| 0.5 | (0.07 M) | ||||||
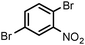
| 18 |
|
SiliaCat Pd0 | MeOH | 1 |

|
54 | 0 |
| 0.5 | (0.1 M) | 4 | 100 | 0f | |||
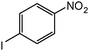
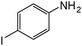
| 19 |
|
SiliaCat Pd0 | MeOH | 4 |
|
40 | 40 |
| 0.5 | (0.05 M) |
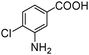
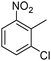
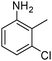
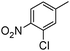
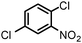
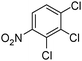
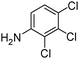
When one chloride is present in the substrate molecule (entries 6–13 in Table 4) fast dechlorination generally takes place along with hydrogenation of the nitro residues; and only in the presence of three chlorine atoms de-halogenation is prevented and selectivity in the nitro group reduction was again >90% (entries 15 and 16). Finally, with bromide (entries 17 and 18) and iodide (entry 19) halo-nitroarenes, selectivity was also poor and now also conversion was not complete pointing to likely catalyst deactivation effect due to iodine and bromine atoms bound to the benzene ring.
Catalyst recycle
The reusability of the catalyst was studied using methyl-4-nitrobenzoate as substrate of choice. The substrate was dissolved in MeOH (HPLC grade, 0.1 M with respect to the substrate) and the hydrogenation was carried out in the presence of 0.5 mol% of the SiliaCat Pd0 under the same conditions reported above (eqn (2)). The resulting mixture was degassed twice replacing each time the vacuum with H2. The reaction mixture, connected to a balloon of hydrogen, was stirred at room temperature for 30 minutes after which the catalyst was recovered by filtration, rinsed with MeOH and dried under vacuum. The conversion was assessed by GC/MS analysis and the filtrate was concentrated to give a crude product.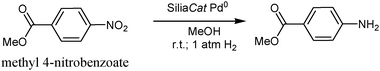 | (2) |
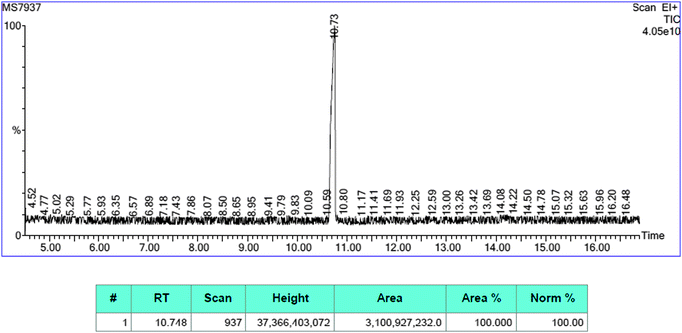 | ||
| Fig. 2 Profile of the GC/MS chromatogram after the 8th run in the hydrogenation of methyl-4-nitrobenzoate over 0.5 mol% SiliaCat Pd0. | ||
| Run | Catalyst/mol% | Solvent/cm | Time/h | Conversiona (%) | Yieldb (%) | Leachingc/ppm | |
|---|---|---|---|---|---|---|---|
| Pd | Si | ||||||
| a Reaction conditions: room temperature under 1 bar H2. Conversion in the final product determined by GS/MS analysis. b Isolated yield of the crude product. c Leaching determined by ICP analysis in solution (DMF solvent, 0.7 M) and in the solid crude product. | |||||||
| 1 | SiliaCat Pd0 | MeOH | 1/2 | 100 | 99.7 | 1.6 | 1.3 |
| 0.5 | 0.1 M | (16) | (12.5) | ||||
| 2 | SiliaCat Pd0 | MeOH | 1/2 | 100 | 99.6 | 0.2 | 0.8 |
| 0.5 | 0.1 M | (2.4) | (8.2) | ||||
| 3 | SiliaCat Pd0 | MeOH | 1/2 | 100 | 99.6 | 0.14 | 0.9 |
| 0.5 | 0.1 M | (1.4) | (8.9) | ||||
| 4 | SiliaCat Pd0 | MeOH | 1/2 | 100 | 99.2 | 0.2 | 0.5 |
| 0.5 | 0.1 M | (2) | (5) | ||||
| 5 | SiliaCat Pd0 | MeOH | 1/2 | 100 | 99.7 | 0.1 | 0.6 |
| 0.5 | 0.1 M | (1.2) | (6) | ||||
| 6 | SiliaCat Pd0 | MeOH | 1/2 | 100 | 99.6 | 0.3 | 1.1 |
| 0.5 | 0.1 M | (3.4) | (11) | ||||
| 7 | SiliaCat Pd0 | MeOH | 1/2 | 100 | 99.3 | 0.2 | 1.1 |
| 0.5 | 0.1 M | (2.6) | (12) | ||||
| 8 | SiliaCat Pd0 | MeOH | 1/2 | 100 | 98.9 | 0.25 | 1 |
| 0.5 | 0.1 M | (2.9) | (11) | ||||
From an applicative viewpoint, it is of crucial importance the fact that reused in 8 consecutive cycles (Table 5) the SiliaCat Pd0 catalyst did not show any loss in catalytic activity with minimal (1–2 ppm) leaching in solution of both Pd and Si (assessed by ICP-MS).
Finally, all nitroarenes hydrogenation reactions were monitored by TLC. In most cases the reactions were very clean, namely showing one product only (single spot on plate) during the reaction and at the end of the conversion. According to the findings in Table 4, however, this was not the case for halide functionalized nitroarenes when the presence of the azoxy compounds was detected both by TLC and in GC/MS analysis.
Conclusions
In brief, we have developed a new catalyst made of an amorphous organosilica matrix entrapping active Pd nanoparticles suitable for the chemoselective hydrogenation of a wide variety of functionalized nitroarenes to the corresponding anilines under remarkably mild conditions (at room temperature and under 1 bar H2) using very low catalyst amount (0.5 mol%) due to fast kinetics and high TON. Trademarked SiliaCat Pd0, the catalyst is highly stable and can be reused several times without loss in activity.The catalyst, furthermore, offers a number of additional advantages over traditional Pd/C catalysts, including lot-to-lot reproducibility due to intrinsically accurate molecular sol–gel loading;11 ease and safe handling (Pd/C is pirophoric); high density (suitable for small volume work); leach-proof nature (ultra low metal contamination of product); and fast, simple recovery of catalyst (the catalyst does not stick to glassware; is mechanically and thermally stable, and solvent independent as organosilica neither shrinks nor swells in any solvent). Given all these features this new catalytic material is amenable to automation in solid phase syntheses, nowadays a well established technology providing with increased productivity in both R&D and manufacturing in the pharmaceutical and fine chemicals industries.12
Acknowledgements
This article is dedicated to Professor John Seddon (Vanguard Consulting, UK) for all he has done for quality in the workplace in all these years.References
- S. C. Mitchell and R. H. Waring, Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 2000 Search PubMed.
- G. Booth, Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag, Weinheim, Germany, 2002 Search PubMed.
- Such as for example the oxo-rhenium complexes recently reported by Fernandes and co-workers: R. G. de Noronha, C. C. Romo and A. C. Fernandes, J. Org. Chem., 2009, 74, 6960 Search PubMed.
- H.-U. Blaser, H. Steiner and M. Studer, ChemCatChem, 2009, 1, 210 Search PubMed.
- M. Takasaki, Y. Motoyama, K. Higashi, S.-H. Yoon, I. Mochida and H. Nagashima, Org. Lett., 2008, 10, 1601 CrossRef CAS.
- F. Chang, H. Kimb, B. Lee, S. Park and J. Park, Tetrahedron Lett., 2010, 51, 4250 Search PubMed.
- M. Lakshmi Kantam, R. Chakravarti, V. Reddy Chintareddy, B. Sreedhar and S. Bhargav, Adv. Synth. Catal., 2008, 350, 822 CrossRef CAS.
- See, for example: F. Figueras and B. Coq, J. Mol. Catal. A: Chem., 2001, 173, 223 Search PubMed.
- R. Ciriminna, M. Pagliaro, G. Palmisano, V. Pandarus, L. Tremblay, F. Béland and M. Simard, WO/2010/015081, 2010.
- M. L. Ferrer, F. dal Monte and D. Levy, Chem. Mater., 2002, 14, 3619 CrossRef CAS.
- M. Pagliaro, R. Ciriminna and G. Palmisano, Chem. Soc. Rev., 2007, 36, 932 RSC.
- A. E. Rubin, S. Tummala, D. A. Both, C. Wang and E. Delaney, Chem. Rev., 2006, 106, 2794 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
