The activity of invertase immobilized on cashew nut shell liquid-templated large pore silica hybrids
Egid B.
Mubofu
*,
James E. G.
Mdoe
and
Grace
Kinunda
Department of Chemistry, University of Dar es Salaam, P.O. Box 35061, Tanzania. E-mail: ebmubofu@gmail.com; Fax: +255-22-2410038; Tel: +255-22-2410038
First published on 22nd August 2011
Abstract
The characterization and activity of invertase enzymes immobilized on large pore micelle templated silica (LP-MTS) hybrid materials is reported. The LP-MTS hybrid materials were prepared by a co-condensation of tetraethoxysilane (TEOS) and 3-aminopropyltrimethoxysilane (AMPTS) in a cashew nut shell liquid (CNSL) template. A commercially available dodecylamine template was also used to afford similar materials, hereinafter abbreviated as DDA-MTS, for comparison purposes. The prepared materials were characterized by different techniques to determine their physicochemical properties. The maximum loading for the amine groups in LP-MTS and DDA-MTS were 3.3 and 2.8 mmol g−1, respectively. Modification of the materials for immobilization of the invertase enzyme was done by reacting them with glutaraldehyde resulting in Glu-LP-MTS or Glu-DDA-MTS. The as-prepared hybrid materials have surface areas ranging from 100 to 214 m2 g−1 with pore diameters ranging from 3.1 to 25 nm. Scanning electron microscopy (SEM) images show that LP-MTS and DDA-MTS materials comprise of roughly spherical particles whereas enzyme and glutaraldehyde supported micelle templated silicas show a rupture of the spherical particles to a fine powder. The activities of free and immobilized invertases have been determined by measuring the amount of reducing sugar produced upon hydrolysis of sucrose at different temperatures, pH and substrate concentrations. Both free and immobilized invertase enzymes showed a maximum activity at a particular optimum temperature, pH and substrate concentration. The maximum activity for the free invertase was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 229 U at pH 5.0 and at 40 °C whereas those of the LP-MTS immobilized invertases were 14
229 U at pH 5.0 and at 40 °C whereas those of the LP-MTS immobilized invertases were 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833 U and 14
833 U and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625 U for covalent and cross-linked invertases, respectively, at pH 4.0 and 40 °C. The maximum activity for DDA-MTS covalently immobilized invertase was 4750 U, at pH 4.0 and 40 °C.
625 U for covalent and cross-linked invertases, respectively, at pH 4.0 and 40 °C. The maximum activity for DDA-MTS covalently immobilized invertase was 4750 U, at pH 4.0 and 40 °C.
Introduction
The application of enzymes as biocatalysts is important in industrial applications, biosensors and drugs.1–6 Unlike metal catalysts, enzymes are highly desirable catalysts when the specificity of the reaction, mild reaction conditions and environmental restrictions are major issues. Despite advantages, the use of enzymes on an industrial scale is hampered by several factors, including the high cost of enzymes and their instability. Furthermore, extreme reaction conditions inactivate enzymes through denaturation due to either changes of conformation or other transformations in their chemical structure. In addition, the utilization of natural enzymes has other processing difficulties such as the problem on reuse of enzymes and product contamination.7–9 Enzymes are soluble in aqueous media and it is therefore difficult and expensive to recover them at the end of the catalytic process. As a result, native enzymes often suffer severe limitations in broader industrial applications. One of the approaches to resolve these difficulties is to immobilize them on solid surfaces which can produce recoverable and stable heterogeneous biocatalysts. Enzyme immobilization with different kinds of matrices have been investigated by several workers.9–13 The fixation of an enzyme into inorganic materials combines the selectivity of the enzyme with improved chemical and mechanical stability of the enzyme. The storage properties of enzymes are also improved by immobilization.Mesoporous silicas have been the subject of intense study5–29 because they offer attractive supports for a range of catalytic complexes due to their wide range of topologies and connectivities with dimensions of up to 3 nm and beyond. However, due to small pore size and non-open pore hindrance, the immobilized enzymes have sometimes shown lower activity than free enzymes, and the non-uniform pore sizes of most silica gel supports make the processes less reproducible. Micelle templated silica (MTS) materials have tunable and functionalizable surfaces with restricted nanospaces that are ideal for enzyme immobilization. Recently, many research groups have immobilized enzymes on MTS which showed improvements on stability, activity, product specificity, and resistance to extreme environmental conditions.6,18,19,26–28 MTS supports show biocompatibility, have a low cytotoxicity, large surface areas, and can be easily functionalized and hence are also attractive for biomedical applications.20–25
Invertase is a widely used enzyme in the food industry particularly in the production of sweeteners used in beverages, jams, and artificial honey30 as it catalyzes the cleaving of the α-1,4 glycosidic bonds of sucrose to produce glucose and fructose.
The enzyme exhibits relatively high activity over a broad range of pH (3.5–5.5) with the optimum31 near pH 4.5 and its activity reaches maximum at about 55 °C. Due to its potential in industries, availability and ease of handling, invertase has been immobilized on a number of carriers.32–38 However, most of these supports are based on soft gels which have low mechanical strength and in some cases low thermal and chemical stability.34 Organic–inorganic mesoporous silica materials are most promising because they offer tunability and most of the required properties of a good enzyme support. A good number of the mesoporous materials that have been studied for bioimmobilization posses a relatively small pore diameter not exceeding 10 nm.1–10,26–28 This pore diameter is smaller than most biomolecules, which are more than 10 kD. Large pore micelle templated silicas (LP-MTS) with pore dimensions in the range of 17 nm to 25 nm are potentially attractive supports for bioimmobilization due to their large pore dimensions. LP-MTS with pore dimensions in this range have previously been prepared in our group using a cashew nut shell liquid (CNSL) template.29 Herein, we report the immobilization protocol, the systemic characterization and catalytic testing of LP-MTS immobilized invertase. For comparison, invertase immobilized on MTS prepared from the commercially available surfactant, dodecylamine (DDA) is also reported.
Results and discussion
Porosity properties of LP-MTS/DDA-MTS and supported glutaraldehyde
The porosity properties of the LP-MTS/DDA-MTS and their derivatives were determined by analysis of nitrogen adsorption isotherms using the BET method. Table 1 gives a summary of the BET surface areas, pore volumes and average pore diameters of these materials.| Material | BET Surface area (m2 g−1) | Average pore diameter (nm) | Total pore volume (cm3 g−1) |
|---|---|---|---|
| LP-MTS | 160 | 24.8 | 2.9 |
| DDA-MTS | 214 | 3.1 | 0.1 |
| Glu-LP-MTS | 189 | 11.7 | 2.4 |
| Glu-DDA-MTS | 212 | 3.1 | 0.1 |
| Covalent immobilized invertase on LP-MTS | 116 | 11.5 | 0.6 |
| Cross-linked invertase on LP-MTS | 123 | 11.5 | 0.7 |
| Free invertase | 30 | 3.1 | 0.4 |
The LP-MTS and modified LP-MTS materials display surface areas ranging from 100 to 190 m2 g−1 with pore diameters ranging from 11 to 25 nm. On the other hand, the DDA-MTS and Glu-DDA-MTS have surface areas of 214 and 212 m2 g−1, respectively, both having pore diameters of 3.1 nm. The pore diameters and pore volumes for LP-MTS decreased upon modification whereas in DDA-MTS it remained the same. This shows that for DDA-MTS, the modification did not take place inside the pores, rather on the void spaces of the particles. The slight decrease in surface areas and pore diameters observed after each step of MTS modification is due to steric effects caused by the functional groups introduced into the interior of the pores which have reduced the area available for nitrogen physisorption and this implies that the modification for LP-MTS takes place inside the pores.39,40
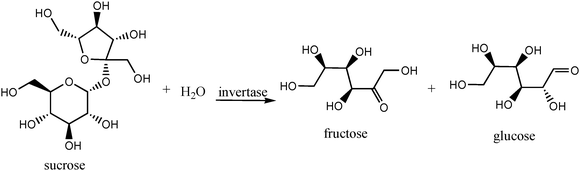
The shapes of the isotherms obtained for all materials are type IV (Fig. 1). This is a typical shape for mesoporous materials with initial gradual increase of nitrogen uptake at low relative pressure (<0.1). Above that, the isotherm forms a plateau followed by another sharp rise of nitrogen uptake starting from about P/Po = 0.8.
 | ||
| Fig. 1 Nitrogen adsorption–desorption isotherms for LP-MTS, Glu-LP-MTS, covalent immobilized invertase and free invertase. | ||
The initial gradual increase of nitrogen uptake at low relative pressure (<0.1) observed in all isotherms is attributed to the formation of nitrogen monolayer on the adsorbent surface. There is a sharp rise of nitrogen uptake from P/Po = 0.8 for these materials and this behavior can be associated with the formation of multilayer of the adsorbate, causing capillary condensation in the pores.40–44 A slightly low nitrogen uptake at the same relative pressure is observed in the LP-MTS derivatives. This implies that materials introduced during modification of LP-MTS to form Glu-LP-MTS and the immobilized invertase occupied some parts of the LP-MTS pore volume. The isotherms of LP-MTS and that of Glu-LP-MTS are similar in that they rise rapidly towards P/Po = 1. On the other hand, the isotherms for the immobilized invertases are similar. Their curves tend to be horizontal towards P/Po = 1 and show mesoporosity characteristics. The significant difference between the isotherms of LP-MTS, Glu-LP-MTS and that of immobilized invertase lies in the amount of nitrogen adsorbed at the same relative pressure. The Glu-LP-MTS isotherm and that of immobilized invertase show slightly low nitrogen uptake compared to the LP-MTS support. The isotherm of LP-MTS and its derivatives clearly show hysteresis loops at a relative pressure above 0.6 whereas free invertase shows a hysteresis loop at a relative pressure above 0.8. The volume of nitrogen adsorbed depends on the pore diameter, pore volume and surface area of the material. A large amount of nitrogen is adsorbed on the parent LP-MTS material and decreases as its modification takes place. This is in line with the decrease in pore sizes and surface areas upon functionalization of the support.
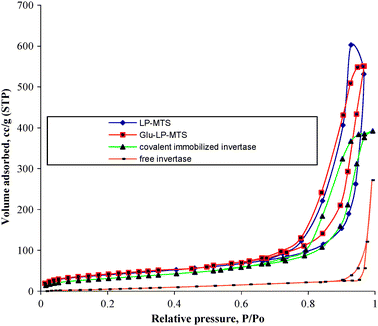
The adsorption–desorption isotherms of MTS prepared by DDA templates are represented in Fig. 2.
 | ||
| Fig. 2 Nitrogen adsorption–desorption isotherms for DDA-MTS and Glu-DDA-MTS. | ||
The isotherms have two substantial initial inflexion points due to sharp nitrogen uptakes; the first is at low relative pressure (<0.1) and the second is at a relative pressure of about 0.9 with some hysteresis loops at a relative pressure of about 0.4. The higher nitrogen uptake at low relative pressure is indicative of monolayer adsorption of these materials. The Glu-DDA-MTS adsorbs less nitrogen relative to DDA-MTS at a given relative pressure.
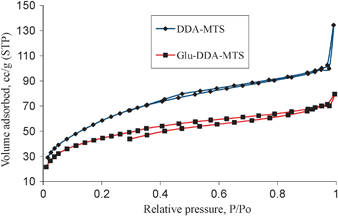
The pore size distributions of LP-MTS, Glu-LP-MTS and immobilized invertases show a wide pore size distribution with the majority of pores ranging from 10 nm to 40 nm as observed in Fig. 3. There is a decrease in the pore size distributions upon fuctionalization of LP-MTS to Glu-LP-MTS. In contrast, the pore size distribution of DDA based materials is narrow with the average pore diameters of 3 nm (Table 1).
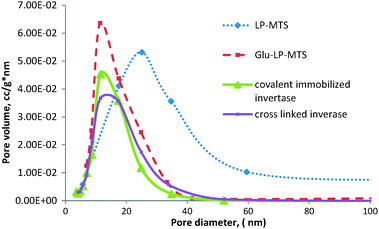 | ||
| Fig. 3 Pore size distributions of LP-MTS, Glu-LP-MTS, covalent immobilized invertase and cross-linked invertase. | ||
The pore size distributions for the LP-MTS are generally wide. The majority of the pores have diameters ranging from 11 to 25 nm and an average pore diameter of 18 nm. Unlike LP-MTS supports, DDA-MTS supports have a narrow pore size distribution. The reason for the unexpectedly large pore diameters observed in CNSL-based hybrids is still unclear, and further studies on the templating mechanism are underway. It was noted that, the greater the modification of LP-MTS, the narrower the pore size distribution and the smaller the pore volume. This decrease in pore diameter may be due to the possibility of the reaction product and/or substrates adsorbing on the support material during the modification process.
Loading of basic functional groups
The materials prepared (LP-MTS/DDA-MTS) are basic in nature and thus acid titration can be used to estimate the amount of basic groups on the surface. This is equivalent to the loading as expressed in mmol of functional groups per gram of silica. Results obtained during titration show that the loading of the amino functional group on LP-MTS material averaged at 3.3 mmol g−1. This loading is higher than that on DDA-MTS (2.8 mmol g−1) and that on others reported elsewhere.29,44 The loading of amino groups onto polyamine-silica hybrids depends on the amino group source (e.g.monoamine, diamine) as well as the type of surfactant used.29SEM analysis of the prepared materials
Scanning electron microscopy (SEM) allowed the visualization of the materials at very high magnifications. Representative electron micrographs for LP-MTS, DDA-MTS, Glu-LP-MTS, Glu-DDA-MTS and the immobilized invertases are presented in Fig. 4(a–f).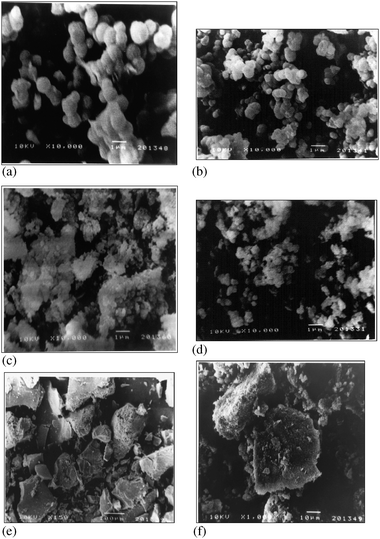 | ||
| Fig. 4 SEM images of (a) LP-MTS (b) DDA-MTS (c) Glu-LP-MTS (d) Glu-DDA-MTS (e)LP-MTS covalent immobilized invertase and (f) LP-MTS cross-linked invertase. | ||
As evident in these micrographs, there is a significant change in the morphology of the materials at different stages when LP-MTS is modified to the supported glutaraldehyde and finally to immobilized invertase enzyme. The LP-MTS (Fig. 4a) and the DDA-MTS (Fig. 4b) are comprised of roughly spherical particles that are loosely bound whereas their supported glutaraldehyde (Fig. 4c and 5d) show an aggregation of the particles. The immobilized invertase displayed a crystalline nature with rough surfaces. These rough surfaces may be a result of coagulation of enzymes after immobilization. It is likely that it is created through the breaking of supported glutaraldehyde into finer particles due to mechanical stirring and hence re-interacting to form clusters. These interactions are likely to be between the aldehyde functionalized groups of different particles or through the immobilization of enzyme molecules on two different silica particles. Moreover, the surface modifications and the effects of the mechanical stirring, creates silica particles with a rougher and finer appearance.
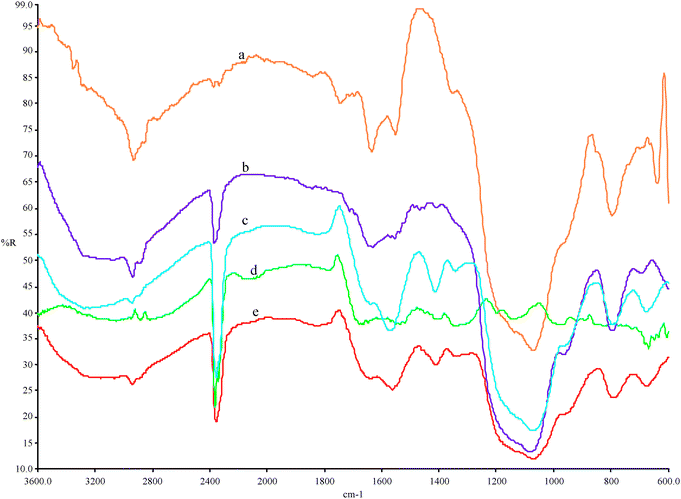 | ||
| Fig. 5 FTIR spectra for (a) Glu-LP-MTS, (b) LP-MTS, (c) covalent immobilized invertase, (d) Free invertase and (e) cross-linked invertase. | ||
DRIFT analysis of LP-MTS, DDA-MTS, Glu-LP-MTS, Glu-DDA-MTS, the immobilized and free invertases
Infrared spectra were obtained using a Perkin Elmer BX FT-IR Spectrophotometer. The samples were diluted using KBr at a ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 5 shows the spectra obtained for LP-MTS, Glu-LP-MTS, covalent immobilized invertase, free invertase and cross-linked invertase.
1. Fig. 5 shows the spectra obtained for LP-MTS, Glu-LP-MTS, covalent immobilized invertase, free invertase and cross-linked invertase.
It is clear from the spectra that the amino group was introduced during the formation of LP-MTS due to the N–H bending mode at 1631 cm−1 and the C–H stretching vibration frequency observed at 2930–2940 cm−1 for all spectra. The corresponding simple C–H bending vibrations occur at 1408 and 1414 cm−1 in the spectra for the cross-linked and covalent immobilized invertases, respectively. An aldehyde group is observed on the spectrum for Glu-LP-MTS at 1744 cm−1 and does not appear in any other spectrum. The amine-glutaraldehyde reaction produces an imine N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond seen at 1634 cm−1 in Glu-LP-MTS. The ethylenic C
C bond seen at 1634 cm−1 in Glu-LP-MTS. The ethylenic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond formed by resonance stabilization of the imine, appears at 1551 cm−1 in Glu-LP-MTS spectrum and at 1561 cm−1 and 1566 cm−1 in cross-linked and covalent immobilized invertase spectra, respectively. Associated with Si–O–Si bonds are the bands, appearing in the range 1074–1081 cm−1 in all spectra except in the free invertase.
C bond formed by resonance stabilization of the imine, appears at 1551 cm−1 in Glu-LP-MTS spectrum and at 1561 cm−1 and 1566 cm−1 in cross-linked and covalent immobilized invertase spectra, respectively. Associated with Si–O–Si bonds are the bands, appearing in the range 1074–1081 cm−1 in all spectra except in the free invertase.
The spectrum of DDA-MTS (Fig. 6) shows weak C–H stretching vibration bands in the 2800–2950 cm−1 region and the corresponding weak H–N–H and H–C–H bending vibrations at 1640 cm−1 to 1550 cm−1.
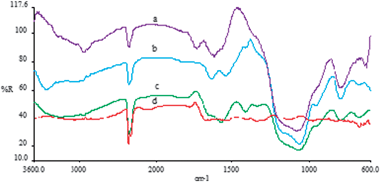 | ||
| Fig. 6 FTIR spectra for (a) Glu-DDA-MTS, (b) DDA-MTS, (c) covalent immobilized invertase, and (d) Free invertase. | ||
The spectrum of Glu-DDA-MTS shows some changes in the regions where apart from the bending vibrations due to the H–N–H and H–C–H, also a new band at about 1740 cm−1 attributed to a C|N stretching vibration is also observed.
Incubation time
The incubation time of the invertase enzyme and its substrate (sucrose) was varied from 10 to 70 min in order to establish the optimum incubation period. The activities obtained for free and immobilized enzymes on LP-MTS are given in Fig. 7. As seen in the Figure, as the incubation time increases, the activity increases for both free and immobilized invertases until it reaches a maximum. For both free and the immobilized invertases, the maximum activity was after 50 min incubation time.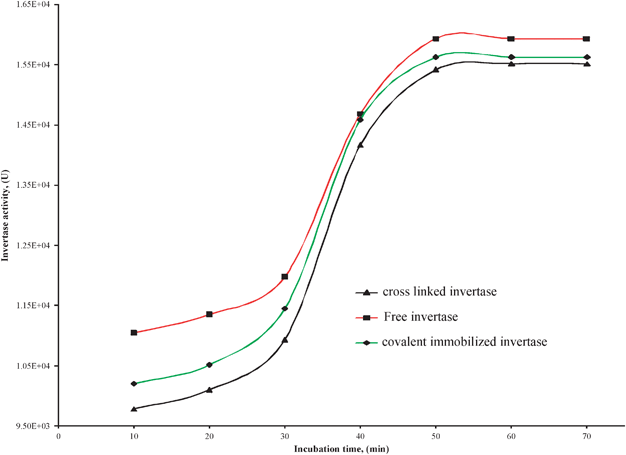 | ||
| Fig. 7 Effect of incubation time on activity of invertase enzymes on LP-MTS derivatives and free invertase. | ||
Effect of temperature on the activities of free and immobilized invertase enzymes
The effect of temperature on the activities of free and immobilized invertase was determined by measuring the hydrolysis of sucrose at incubation temperature ranging from 25 to 60 °C. Initially, the activity of each biocatalyst increased with increasing temperature to a maximum value at which it started decreasing with increasing temperature. It is interesting to note that both the free and LP-MTS immobilized invertases exhibited a very similar temperature profile with a maximum activity between 40–43 °C (Fig. 8).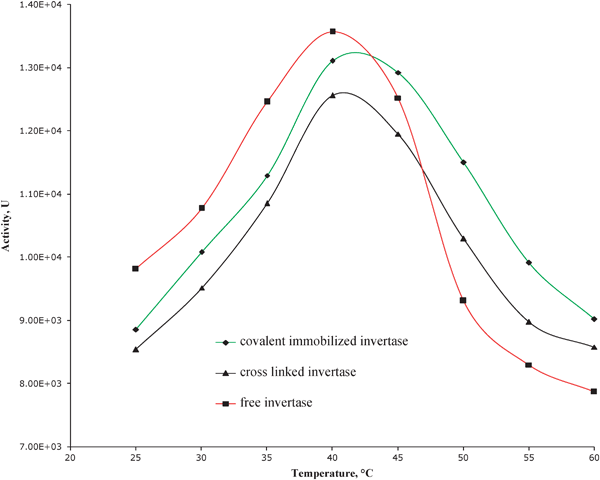 | ||
| Fig. 8 Effect of temperature on the activities of free and LP-MTS immobilized invertases at pH 4.7, 50 min reaction time and 0.3 M sucrose. | ||
The main difference between the immobilized and the free invertases is that the immobilized invertases were stable up to slightly above 45 °C. It was noted that cross-linked invertase was slightly less stable than covalently immobilized invertase. Covalent DDA-MTS immobilized invertase generally showed very little activity compared to covalent LP-MTS immobilized and free invertase (Fig. 9).
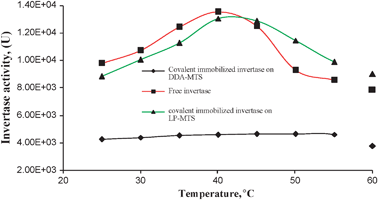 | ||
| Fig. 9 Effect of temperature and type of support on the activities of free and covalent immobilized invertases. | ||
Reuse studies of immobilized invertases
The reuse studies of the immobilized invertase was done at 40 °C and pH 4.7 at five-day intervals over a range of 30 days so as to investigate both their shelf life and reuses (Fig. 10). As evident from the Figures, the immobilized enzymes show a high stability and they were used for up to 10 cycles in a day (Fig. 10). Both forms of the immobilized invertases were stable for about 20 days and after that, the activity started to decrease slowly (Fig. 10 cycle 7). It is evident from the reuse studies that DDA based invertase maintains its activities upon reuse whereas LP-MTS decreases in activity. This shows that in DDA supports, the enzyme is attached on the outer surface whereas in LP-MTS the enzyme is supported inside the pores and hence upon reuse pore blockage takes place reducing the access of substrate to the active sites. The blockage of catalyst pores, leading to a drop in the surface area of the catalyst from 248 m2 g−1 before use to 84 m2 g−1 after catalyst use, has previously been reported in non-enzymatic catalysis.45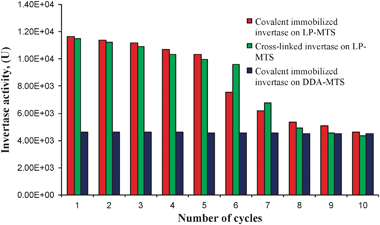 | ||
| Fig. 10 Reusability of the LP-MTS and DDA-MTS immobilized invertase enzyme. | ||
Experimental
Reagents and chemicals
Yeast invertase (E.C.2.3.1.26) from baker's yeast (S. cerevisiae) 157 U mg−1, glutaraldehyde (50%), tetraethylorthosilicate (TEOS, 98%), dodecylamine (DDA, 98%) and dinitrosalicylic acid (DNSA) were purchased from Fluka. Ethanol, potassium sodium tartrate tetrahydrate, sodium hydroxide, sodium acetate, glacial acetic acid, hydrochloric acid (assay 32%), sodium phosphate, phosphoric acid and phenol were supplied by BDH laboratory. 3-Aminopropyltrimethoxylsilane (APTMS, 97%), sucrose and glucose were supplied by Sigma-Aldrich. Cashew nut shell liquid (CNSL) was obtained from the cashew nut processing factory, Dar es Salaam, Tanzania. Distilled water was obtained from the chemistry laboratory, University of Dar es salaam. All chemicals were used as received without further purification.Preparation of micelle templated silicas (LP-MTS or DDA-MTS)
The one pot co-condensation method was used to synthesize the LP-MTS and DDA-MTS using CNSL or DDA surfactants, respectively. The synthesis was done as described elsewhere29 where a solution of CNSL (2.5 g) was poured into stirred aqueous ethanol (46 ml of absolute ethanol and 53 ml of distilled water) at ambient conditions of temperature and pressure. To this solution, TEOS (8.3 g) and APTMS (1.9 g) were separately but simultaneously and rapidly added. The APTMS and TEOS were added at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 or 1
4 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. A brown solution started to thicken three minutes after addition but stirring continued for 18 h. After 18 h, the thick paste was vacuum filtered, and the brown solid was thoroughly washed with ethanol. The wet solid product was refluxed by Soxhlet using ethanol as a solvent for 10 h so as to remove the template. The final solid (LP-MTS) obtained after the removal of the template was dried in an oven at 100 °C for 8 h and then stored in sealed bottles and stored in a desiccator for further characterization and derivatization. The same procedure was followed for the DDA surfactant but 5 g of DDA was used and the resultant material is denoted DDA-MTS.
9. A brown solution started to thicken three minutes after addition but stirring continued for 18 h. After 18 h, the thick paste was vacuum filtered, and the brown solid was thoroughly washed with ethanol. The wet solid product was refluxed by Soxhlet using ethanol as a solvent for 10 h so as to remove the template. The final solid (LP-MTS) obtained after the removal of the template was dried in an oven at 100 °C for 8 h and then stored in sealed bottles and stored in a desiccator for further characterization and derivatization. The same procedure was followed for the DDA surfactant but 5 g of DDA was used and the resultant material is denoted DDA-MTS.
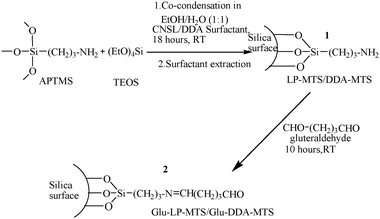 | ||
| Scheme 1 Functionalization of LP-MTS and DDA-MTS with glutaraldehyde. | ||
Immobilization of invertase on modified support material
Immobilization of invertase on the modified support material was done by using covalent and cross-linking methods.Invertase immobilization by covalent method
In order to covalently immobilize an enzyme on LP-MTS or DDA-MTS, the amino modified silica was first reacted with glutaraldehyde and then, after extensive washing with distilled water, it was added to a buffered enzyme solution. The covalent immobilization of invertase on supported glutaraldehyde (Scheme 2) was done as described in the literature with some minor changes.46 In a typical experiment, 0.05 g of supported glutaraldehyde (ca. 1 mmol) was put in 10 mL of 0.05 M sodium acetate buffer (pH 4.7), 0.03 g (ca. 1 mmol) of invertase enzyme was added and stirred for 5 h at room temperature followed by overnight incubation at 4 °C. The Glu-LP-MTS/Glu-DDA-MTS-invertase catalyst obtained denoted as Glu-LP-MTS-Enz/Glu-DDA-MTS-Enz (3) was filtered, washed and assayed for determination of immobilized invertase and characterization.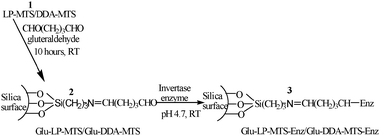 | ||
| Scheme 2 Schematic representation of the covalent binding of the invertase enzyme onto Glu-LP-MTS/Glu-DDA-MTS (2). | ||
Invertase immobilization by cross-linking
Cross linking of the invertase enzyme by glutaraldehyde was done as explained elsewhere47 with minor modifications. About 0.05 g of synthesized amine functionalized LP-MTS (1) in a 250 mL volumetric flask containing 10 mL of sodium acetate buffer (pH 4.7) was mixed with 0.03 g (ca. 1 mmol) of invertase. The mixture was then shaken in a water bath shaker for 4 h at room temperature. Thereafter, 10 mL of 25% aqueous glutaraldehyde was added and held in the water bath shaker for 3 h before keeping it at 4 °C in a refrigerator for 16 h. The immobilized invertase was then washed with excess distilled water and kept for further characterization. Unbound glutaraldehyde was removed by vacuum filtration and then washed extensively with distilled water.Characterization of LP-MTS/DDA-MTS and supported glutaraldehyde
The LP-MTS/DDA-MTS support and supported glutaraldehyde (Glu-LP-MTS/Glu-DDA-MTS) were characterized by standard techniques including nitrogen physisorption, DRIFT, acid titration and SEM.Activity studies of free and immobilized invertases
The activity of free and immobilized invertase was done by measuring the rate of formation of reduced sugars during the hydrolysis of sucrose (0.3 M) in 0.05 M sodium acetate buffer (pH 4.7) at 40 °C. The optimum incubation time for the enzyme and its substrate was found to be 50 min and 10 min for the LP-MTS and DDA-MTS derivatives respectively. All other experiments were done under these conditions except when a specific parameter was under investigation. The total reduced sugar produced was measured calorimetrically in triplicate, using a dinitrosalicylic acid (DNSA) method. The activities of the free and immobilized invertases were performed at different temperatures, pH values and substrate concentration to study their effects on the enzymes. A unit of invertase activity (U) corresponds to the amount of enzyme hydrolyzing sucrose at 1 μmol min−1.The activities of the free and immobilized invertases were determined according to the method described in the literature48 with some minor modifications. The free invertase (ca. 1 mmol) and immobilized invertase (ca. 1 mmol) were each incubated with a sucrose solution (0.3 M) in 10 mL of 0.05 M sodium acetate buffer (pH 4.7) at a temperature range of 25 °C to 60 °C for 50 min in order to investigate the effect of temperature and was followed colorimetrically at A430 with a light path length of 1 cm. This was done in triplicate and the reducing sugar produced was determined by the DNSA method described elsewhere.49 The same procedure was followed but at the pH range 1–9 at 40 °C to investigate the effect of pH. Different concentrations of sucrose solution (0.1–0.6 M) in 10 mL of 0.05 M sodium acetate buffer were also done so as to establish the effect of substrate concentration on invertase activity.
The reusability of immobilized invertases
The reusability of the immobilized invertases was done as described in the literature.50 The activity of a given immobilized invertase was tested several times in a day and after each test, the immobilized invertase was washed with sodium acetate buffer (pH 4.7) before being re-tested. The insoluble invertase was stored in a refrigerator at 4 °C in 10 mL of sodium acetate (0.05 M) buffer (pH 4.7). The storage tests were done in the interval of five days and were done for a period of one month. There was a notable decrease in the activity after one month of shelf life. The thermal and pH stability of immobilized invertase was tested using a similar procedure but at different temperatures (from 25 °C to 60 °C) and pH values ranging from 1 to 9.Conclusion
A new mesoporous micelle-templated silica route for enzyme immobilization has been developed using large pore micelle templated silica prepared using a natural and renewable surfactant, a CNSL template. The results of this study show that large pore micelle templated silicas (LP-MTS) derived from a CNSL templates have large pore diameters up to 25 nm depending the condition of preparation. These materials are suitable for immobilization of biomolecules compared to dodecylamine based micelle templated silica, DDA-MTS. In general large pore micelle templated silicas prepared using CNSL templates produced materials with larger pore dimensions than those of DDA derivatives. The LP-MTS immobilized enzymes are active, stable and can be stored for over twenty days and reused without significant loss of activity.Acknowledgements
Sida-Sarec and Faculty of Science-University of Dar es Salaam under the Capacity Building in Material Science and Solar Energy Project is acknowledged for sponsoring this work.References
- J. M. Woodley, Trends Biotechnol., 2008, 26, 321–327 CrossRef CAS.
- L. Que and W. B. Tolman, Nature, 2008, 455, 333–340 CrossRef.
- J. S. Dordick and A. Freeman, Curr. Opin. Biotechnol., 2006, 17, 559–561 CrossRef CAS.
- H. E. Schoemaker, D. Mink and M. G. Wubbolts, Science, 2003, 299, 1694–1697 CrossRef CAS.
- C.-H. Lee, T.-S. Lin and C.-Y. Mou, NanoToday, 2009, 4, 165–179 CrossRef.
- S. Pankel and M. Wubbolts, Curr. Opin. Chem. Biol., 2005, 9, 188–194 CrossRef CAS.
- Y. Li, G. Zhou, W. Qiao and Y. Wang, Mater. Sci. Eng., B, 2009, 162, 120–126 CrossRef CAS.
- H. H. P. Yiu, P. A. Wright and N. P. Botting, J. Mol. Catal. B: Enzym., 2001, 15, 81 CrossRef CAS.
- H. H. P. Yiu and P. A. Wright, J. Mater. Chem., 2005, 15, 3690–3700 RSC.
- M. Mureseanu, A. Galarneau, G. Renard and F. A. Fajula, Langmuir, 2005, 21, 4648–4655 CrossRef CAS.
- A. L. Crumbliss, J. Stonehuerner, R. W. Henkens, J. P. O'Daly and J. Zhao, New J. Chem., 1994, 18, 327–339 Search PubMed.
- W. Tischer and V. Kasche, Trends Biotechnol., 1999, 17, 326–334 CrossRef CAS.
- T. Nagayasu, M. Miyanaga, T. Tanaka, T. Sakiyama and K. Nakanishi, Biotechnol. Bioeng., 1994, 43, 1108–1117 CrossRef CAS.
- Y.-F. Chang and D.-F. Tai, Tetrahedron: Asymmetry, 2001, 12, 177–179 CrossRef CAS.
- J. Livage, T. Coradin and C. Roux, J. Phys.: Condens. Matter, 2001, 13, 673–691.
- C. Lei, T. A Soares, Y. Shin, J. Liu and E. J Ackerman, Nanotechnology, 2008, 19, 125102–125111 CrossRef.
- H. Ikemoto, Q. Chi and J. Ulstrup, J. Phys. Chem. C, 2010, 114, 16174–16180 CrossRef CAS.
- X. Bai, Z. Ye, Y. Li, L. Yang, Y. Qu and X. Yang, Biochem. Eng. J., 2010, 49, 264 CrossRef CAS.
- X. S. Zhao, Xiao Y. Bao, W. Guo and F. Y. Lee, Mater. Today, 2006, 9, 32–39 CrossRef CAS.
- K.-C. Kao, C.-H. Lee, T.-S. Lin and C.-Y. Mou, J. Mater. Chem., 2010, 20, 4653–4662 RSC.
- M. Vallet-Regi, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548 CrossRef CAS.
- I. I. Slowing, J. L. Vivero-Escoto, C. W. Wu and V. S. Lin, Adv. Drug Delivery Rev., 2008, 80, 1278–1288 CrossRef.
- J. M. Rosenholm, C. Sahlgren and M. Lindèn, Nanoscale, 2010, 2, 1870–1883 RSC.
- A. Macario, M. Moliner, A. Corma and G. Giordano, Microporous Mesoporous Mater., 2009, 118, 334–340 CrossRef CAS.
- A. Corma, V. Fornes and F. Rey, Adv. Mater., 2002, 14, 71–74 CrossRef CAS.
- E. Serra, E. Díez, I. Díaz and R. M. Blanco, Microporous Mesoporous Mater., 2010, 132, 487–493 CrossRef CAS.
- E. Serra, A. Mayoral, Y. Sakamoto, R. M. Blanco and I. Díaz, Microporous Mesoporous Mater., 2008, 114, 201–213 CrossRef CAS.
- S. Urrego, E. Serra, V. Alfredsson, R. M. Blanco and I. Díaz, Microporous Mesoporous Mater., 2010, 129, 173–178 CrossRef CAS.
- A. Hilonga, J. E. G. Mdoe and L. L. Mkayula, Int. J. BioChemiPhysics, 2009, 17, 26–31 Search PubMed.
- P. S. J. Cheetham, The applications of enzymes in industry. Handbook of Enzyme Biotechnology, Ellis, London, 1995, p. 420 Search PubMed.
- K. Nakane, T. Ogihara, N. Ogata and Y. Kurokawa, J. Appl. Polym. Sci., 2001, 81, 2084 CrossRef CAS.
- G. Bayramoglu, S. Akgol, A. Bulut, A. Denizli and M. Y. Arica, Biochem. Eng. J., 2003, 14(2), 117 CrossRef CAS.
- E. H. Mansour and F. M. Dawoud, J. Sci. Food Agric., 2003, 83(5), 446 CrossRef CAS.
- S. Kiralp, L. Toppare and Y. Tagci, Synth. Met., 2003, 135(1–3), 79 CrossRef.
- C. Airoldi and O. A. C. Monteiro, Polym. Bull., 2003, 50(1–2), 61 CrossRef CAS.
- M. Fuentes, J. V. Maquiese, B. C. C. Pessela, O. Abian, R. Fernandez-Lafuente, C. Mateo and J. M. Guisan, Biotechnol. Prog., 2004, 20(1), 284 CAS.
- G. Sanjay and S. Sugunan, Catal. Commun., 2005, 6, 81 CrossRef CAS.
- S. A. Camperi and M. Grasselli, Process Biochem., 2004, 39(8), 1017 CrossRef CAS.
- I. S. Khatib and R. V. Parish, J. Organomet. Chem., 1989, 369, 9–16 CrossRef CAS.
- J. E. G. Mdoe and D. J. Macquarrie, Int. J. Sci. Res., 2005, 14, 19–25 Search PubMed.
- E. F. Vansant, P. Van Der Voort and K. C. Vranken, in Characterisation and Chemical Modification of the Silica Surface, Elsevier, Amsterdam, 1995 Search PubMed.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press, London, 1982 Search PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- E. B. Mubofu, PhD Thesis, University of York, UK, 2001 Search PubMed.
- J. H. Clark, D. J. Macquarrie and E. B. Mubofu, Green Chem., 2000, 2, 53–56 RSC.
- A. E. David, N. S. Wang, V. C. Yang and A. J. Yang, J. Biotechnol., 2006, 125, 395–407 CrossRef CAS.
- W. L. Stanley and A. C. Oslon, J. Food Sci., 1974, 39, 660–666 CrossRef CAS.
- Immobilized cells and enzymes, A practical approach, ed. J. Woodward, IRL Press, Oxford, Washingtone DC, 1985, p. 3 Search PubMed; Bergmeyer, Methods of Enzymatic Analysis, 1974, I, 450–451 Search PubMed.
- G. L. Miller, Anal. Chem., 1959, 31, 426 CrossRef CAS.
- A. Nighojkar, S. Srivasttva and A. Kumar, Indian J. Exp. Biol., 1996, 34, 1248–1253 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |