Dye and bacteria photodegradations with anatase-loaded microporous poly(vinylidene fluoride) membranes
Ouafa Tahiri
Alaoui
a,
Quang Trong
Nguyen
*a,
Pierre
Schaetzel
b and
Chamekh
Mbareck
c
aPolymers, Biopolymers and Surfaces Department, CNRS-UMR 6270, Chemistry Federation FR 3038, Rouen University, 76821 Mont-Saint-Aignan Cedex, France. E-mail: trong.nguyen@univ-rouen.fr
bDepartment of Catalysis and Spectrochemistry, UMR 6506—Caen University, 14000 Caen, France
cUniversité de Nouakchott, Faculté des Sciences et Techniques, B.P. 5026, Nouakchott, Mauritania
First published on 15th August 2011
Abstract
Non-skinned microporous membranes containing anatase particles were fabricated without anatase loss with the dry cast technique. The membranes were characterized by scanning electron microscopy, X-ray diffraction (XRD), infrared spectroscopy, thermal gravimetry analysis and contact angle measurements. Anatase particles were fairly well distributed in the crystallized-PVDF microporous matrix, mainly as aggregates. The membrane porosity, as well as their nitrogen and water permeabilities, reached a maximum at 0.5 TiO2/PVDF weight ratio. The anatase-loaded PVDF membranes were successfully used in the dye and bacteria photodegradations catalyzed by anatase particles. The dye sorption and the photodegradation kinetics with the membrane of 0.5 TiO2/PVDF weight ratio obeyed the Freundlich and the first-order laws, respectively. In the photodegradation of a dye mixture, the better sorbed dye was preferentially photodegraded, suggesting the key role of dye sorption in the photodegradation mechanism. The technical feasibility of the supported photocatalyst in a continuous discoloration of a dye solution was shown in runs of a flat-type reactor under a black-light lamp or direct solar light.
1. Introduction
Nanoparticles have unique electronic, magnetic, catalytic and optical properties because of their nanometric sizes, large surface areas and strong activities. During the last few decades, powders of several photosemiconductors have been shown to be effective in photocatalytic degradation of organic pollutants. Among them, titanium dioxide is the most promising photocatalyst for complete mineralization of organic pollutants. Dispersions of nano-sized particles of titanium dioxide show high efficiency in the pollutant degradation with UV irradiation or free solar radiation, without any added chemicals. However, their difficult recovery from the treated water strongly limits the industrial application development of the process. Due to this, photocatalytic processing of aqueous effluents has not yet achieved a breakthrough, despite intensive research efforts.The immobilization of TiO2 can eliminate the catalyst separation step. Several characteristics have to be met for any support to be used as a photo-catalyst for photodegradation: (i) TiO2 nanoparticles should not leach out of the support during operation, (ii) the support should not be attacked by the highly oxidative species produced on TiO2 particles under irradiation, (iii) easy recovery and negligible light shielding effect towards the catalyst, and (iv) the composite catalyst should present acceptable reaction kinetics for the target pollutants and a low cost.
There has been intensive research work dealing with TiO2 immobilized in composite structures of different structural and textural properties1,2 but definitive solutions have not been yet achieved. In the case of a process without catalyst separation, i.e. with a catalyst fixed on a bulk carrier material such as glass, quartz or stainless steel,3 there is generally a significant reduction of the active surface accessible to components of the solution, which results in a loss in the photocatalytic activity.4 Moreover, the sol–gel technique (using titanium tetraalkoxide), which is currently used for anatase immobilization, required several carefully-controlled steps, especially the heat treatment one;4 this would make the catalyst preparation expensive. In fact, the most promising supported photocatalysts would be made by entrapping commercially-available anatase particles in inorganic binders.5–7 Some systems are commercialized as silica–anatase composites, either on nonwoven paper or glass-fiber mats. They are currently used in the removal of organic odorous molecules and bacteria in air.8 Note that new inorganic solids with high photocatalytic activities such as Ag–TiO2 nanocomposite films9 and TiO2 nanotubes10 are recently reported in the literature.
TiO2 particles were also loaded in ultrafiltration and nanofiltration membranes to improve their membrane permeability and fouling resistance by imparting their hydrophilicity to the membrane surface.11,12 The main inconvenience of anatase-loaded organic membranes is the risk of anatase-catalyzed degradation of their polymer matrix under light irradiation.13 Nevertheless, fluorinated polymers like poly(vinylidene fluoride) resist photocatalytic degradation. TiO2 was incorporated into fluorinated-copolymer membranes to improve the mechanical and conductivity properties of the membranes for lithium batteries applications.14
The objective of the present paper was to prepare anatase-loaded PVDF membranes which would meet the characteristics of a good anatase-supported photocatalytic system. The pore size, which is the biggest in the category of filtration membranes (micrometre size), favors the transport of pollutants from the external fluid towards the catalytic particles. The film form would reduce light screening in the reactor, since the catalytic membrane can be put face to the radiations, for a direct access of the radiations to the anatase particles (e.g. with membranes wrapping a black-light lamp, or plane membranes perpendicular to sun beam…). PVDF was chosen as the polymer material for the support membrane because of its excellent resistance to UV-light and to oxidation of radicals,14 contrary to other common polymer materials for membrane making. Moreover, its semi-crystalline and fluorinated nature provides a good thermal stability (with the PVDF crystalline phase), and flexibility (with the amorphous PVDF phase in the rubbery state), together with other desirable characteristics for membrane applications, like excellent film forming properties, and excellent resistance to corrosive chemicals.
The originality of our work resides first in the choice of PVDF polymer as polymer material for anatase particle entrapment, and that of a membrane formation technique15,16 which produces in one step an un-skinned microporous membrane, without waste stream or toxic chemicals. Such a technique could be easily extended to the deposition of a thin catalyst-supported film on large-scale substrates of various forms and natures. Secondly, the TiO2-supported PVDF membranes developed in the present work differ from those reported by Losito et al.17 and by Yu et al.,18 in that the membranes are microporous and not dense or with a dense skin, so that they must offer low resistance to the pollutant transport from and towards the catalyst particle, thus higher overall degradation rates. They differ from the majority of the other TiO2-supported inorganic systems by the inherent flexibility of a polymer in the rubbery state for its amorphous phase, which would make easier the design of the photocatalytic reactor and the catalytic membrane reuse (lesser risk of system breakage).
We report in the first section the membrane characteristics that are of interest for their applications in photocatalysis. In the next sections, we focus on the photocatalytic effects of the anatase-supported microporous membranes on the inactivation of two bacteria (Escherichia coli and Pseudomonas aeroginosa) and the degradation of Brilliant Green and Indigo Carmine dyes in aqueous solutions. We also report on the kinetics of degradation of a dye mixture, which were much less studied in the literature than those of single dyes. They give us an interesting insight in the mechanism of photodegradation of organic molecules with anatase-based catalytic membranes.19–22
2. Experimental
2.1. Products and materials
Poly(vinylidenedifluoride) (PVDF), Kynar 741, was supplied by Arkema Inc., Serquigny (France). Its weight averaged molecular weight was 245![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. The polymer sample had a glass transition temperature of −38 °C, and a melting temperature of 167 °C. Special titanium dioxide (TiO2) with 98% of anatase was obtained from Degussa (Germany). It was in the form of a powder of aggregated particles, but the size of individual particles as determined from X-ray diffraction data with the Scherrer equation was ca. 16 nm.
000 g mol−1. The polymer sample had a glass transition temperature of −38 °C, and a melting temperature of 167 °C. Special titanium dioxide (TiO2) with 98% of anatase was obtained from Degussa (Germany). It was in the form of a powder of aggregated particles, but the size of individual particles as determined from X-ray diffraction data with the Scherrer equation was ca. 16 nm.
Table 1 summarizes the structure and some properties of PVDF and Congo Red (CR), Brilliant Green (BG) and Indigo Carmin (IC). Dye solutions were prepared by using ultra pure water (Milli Q with a resistance of 18.6 MΩ cm).
2.2. Microporous PVDF–TiO2 membrane preparation
The microporous membranes were prepared according to a proprietary technique, which was analyzed in a previous paper.16 Briefly, a solution of 13 wt% PVDF was prepared by heating PVDF powder at 60 °C for 2 hours in an acetone–butan-1-ol mixture of 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 wt. ratios under the mixture vapor pressure in a closed kettle. The solution was rapidly cooled and an appropriate amount of anatase was dispersed under stirring for 10 min. The dispersion was ultrasounded for 15 min under stirring, then quickly spread with a Gardner-type knife onto a glass plate into a liquid film of thickness of ca. 200 μm. The solvents of the liquid film were left to evaporate from the film free surface under atmospheric pressure at ambient temperature in a vented fumed hood (ca. 25 °C). Selective solvent evaporation resulted in a fast phase separation in the concentrated polymer solution and a rapid setting of PVDF into a white microporous membrane (ca. 3 min). An additional evaporation of the residual liquid in the pores (mainly butanol) was carried out in the ambient atmosphere or in a vented oven at 35 °C.
19 wt. ratios under the mixture vapor pressure in a closed kettle. The solution was rapidly cooled and an appropriate amount of anatase was dispersed under stirring for 10 min. The dispersion was ultrasounded for 15 min under stirring, then quickly spread with a Gardner-type knife onto a glass plate into a liquid film of thickness of ca. 200 μm. The solvents of the liquid film were left to evaporate from the film free surface under atmospheric pressure at ambient temperature in a vented fumed hood (ca. 25 °C). Selective solvent evaporation resulted in a fast phase separation in the concentrated polymer solution and a rapid setting of PVDF into a white microporous membrane (ca. 3 min). An additional evaporation of the residual liquid in the pores (mainly butanol) was carried out in the ambient atmosphere or in a vented oven at 35 °C.
2.3. Dye adsorption analysis and photodegradation experiment
The dye adsorption tests were performed using 10 mL aqueous solutions at different concentrations (1, 2.5, 5, 10, 15, and 20 mg L−1) of dye which were put into contact with ca. 0.1 g of TiO2/PVDF membrane of 0.5 TiO2/PVDF ratio at 23 ± 0.5 °C until equilibrium.The amount of adsorbed dye was calculated using eqn (1):
| Q = (C0 − C)V/W | (1) |
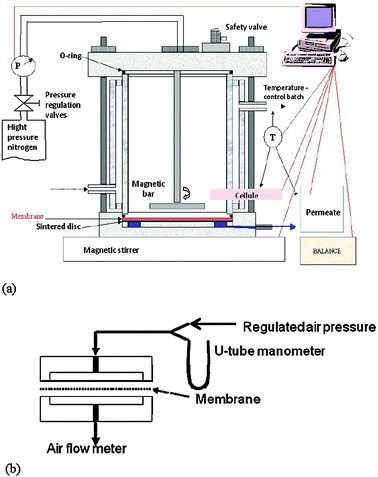
The photodegradation reaction was carried out in a glass reactor containing 400 mL of a model solution and a fixed amount of a photocatalyst, either as dispersed TiO2 powder or a standing composite membrane. The membranes were first wetted with ethanol, then thoroughly rinsed in pure water and blotted with a paper tissue before being used in the photoreactor. UV irradiation was carried out with a Swig® black lamp (Bioblock) at 365 nm wavelength. The nominal light flux of the lamp was 4750 lumens.
The UV lamp was located at the top of a reactor protected from external irradiation sources and thermostated at 26 °C. Aqueous solutions of the BG dye (pH = 4.5) and IC dye (pH = 6) were prepared at 20 mg L−1 for the photodegradation with 2.5 g L−1 of a TiO2/PVDF membrane sample.
The free TiO2 powder was used as a reference for the comparison of the photocatalytic efficiency of the catalytic membrane. The dye–photocatalyst suspensions were stirred in the dark to ensure adsorption equilibrium prior to irradiation; 3 mL of aliquots were taken from the reactor at different times, filtered through a Millipore filter (0.45 μm), and monitored in the dye content with a spectrophotometer (Uvicon 860 from Kontron). The quantity of free TiO2 and that of TiO2 entrapped in the PVDF membrane were the same in the experiments. A blank experiment (irradiation of dye solutions without TiO2) indicated an absence of UV action on the BG degradation, after 10 h of irradiation. The decrease in the dye concentration in the presence of the membrane-supported TiO2, after adsorption/desorption equilibrium, was attributed to the photocatalytic reaction.
The kinetics of degradation of Rhodamine B dye adsorbed on the membrane faces were followed with a Fluoromax-4 spectrofluorometer (Horiba Jobin-Yvon, France) (monitoring of the peak at 397 nm).
2.4. Characterisation of the composite membranes
A Jeol 8030 X-ray diffractometer was used at room temperature (power: 30 kV/60 mA, with CuKα radiations, in the scan range of 2θ = 3–60°, and with a scan speed of 3° min–1, and a step size of 0.01°) to determine the crystalline characteristics in the TiO2/PVDF membranes.
The overall TiO2/PVDF ratio in the composite membranes was determined from the weight of the inorganic anatase phase left after a complete pyrolysis, at 800 °C, of the polymer phase in 100 mg samples in a thermal gravimetric analysis (TGA7 from Perkin-Elmer).
The contact angle of the membrane surface was measured with a multi-purpose optical instrument (Multiskop, from Optrel, Germany). The measurements were done on membrane samples conditioned in water, or in ethanol for 5 min then rinsed with water, wiped with a paper tissue and dried in ambient air for 10 min.
The degree of crystallinity (Xc) was determined from the crystallite melting enthalpy thermograms at a rate of 10 °C min−1 with a Differential Scanning Calorimeter (Perkin-Elmer DSC-7 calibrated with indium standard) by using a value of the melting enthalpy of 100% crystalline PVDF of 105 J g−1.23
| B = QL/(AΔp) | (2) |
The membrane permeability in microfiltration of pure water or dye solutions was determined in a similar way using a stirred cell of 42 cm2 of membrane surface area equipped with a glass body for an eventual irradiation from the outside. The permeate (water) flow rate was determined from the time required to fill a fixed volume of a buret.
The reactor set-up is schematized in Fig. 2. It consists of a rectangular anatase membrane that fits the bottom surface of rectangular glassware. A solution of 6.2 mg L−1 of IC dye was continuously fed from a tank to the reactor at a corner, and extracted from the reactor at the opposite corner, with a multicanal peristaltic pump working at the same flow rate for the feed and the extraction lines. The liquid extracted from the reactor was sampled at different times to monitor the dye concentration after its photodegradation.
 | ||
| Fig. 2 Schematic representation of the flat membrane-reactor operating continuously with a flow-through dye solution. | ||
The flow was mainly laminar in the reactor, but the flow pattern in the reactor was not ideal. It was between a plug-flow and continuously stirred reactor pattern. Since the flow rate was small, the dye diffusion between zones of different concentrations was significant. No study was done on the hydrodynamics or the reactor modelling, as our aim was not a process calculation but a feasibility test of the simplest form of a solar photoreactor with an immobilized anatase catalyst, for organic-compound abatement in aqueous effluents (i.e. a kind of shallow solar pond).
3. Results and discussions
3.1. Thermal properties of PVDF/TiO2 composites
The preliminary tests of entrapment, in different types of polymers (polydimethylsiloxane, cellulose triacetate, polysulfone, PVDF, polyacrylonitrile), of the same content in anatase particles (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), using different techniques of membrane casting (evaporation, coagulation of a suspension of anatase in polymer solutions), led to membranes with negligible photocatalytic effects on organic solutes like dyes. We explained this by a limited solute access to the anatase particles entrapped in a dense polymer matrix or asymmetric membranes. Moreover, there was a loss of TiO2 of ca. 5–20 wt% (depending on component natures) that occurred in the coagulation step of the conventional wet-phase technique of asymmetric membrane fabrication due to particle leakage with the water-rich droplets in the nascent big pores. On the contrary, there was no catalyst loss with our dry-cast technique, due to the absence of external liquid for nonsolvent phase escape. We also noticed that membranes other than PVDF ones underwent disintegration in water under exposure of UV or daylight, due to polymer photodegradation catalyzed by the entrapped anatase particles.
1 ratio), using different techniques of membrane casting (evaporation, coagulation of a suspension of anatase in polymer solutions), led to membranes with negligible photocatalytic effects on organic solutes like dyes. We explained this by a limited solute access to the anatase particles entrapped in a dense polymer matrix or asymmetric membranes. Moreover, there was a loss of TiO2 of ca. 5–20 wt% (depending on component natures) that occurred in the coagulation step of the conventional wet-phase technique of asymmetric membrane fabrication due to particle leakage with the water-rich droplets in the nascent big pores. On the contrary, there was no catalyst loss with our dry-cast technique, due to the absence of external liquid for nonsolvent phase escape. We also noticed that membranes other than PVDF ones underwent disintegration in water under exposure of UV or daylight, due to polymer photodegradation catalyzed by the entrapped anatase particles.
We inferred that the entrapment of anatase nanoparticles in microporous PVDF membranes with the dry-cast technique would be the best way to fabricate anatase-supported membranes for organics photodegradation. It allowed us to obtain a symmetric, un-skinned, highly porous membrane, in a very simple and fast process.
For the photodegradation of organic pollutants, the reaction rate depends on how easy pollutant molecules and the light reach the anatase particles for the reaction to take place, i.e. on the internal morphologies of the composite membranes.
The prepared composite membranes had surface pores well connected with the inner matrix in a “bicontinuous” structure of pores and a lacy polymer network. Such a “bicontinuous” pore morphology would make the pore-entrapped anatase particles accessible to the external fluid, in contrast with the honey-comb or finger-like morphology commonly observed in the membranes prepared with other techniques (Fig. 3). The anatase particles appeared not covered by any adherent layer of polymer (enlarged SEM, Fig. 3c), so that one can expect that all parts of the particle surface can come into contact with the pollutants for an effective action in a catalytic process.
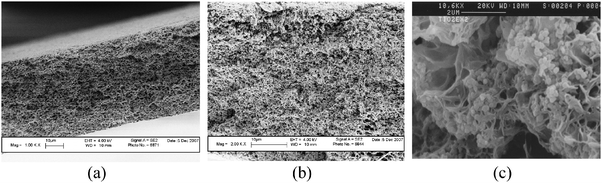 | ||
Fig. 3
SEM images of the sections of the PVDF membranes (a) without and (b) and (c) with TiO2 particles at 2000× and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800× magnification factors. 800× magnification factors. | ||
A TGA study of the composite membranes showed an increase in the onset of PVDF thermal decomposition, from 340 °C to ca. 366 °C (Table 2). This indicates a slight thermal stabilization of the polymer by the anatase particles. The amounts of the anatase phase that remained after total degradation of PVDF (at ca. 600 °C in the TGA scan) were the same as those in the initial casting dopes, thus confirming negligible loss of catalytic particles in the composite fabrication with the dry-cast process.
| TiO2/PVDF ratio | Onset temperature of PVDF degradation/°C |
|---|---|
| 1 | 370 |
| 0.7 | 368 |
| 0.5 | 367.6 |
| 0.3 | 366.6 |
| 0 | 340 |
WAXS (wide angle X-ray scattering) experiments showed no change in the TiO2 crystalline structure after immobilization in the PVDF matrix (same diffraction peaks for initial and immobilized anatase particles). This was expected because of the mild conditions of particle immobilization: there were no chemical reactions nor thermal treatment as in the sol–gel immobilization technique. The PVDF crystallite structure and proportion in the membrane were evidenced by IR spectroscopy in the ATR mode. For all the membranes (with and without anatase particles), the data indicate a PVDF polymer crystallization from the cast solution into an α-type structure (i.e. with IR peaks at 795, 764, 857 and 1210 cm−1) in the solvent evaporation step (Fig. 4). WAXS crystal-structure data of PVDF were discussed in a previous paper.16 The stable α-crystallite structure (compared with the β and the γ ones) would insure a use of the catalytic membranes without mechanical-property loss in applications.
 | ||
| Fig. 4 FTIR in attenuated total reflectance mode of the composite membranes of different TiO2/PVDF TiO2 ratios. The arrows show the position of the peaks characteristics of the α-crystalline phase of PVDF. | ||
From the viewpoint of the membrane formation mechanism, the anatase particles may affect the PVDF phase separation and the membrane “setting” kinetics. The particles could act as starting points for phase-separated nuclei, and affect the phase separation kinetics growth in increasing the medium viscosity, or creating physical “obstacles” to the component migration. The PVDF crystallinity (ca. 46%) and melting point (167 °C) were the same for all the membranes. The absence of crystallization exotherms in all membrane DSC scans (i.e. no crystallizable PVDF) indicates that the maximum crystallinity was reached in all cases. It appears that the crystallization process dominated the phase separation, and led to the opened, bicontinuous pore structure. In fact, the phase separation by a liquid–liquid binodal process, if it dominates the membrane formation, would lead to a cellular morphology.
3.2. Membrane characterization
As our goal is to obtain a supported-photocatalytic system with a microporous membrane, and not filtration membranes with improved properties, the membranes were not focused of the changes in permeability and anti-fouling properties with the TiO2 content. Instead, we studied the membrane porosity and permeation properties (as well as the structure of TiO2 and PVDF in the membranes), since they are more important for the membrane performances in their use in pollutant photodegradation.We reported in a previous paper25 that the membrane porosity increases from 42 vol% to 67 vol% when the anatase/PVDF ratio varies from 0.15 to 1, while the membrane mean pore diameter increases from 0.26 to 0.7 μm. A maximum was observed at the value of anatase/PVDF ratio of 0.5 for both the porosity and the mean pore size, whose values were 0.72 and 0.96 μm, respectively. The increase in porosity with the TiO2/PVDF ratio was attributed to an anatase-promoted increase in the number of the nuclei of polymer-lean phase (membrane pore precursor) and also to the presence of inter-granular spaces in the embedded anatase aggregates. The decrease in porosity beyond a TiO2/PVDF ratio of 0.5 was explained by an insufficient polymer material to form the porous scaffold (the main membrane porous structure) that confined the particles; without sufficient polymer material to separate the particles, the surface attraction forces induce a particle aggregation and the membrane structure collapse (Fig. 5).
 | ||
Fig. 5
SEM micrographs of the surface of three composite membranes of different TiO2/PVDF TiO2 ratios of 0.15, 0.5 and 1 (membranes made from a dope of 15 wt% of Kynar 741 PVDF in an acetone/butan-1-ol mixture of 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 wt ratio). 13 wt ratio). | ||
The TiO2/PVDF microporous membranes behave like a hydrophobic solid. Their contact angle with water was ca. 80°, and their water vapor sorption at a relative humidity of 0.98 (quasi-saturation in water vapor) was very small (less than 0.8 wt% for the composite membranes). In a previous paper,26 the higher contact angle for the PVDF membrane loaded with hydrophilic TiO2 particles compared with the pristine membrane was attributed to the particle-imprinted surface rugosity which enhances the hydrophobicity of the PVDF surface (hydrophobic by its chemical nature). Surface rugosity is known to cause the “lotus effect”, whose water repellency stems from the fact that water cannot touch the air-filled-grooves of the surface.27 Such a situation contrasts with the case in which a hydrophilic polymer is adsorbed on PVDF without imprinting a rugosity, where water permeates through the PVDF microporous membranes after polymer adsorption.26
Due to its surface hydrophobicity, the membrane showed no liquid water permeation at low pressures: this is generally the case for non-treated hydrophobic microporous PVDF membranes. Gholap et al.28 made PVDF water-permeable by adsorbing irreversibly a hydrophilic polymer. However, such an adsorbed polymer, which is not resistant to oxidants and UV light as PVDF, would be degraded in the photocatalytic process, resulting in a loss in pollutant degradation yield. We chose to work with a wetting solvent whose effect is permanent even after its washing from the membrane. In fact, we observed that, when the hydrophobic membranes are soaked in ethanol then rinsed with water, the membranes pre-wetted with ethanol become wetted by water and fully permeable to aqueous media. Interestingly, they remained permanently water-permeable (if not re-dried); a simple storage in water was sufficient for their re-use in aqueous media.
The water flux through the membranes also went through a maximum value at the particle/polymer ratio of ca. 0.5, where the porosity and the pore size were maxima (Fig. 6).
 | ||
| Fig. 6 Variations of the membrane porosity and the permeabilities of liquid water and nitrogen gas through the composite membranes as a function of the TiO2/PVDF ratio. | ||
As the permeability of a porous medium is solely a function of the medium characteristics (nature, porosity, pore-size distribution, pore shape tortuosity and connectivity), the gas and the water permeability should be the same, as the convective transport of both fluids obeys to the Darcy law, insofar as the flows are laminar. The similar permeabilities found for both water and nitrogen in their permeation through the anatase-loaded membranes (Fig. 7), if one takes into account the heterogeneous nature of the membranes, confirm the validity of the Darcy law for the two quite different permeating media: a hydrophobic gas, and a hydrophilic liquid, water. Such a result also indicates that the hydrophobic nature of the PVDF matrix did not reduce the water permeability compared with the nitrogen permeability, once the membranes are pre-wetted in a water-miscible organic solvent. In other words, there were no air-blocked spaces in the fine mixed matrices, so that solute molecules in an aqueous medium that flows through the membrane will have a full access to the loaded particles and polymer-pore surface for adsorption. The membrane with the largest pore size and porosity was chosen for further studies, since its photocatalytic properties were also the highest,25 in agreement with the inferred full access of the internal structure to the fluid.24
3.3. Dye adsorption
A dye adsorption on the solid catalyst is required for an efficient dye degradation by the active species formed on the catalyst. Without adsorption, the dyes are solely degraded via the soluble oxidant species (e.g.hydroxyl radicals) photo-generated by the oxidation of water molecules on the anatase particles under UV-light irradiation, while with adsorption, dye molecules are photo-degraded at the same time directly and via these soluble oxidant species. The dye adsorption was compared visually and with an optical microscope. Fig. 8 shows that Brilliant Green (BG) adsorbs well on both components of the PVDF/TiO2 composite catalyst, Congo Red (CR) adsorbs only slightly on the membrane, while Indigo Carmine (IC) adsorbs only on the TiO2 phase, and much less on the PVDF phase. Adsorption of an organic compound on a surface is due to attractive van der Waals interactions, hydrogen bonding and/or ion type. A high adsorption on the PVDF component is probably due to non-specific van der Waals interactions between the hydrocarbon polymer backbone with hydrocarbon moieties. The strong BG adsorption on the anatase particles compared with IC and CR dyes can be explained by the presence of cationic groups in the BG molecule (not present in the other dyes), which can interact with the polar Ti–OH groupings on the TiO2 particles surface. | ||
| Fig. 8 Differences in dyeing of the two phases (TiO2 aggregates, PVDF matrix) of the composite membrane of TiO2/PVDF ratio of 0.5 with Congo Red, Brilliant Green and Carmin Indigo dyes (BG and IC dye concentration: 20 mg L−1). Note that a five time larger value for the Congo Red dye concentration had to be used for an observable dyeing of the membrane. | ||
Two sorption equations were tested: the linearized Langmuir and Freundlich equations 1/q = (1/Kadsqmax)(1/Ceq) + 1/qmax and log q = log F + (1/n) log Ceq, respectively, where q is the amount of dye adsorbed on the photocatalyst in mg g−1, Ceq is the dye concentration in solution at equilibrium in mg L−1, Kads the equilibrium constant, qmax the maximum amount of dye adsorbed on the adsorbent; Kads, and qmax represent the parameters of the Langmuir equation, while F and 1/n represent those of the Freundlich equation. The fitting of the linearized forms of the dye sorption isotherms with the empirical Freundlich equation was good (Fig. 9) while that with the Langmuir equation led to unrealistic parameter values (negative values for the maximum dye sorption amounts). The values of the Freundlich parameters reveal a much smaller sorption of Congo Red dye in the composite membrane than that of the other two dyes, especially at higher dye concentrations. This could be due to the heterogeneous nature of the system or a multilayer sorption mechanism rather than a monolayer one. The sorption time (e.g. the time to reach ca. 95% equilibrium level) was large (30–60 min); this time would reflect both the dye sorption and diffusion kinetics from the external liquid towards the sorption sites.
 | ||
| Fig. 9 Fitting of the data of the sorption, in the composite membrane of TiO2/PVDF ratio of 0.5, of Congo Red, Brilliant Green and Indigo Carmin dyes with the Freundlich model. Experimental conditions: C0 = 20 mg L−1, T = 25 ± 1 °C, V = 400 mL, natural pH, overall composite catalyst content = 1.25 g L−1. | ||
The dye adsorbed in the membranes can be degraded under UV irradiation. This makes possible a regeneration of organics-contaminated or fouled membranes. On the contrary, irradiation of the dye solution and the membrane in the glass cell with an external lamp did not improve the dye abatement during the filtration, probably because of the too-small dye degradation rate under filtration conditions (due to a small irradiated surface, short contact time of the dye with the catalyst, and/or low amount of catalyst). Note that the present technique can be used to prepare membranes that capture more efficiently organic molecules in filtration, by immobilizing inorganic sorbents with faster organic sorption rates: we succeeded in immobilizing silicalites in PVDF microporous membranes by using the same protocol.
3.4. Photodegradation of single dyes
As a direct filtration was not effective for dye removal, we studied the dye removal by irradiation with a large amount of membrane immersed in the dye solution. The photocatalytic activity of TiO2 immobilized in the microporous PVDF membrane was compared with that of the Degussa anatase powder. As the degradation rates of dyes increased, then levelled-off at a certain threshold when the dye concentration increases due to the saturation of the catalyst surface, we chose to study the photodegradation of dyes under the conditions of sub-saturation of the catalyst surface.Fig. 10 and Table 3 compared the degradation kinetics of CR dye with those of BG and IC dyes. The dye degradation rate increased according to the sequence BG > IC > CR, which is also the sequence of dye adsorption in the membrane. Such parallel sequences suggest the role of adsorbed molecules on their efficient degradation by the activated species created on the anatase surface by irradiation in the presence of water.29 However, the mechanism of photodegradation of large organic molecules like dyes may be more or less complex, depending on their chemical structure. For instance, a study of the chemical mechanism of the photodegradation of BG showed that BG molecules are degraded faster because of a degradation by both de-ethylation of the N-linked ethyl groups in the quaternary ammonium and by cleavage of the BG aromatic ring structure.30
 | ||
| Fig. 10 Fitting of the kinetics data with the first-order kinetics model for the photocatalytic degradation of Congo Red, Brilliant Green and Carmin Indigo dyes. Experimental conditions: C0 = 20 mg L−1, T = 25 ± 1 °C, V = 400 mL, natural pH, overall composite catalyst content = 1.25 g L−1. | ||
| Dye | BG | IC | CR |
|---|---|---|---|
| First-order kinetic constant for membrane TiO2/PVDF 0.5 ratio (min−1) | 3.7 × 10−3 | 4.8 × 10−3 | 1.2 × 10−3 |
| Correlation coefficient | 0.9701 | 0.9929 | 0.8351 |
| L–H kinetic constant for membrane TiO2/PVDF 0.5 ratio (mg L−1 h−1) | 0.0605 | 0.0986 | 0.0015 |
Both the Langmuir–Hinshelwood (L–H) and the common first-order kinetics models were used to fit the reaction kinetics. Although the L–H model is known to describe well the kinetics of photocatalytic degradation with the TiO2 catalyst,29 we found that its simplified form, the first-order reaction model (C = C0 exp (−kappt)) (where C and C0 are the dye concentration at time t and the initial dye concentration after sorption pre-equilibrium, respectively, and kapp the apparent reaction-rate constant) represents rather correctly the dye photodegradation of BG and IC dyes by the catalytic microporous membranes (Fig. 10, Table 3).
One may raise the question whether the light scattering by the TiO2 and porous PVDF components in the composite membrane forms a strong barrier for the light in the photocatalytic reaction. To study this effect, we first adsorbed in the membrane a fluorescent dye, rhodamine B, then monitored the dye concentration on both faces of the membrane submitted to the sunlight only on one face. Fig. 10 shows that, when the membrane with adsorbed rhodamine B is irradiated by the sunlight from one side, there was negligible difference in the dye-degradation rate for the two faces (Fig. 11). The efficient photocatalytic reaction on the face non-exposed to the light could be explained by the known (and checked) transparency of PVDF to visible and near-UV lights, and by the fact that the light scattered by the anatase particles and the PVDF ribbons in the membranes can be used to activate the catalyst particles behind. Further experiments with analyses of the reaction intermediates at different times would be required if one is interested in the photodegradation mechanism involved at the differently irradiated faces of the membrane.
 | ||
| Fig. 11 Decay of fluorescence emission of a Rhodamine-adsorbed membrane with time from the two faces of the membrane of a TiO2/PVDF ratio of 0.5, submitted to the daylight from one side. | ||
The photocatalytic activity of the membrane re-used after simple rinsing with water was reduced about 10% for the BG dye. Nevertheless, a determination of the photocatalytic activity of the membranes in long-term uses would be necessary to evaluate the potential of the composite membranes for industrial uses. Indeed, Rao et al.31 showed that anatase particles immobilized on different supports exhibit a significant loss in their activity.
In order to assess the technical feasibility of the supported photocatalyst, we carried out some runs of a continuous reactor with the microporous membrane bearing anatase particles. Although the membrane-borne photocatalyst can be used either in a cylindrical (rolled membrane) or flat configuration, we chose to work with a flat-type reactor due to its simplicity and its suitability for direct sunlight use in pollutant photodegradation. The runs were performed to discolor an IC dye solution under single pass through a horizontal flat-membrane reactor irradiated with either a blacklight lamp or a winter daylight through a double paned glass window. Fig. 12 compares the influence of light sources on the kinetics of changes in dye concentration at the exit of the reactor: it decreased with time down to a steady residual concentration of ca. 0.2 mg L−1 from a value of 6.2 mg L−1 at the reactor entrance. The IC-dye abatement in the reactor after a transition time of ca. 12 h was 90%, for a membrane surface of 0.0166 sq. m and under a flow rate of 9 mL h−1.
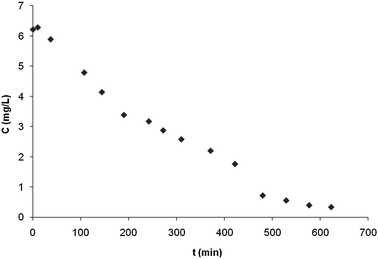 | ||
| Fig. 12 Changes in IC-dye concentration at the exit of the reactor under irradiation with a blacklight lamp or winter daylight (through a double paned glass window). The reactor was fed with a 20 mg L−1 IC-dye solution at a flow rate of 6.6 × 10−11 mL h−1. The membrane surface area was 0.0166 sq. m (corresponding to 0.5 mg of anatase). | ||
Although a modeling of the reactor could be done via a mass balance, no quantitative analysis of the dye removal was tried due to the lack of data (e.g. those on true kinetics, flow patterns and stability, light power variation with time…). Nevertheless, the continuous abatement data showed that the membrane-supported catalyst under daylight in Normandy was as efficient as a UV lamp of 160 W. Apparently, the membrane-supported catalyst made good use of daylight whose UV parts (UV A, B, and C) represent only a maximum power of ca. 50 W m–2.
3.5. Photodegradation of dye mixtures
There have been very few studies on the photodegradation kinetics of mixtures of dyes, although real effluents generally contain several organic components whose kinetics need to be known for the prediction of industrial operating conditions. We therefore studied the photodegradation kinetics of mixtures of BG and IC dyes with the same protocol as that of the single dyes. Fig. 13 shows that BG was degraded more rapidly than IC in the mixture. This situation contrasts with the case of single dyes, where IC dye had faster degradation kinetics than BG. The degradation kinetics for BG dye still obeyed to the first-order law, but that for IC shows clearly two regimes, with a slow degradation rate at early times (Fig. 13). The IC degradation rate becomes normal (i.e. with a fast concentration decay with time) when BG dye is completely degraded. | ||
| Fig. 13 The degradation rates of IC and BG dye mixture solution under UV irradiation in the presence of a sample of membrane of 0.5 TiO2/PVDF ratio. Experimental conditions: CIC+GB = 10 mg L−1, T = 25 ± 1 °C, natural pH, overall composite catalyst content = 2.5 g L−1. | ||
Such a behavior in the photodegradation of the BG and IC mixture can be interpreted if one admits that dye adsorption is a pre-requisite step for the TiO2-assisted photodegradation, as suggested by Zhang et al.32 The stronger BG dye adsorption onto the catalyst would prevent IC molecules from being well adsorbed on the same sorption sites, thus from being directly degraded on the catalyst surface. The slow IC photodegradation in the presence of BG which is preferentially degraded was probably due to the oxidant radicals generated in the solution, or the IC amount that can competitively adsorb onto the catalyst in the presence of BG.
As a similar behavior was observed for the TiO2-assisted photodegradation of the methyl orange and IC mixture,33 we speculate that there is one (main) type of adsorption site where different dyes have to compete for adsorption first, then for photodegradation.
3.6. Photodegradation of bacteria
Since the first report on the bactericide effect by Masunaga et al. of anatase particles under near UV irradiation,34 a great deal of works have been devoted to the disinfection effect, including that on air-borne H5N2 virus.35 Air disinfection devices with anatase-supported nonwoven are fabricated, but water disinfection devices are not yet commercially available, probably because of the lack of adequate anatase-supported materials for long term uses in water flows.The photodegradation effects of our anatase/PVDF membrane were studied on suspensions of Escherichia (E.) coli and Pseudomonas (P.) aeroginosa, respectively. Fig. 14 shows the decrease in the proportion of viable E. coli with time of the suspensions in contact with the anatase/PVDF membrane and with anatase powder under UV irradiation, together with that of an irradiated cell suspension as a blank test. Compared with the bactericide effect of UV light alone, the contact with the anatase/PVDF membrane has a strong bactericide effect but slightly less strong than that with the anatase powder. We infer that the anatase particles entrapped in a microporous membrane still have a bactericide effect, which is slightly decreased from that of pure anatase, probably due to a reduction in the access of the suspended bacteria to the catalyst surface. The abatements of viable bacteria in the suspension at fixed times were higher for P. aeroginosa than for E. coli (Table 4); no clear explanation can be given for this difference because the mechanism of anatase-aided inactivation that involves several processes36 which are not yet totally elucidated. The optical microscopy picture of the membrane surface early in contact with E. coli suspensions shows a large density of the rod-shaped bacteria, while in that at late contact time, there are quite few intact rod-shaped cells on the surface, but a large number of cell debris (Fig. 15). We think that the cell attachment onto the membrane surface would be a main parameter for the degradation kinetics. Indeed, Gumy et al.37 showed that the bacterial inactivation by TiO2 particles increased when the TiO2 surface charge became more positive, for larger electrostatic attraction with the negatively charged E. coliouter membrane.
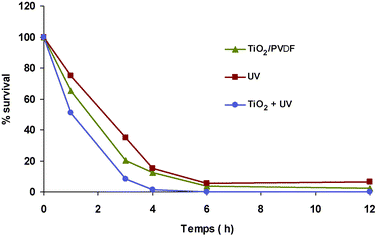 | ||
| Fig. 14 Effect of TiO2/PVDF, TiO2/SiO2, UV, only TiO2 and the TiO2 to the darkness on the destruction of E. coli of 107 C mL−1 in the presence of a 2.5 g L−1 sample of membrane of 0.5 TiO2/PVDF ratio at different irradiation times. | ||
| Time/h | 1 | 3 | 4 | 6 |
|---|---|---|---|---|
| Viable P. aeroginosa (%) | 60 | 77 | 96 | >99 |
| Viable E. coli (%) | 35 | 78 | 88 | >99 |
 | ||
| Fig. 15 Optical microscopy pictures of the membrane surface in contact with E. coli suspensions of 107 C mL−1 at early (a) and at late, 120 min contact times (b) and after 24 h of irradiation (c) in the presence of a 2.5 g L−1 sample of membrane of 0.5 TiO2/PVDF ratio at different irradiation times. | ||
4. Conclusion
Composite membranes consisting of anatase particles dispersed in a microporous PVDF matrix were fabricated without particles loss with a dry cast technique. The composite microporous membranes were characterized by different techniques; they exhibited a high porosity and had well inter-connected pores of sub-micron mean size, in which anatase-particle aggregates were well entrapped. When the TiO2/PVDF ratio in the composite membranes increases, the pore size and the membrane porosity increases first, then decreases beyond a ratio of ca. 0.5. A pre-conditioning of the hydrophobic composite membranes in ethanol made them wettable by aqueous media, thus making TiO2 particles in the internal structure accessible to the dye molecules to be degraded by the entrapped catalyst under near-UV irradiation. The dye degradation rate followed the same order as the dye-adsorption capacity in TiO2/PVDF membranes, i.e. Brilliant Green > Indigo Carmine > Congo Red dye, suggesting a requirement of dye adsorption in the composite membranes for an efficient dye photodegradation. The dye sorption obeyed the Freundlich equation, and the degradation kinetics under near-UV irradiation could be predicted with a simple first-order kinetic law for Congo Red and Indigo Carmine dyes, and the Langmuir–Hinshelwood law for Brilliant Green dye.The use of such TiO2/PVDF composite membranes makes possible an efficient photocatalytic degradation/mineralization of organic molecules without additional steps for catalyst separation.
References
- Z. P. Fang, Y. Z. Xu and C. W. Xu, Modification mechanism of nanoparticles on polymers, J. Mater. Sci. Eng., 2003, 21, 279–282 CAS.
- A. C. Balazs, T. Emrick and T. P. Russell, Nanoparticle polymer composites: where two small worlds meet, Science, 2006, 314, 1107–1110 CrossRef CAS.
- M. F. J. Dijkstra, H. Buwalda, A. W. F. de Jong, A. Michorius, J. G. M. Winkelman and A. A. C. M. Beenackers, Experimental comparison of three reactor designs for photocatalytic water purification, Chem. Eng. Sci., 2001, 56, 547–556 CrossRef CAS.
- J. Yu, X. Zhao and Q. Zhao, Photocatalytic activity of nanometer TiO2 thin films prepared by the sol–gel method, Mater. Chem. Phys., 2001, 69, 25–29 CrossRef.
- D. Dumitriu, A. R. Bally, C. Ballif, P. E. Schmid, R. Sanjines, F. Levy and V. I. Parvulescu, Photocatalytic degradation of phenol by TiO2 thin films prepared by sputtering, Appl. Catal., B, 2000, 25, 83–92 CrossRef CAS.
- R. Huchon, Activité photocatalytique de catalyseurs déposés sur différents supports médias, applications à la conception d'un photoréacteurs pilote, Université Claude Bernard—Lyon 1, 2006 Search PubMed.
- O. Tahiri Alaoui, Q. T. Nguyen and T. Rhlalou, Preparation and characterization of a new TiO2/SiO2 composite catalyst for photocatalytic degradation of indigo carmin, Environ. Chem. Lett., 2009, 7, 175–181 Search PubMed.
- Purification de l'air par photocatalyse, Saint Gobain, T 0116/05—novembre 2007.
- J. Yu, J. Xiong, B. Cheng and S. Liu, Fabrication and characterization of Ag–TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity, Appl. Catal., B, 2005, 60, 211–221 CrossRef.
- J. Yu and B. Wang, Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays, Appl. Catal., B, 2010, 94, 295–302 CrossRef CAS.
- M. Luo, J. Zhao, W. Tang and C. Pu, Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles, Appl. Surf. Sci., 2005, 249, 76–84 CrossRef CAS.
- X. Cao, J. Ma, X. Shi and Z. Ren, Effect of TiO2 nanoparticle size on the performance of PVDF membrane, Appl. Surf. Sci., 2006, 253, 2003–2010 CrossRef CAS.
- T. J. Kemp and R. A. McIntyre, Influence of transition metal-doped titanium(IV) dioxide on the photodegradation of polystyrene, Polym. Degrad. Stab., 2006, 91, 3010–3019 CrossRef CAS.
- K. W. Kim, N. Park, K. S. Ryu and S. H. Chang, Characteristics of PVDF–HFP/TiO2 composite membrane electrolytes prepared by phase inversion and conventional casting methods, Electrochim. Acta, 2006, 51, 5636–5644 CrossRef CAS.
- Q. T. Nguyen, Process for microporous membrane preparation, Fr. Pat no. 2847175, 2004 Search PubMed.
- Q. T. Nguyen, O. Tahiri Alaoui, H. Yang and C. M'Bareck, Dry-cast process for synthetic microporous membranes: Physico-chemical analyses through morphological studies, J. Membr. Sci., 2010, 358, 13–25 CrossRef CAS.
- I. Losito, A. Amorisco, F. Palmisano and P. G. Zambonin, X-Ray photoelectron spectroscopy characterization of composite TiO2–poly(vinylidenefluoride) films synthesised for applications in pesticide photocatalytic degradation, Appl. Surf. Sci., 2005, 240, 180–188 CrossRef CAS.
- L. Yu, H. Shen and Z. Xu, PVDF–TiO2 composite hollow fiber UF membranes prepared by TiO2 sol–gel method and blending method, J. Appl. Polym. Sci., 2009, 113, 1763–1772 CrossRef CAS.
- J. F. Li, Z. L. Xu, H. Yang, L. Y. Yu and M. Liu, Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane, Appl. Surf. Sci., 2009, 255, 4725–4732 CrossRef CAS.
- Y. Yang and P. Wang, Preparation and characterizations of a new PS/TiO2 hybrid membranes by sol–gel process, Polymer, 2006, 47, 2683–2688 CrossRef CAS.
- G. Gogniat, M. Thyssen, M. Denis, C. Pulgarin and S. Dukan, The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity, FEMS Microbiol. Lett., 2006, 258, 18–24 CrossRef CAS.
- S. H. Kim, S. Y. Kwak, B. H. Sohn and T. H. Park, Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem, J. Membr. Sci., 2003, 211, 157–165 CrossRef CAS.
- M. G. Buonomenna, P. Macchi, M. Davoli and E. Drioli, Poly(vinylidene fluoride) membranes by phase inversion: the role the casting and coagulation conditions play in their morphology, crystalline structure and properties, Eur. Polym. J., 2007, 43, 1557–1572 CrossRef CAS.
- T. H. Young, L. P. Cheng, D. J. Lin, L. Fane and W. Y. Chuang, Mechanisms of PVDF membrane formation by immersion–precipitation in soft (1-octanol) and harsh (water) non-solvents, Polymer, 1999, 40, 5315–5323 CrossRef CAS.
- O. Tahiri Alaoui, Q. T. Nguyen, C. Mbareck and T. Rhlalou, Elaboration and study of poly(vinylidene fluoride)–anatase TiO2 composite membranes in photocatalytic degradation of dyes, Appl. Catal., A, 2009, 358, 13–20 CrossRef CAS.
- D. M. Spori, T. Drobek, S. Zurcher, M. O. Chsner, C. Sprecher, A. Muhlebach and N. D. Spencer, Beyond the lotus effect: roughness influences on wetting over a wide surface-energy range, Langmuir, 2008, 24, 5411–5417 CrossRef CAS.
- J. Genzer and K. Zimenko, Recent developments in superhydrophobic surfaces and their relevance to marine fouling: a review, Biofouling, 2006, 22, 339–360 CrossRef CAS.
- S. G. Gholap, M. V. Badiger and C. S. Gopinath, Molecular origins of wettability of hydrophobic poly(vinylidene fluoride) microporous membranes on poly(vinyl alcohol) adsorption: surface and interface analysis by XPS, J. Chem. Phys., 2005, 109, 13941–13947 CAS.
- D. F. Ollis, Kinetic disguises in heterogeneous photocatalysis, Top. Catal., 2005, 35, 217–223 CrossRef CAS.
- A. Fujishima and K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature, 1972, 238, 37–38 CAS.
- K. V. Subba Rao, M. Subrahmanyam and P. Boule, Immobilized TiO2 photocatalyst during long-term use: decrease of its activity, Appl. Catal., B, 2004, 49, 239–249 CrossRef.
- F. Zhang, J. Zhao, T. Shen, H. Hidaka, E. Pelizzetti and N. Serpone, TiO2-assisted photodegradation of dye pollutants II. Adsorption and degradation kinetics of eosin in TiO2 dispersions under visible light irradiation, Appl. Catal., B, 1998, 15, 147–156 CrossRef CAS.
- N. Baraka, A. Assabane, A. Nouah, A. Albourine and Y. Ait-Ichou, Degradation photocatalytique de deux colorants separés et en mélange binaire par TiO2 supporté, Sci. Technol. A, 2008, 27, 9–16 Search PubMed.
- T. Matsunaga, R. Tomoda, Y. Nakajima, N. Nakamura and T. Komine, Continuous-sterilization system that uses photosemiconductor powders, Appl. Environ. Microbiol., 1988, 54, 1330–1333 CAS.
- C. Guillard, T. H. Bui, C. Felix, V. Moules, B. Lina, P. Lejeune, Comptes Rendus de l'Académie des Sciences, Série IIC Chemistry.
- D. M. Blake, P. C. Maness, Z. Huang, E. J. Wolfrum and J. Huang, Application of the photocayalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells, Sep. Purif. Methods, 1999, 28, 1–50 CrossRef CAS.
- D. Gumy, C. Morais, P. Bowen, C. Pulgarin, S. Giraldo, R. Hajdu and J. Kiwi, Catalytic activity of commercial of TiO2 powders for the abatement of the bacteria (E. coli) under solar simulated light: influence of the isoelectric point, Appl. Catal., B, 2006, 63, 76–84 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |