Photocatalytic degradation of Amaranth and Brilliant Blue FCF dyes using in situ modified tungsten doped TiO2 hybrid nanoparticles
B.
Shahmoradi
*a,
A.
Maleki
a and
K.
Byrappa
b
aEnvironmental Health Research Center, Faculty of Health, Kurdistan University of Medical Science, Sanandaj, Kurdistan, Islamic Republic of Iran
bDepartment of Earth Science, University of Mysore, Mysore, Karnataka, India
First published on 29th July 2011
Abstract
This study focuses on the process of photocatalytic degradation of popular dyes like Amaranth and Brilliant Blue, using reagent grade TiO2 and in situ modified tungsten doped TiO2 hybrid nanoparticles. One of the drawbacks of nanoparticles is their agglomeration and poor dispersion in the medium as well as their limited activity in the ultra violet region, which makes them less efficient. In order to overcome such drawbacks, for the first time, in situ surface modification and doping of TiO2 nanoparticles were carried out employing n-butylamine as surface modifier and tungsten oxide as dopant. Modification was conducted under mild hydrothermal conditions (T = 150 °C, P = autogenous). Nanoparticles obtained were characterized using Powder XRD, FTIR, DLS, Zeta potential, UV-Vis spectroscopy, and TEM. The characterization indicated the desired results with respect to morphology, particle size distribution and less agglomeration. The results of the process of photodegradation of Brilliant Blue FCF and Amaranth dyes showed a higher efficiency for in situ modified tungsten doped TiO2 hybrid nanoparticles than for reagent grade TiO2.
1. Introduction
Photodegradation of organic and inorganic pollutants using semiconductor metal oxides has been a very popular topic of research in recent years. Dyes form an important class of aquatic pollutants, which have begun to prove a major source of environmental contamination.1 More than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 commercial dyes based on ca. 8000 different chemical structures are widely used in the textile, food, pharmaceutical, paper and ink industries.2 These dyes are usually discharged into water bodies without complete or partial treatment which can reduce the auto-purification capacity of the receiving water bodies and lead to eutrophication. The textile industry is the largest consumer of dyes (60%), using them in conjunction with a wide range of auxiliary reagents for various dyeing, printing and finishing processes. Textile industrial effluents, irrespective of dye or fiber class, are invariably deeply colored by unabsorbed dyes, the extent of which varies according to the dye-fiber system.3
000 commercial dyes based on ca. 8000 different chemical structures are widely used in the textile, food, pharmaceutical, paper and ink industries.2 These dyes are usually discharged into water bodies without complete or partial treatment which can reduce the auto-purification capacity of the receiving water bodies and lead to eutrophication. The textile industry is the largest consumer of dyes (60%), using them in conjunction with a wide range of auxiliary reagents for various dyeing, printing and finishing processes. Textile industrial effluents, irrespective of dye or fiber class, are invariably deeply colored by unabsorbed dyes, the extent of which varies according to the dye-fiber system.3
Over the period of the last few decades, the increasing industrial demand for dyes has proved to possess a high pollutant potential, especially the use of azo dyes such as Amaranth and Brilliant Blue FCF. The decolouration of water is the major indicator of its quality.
In industries, the process of dyeing results in the production of large amounts of wastewater that exhibit intense colouration. The extent of colouration has to be radically reduced before the effluents are released into natural water streams. Wastewater released from dye-using industries contains different types of synthetic dyes, which are mostly toxic, mutagenic and carcinogenic. Moreover, they are very stable to light and microbial attack, which makes them recalcitrant compounds.4 Amaranth—an anionic dye made of tar—is a dark red to purple azo dye that is predominantly used to colour cosmetics. It could be used to colour natural and synthetic fibers, leather, paper, and phenol–formaldehyde resins. Brilliant Blue FCF is often found in ice creams, tinned processed peas, dairy products, sweets and drinks. It is also used in soaps, shampoos, and other hygiene and cosmetics applications. Table 1 shows the characteristics of these two dyes.
| Characteristics | Amaranth | Brilliant Blue FCF |
|---|---|---|
| Synonym |
FD&C Red No. 2, E123,
C.I. Food Red 9, Acid Red 27, Azorubin S, C.I. 16185 |
Acid Blue 9,
Alzen Food Blue No.1, Atracid Blue FG, Erioglaucine, Eriosky blue |
| Color index no. | 16185 | 42090 |
| Chemical formula | C20H11N2Na3O10S3 | C37H34N2Na2O9S3 |
| M.W. (mol g−1) | 604.48 | 792.84 |
| λ max (nm) | 521 | 630 |
| Structure |
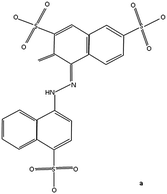
|

|
An ideal waste treatment process should completely mineralize all the toxic species present in the waste stream totally removing all hazardous residues. Any efficient treatment process must be cost-effective. Most of the conventional treatments require subsequent treatments that are expensive.
To date, many kinds of semiconductor metal oxides have been studied as photocatalysts that include TiO2, ZnO, WO3, and so on. TiO2 has been found to be the most widely used and most effective photocatalyst as it has a high degree of efficiency, photochemical stability, non-toxic nature and low cost. Some researchers have highlighted the performance of TiO2 in the process of degradation of some organic compounds.5,6
The photodegradation process, in general, occurs with the attack of organic substances activated by oxygen species. These substances include hydroxyl radicals and super oxide radicals, generated on TiO2 particulate surface by the reduction of dissolved oxygen in solution and/or oxidation of surface hydroxyl groups using TiO2.7
The photocatalytic efficiency of TiO2 catalysts depends entirely upon the relative degree to which the reactive electron–hole pairs branch into interfacial charge transfer reactions. On absorption of a photon of energy greater than or equal to the band gap energy by TiO2, an electron moves to a conduction band from a valence band. As a result, an electron gets distributed in the conduction band, creating an electron vacancy or “hole” that becomes distributed in the valence band. If charge separation is maintained, the electron and hole may migrate to the catalyst surface where they participate in redox reactions with adsorbed species. A hole and an electron range correspondingly and respectively at the valence band and conduction band by the irradiation of light. A hydroxyl radical (˙OH) and superoxide radical (˙O), consequently, come into being in reaction with water and oxygen present in the atmosphere. Therefore, the factor that limits the photocatalytic reaction is the process of recombination of the electron and the hole, preceding the superoxide activation step.8–10 Thus, one of the most crucial measures lies in enhancing the interfacial charge-transfer reaction. Many research groups have recently improved this technology by implanting,11 doping and depositing suitable transitional metal ions,12 noble metals,13 semiconductor metallic oxides,14 and nonmetal ions15 into the TiO2 structure.
A number of methods of TiO2 nanoparticles preparation have been reported that range from sol–gel,16 to supercritical carbon dioxide,17 to photodeposition,18 and hydrothermal.19 The preparation of titanium dioxide nanoparticles is a challenging task as its properties greatly depend upon its size and morphology. The conventional synthesis routes yield highly agglomerated TiO2 particles without any control over their morphology and size, and in some of the preparation processes require post-treatment in order to achieve crystalline products with the desired properties. Though hydrothermal and solvothermal techniques are more effective in obtaining high quality nanoparticles because of the highly-controlled diffusion during the synthesis process, the problem of size and morphology control cannot yet be achieved through normal routes. Hence, an appropriate capping agent or organic ligand or surfactant or chelating agent must be added to achieve control over the size and morphology of these TiO2 particles, thereby allowing their properties to be effectively tailored.
The present paper deals with the modification of the surface of tungsten doped TiO2 hybrid nanoparticles under mild hydrothermal conditions, using n-butylamine with different molar ratios. n-Butylamine was used as surface modifier because it has low toxicity, low density and a low melting temperature and is eco-friendly. The effect of the surfactant on the in situ modification of the doped TiO2 hybrid nanoparticles, and the effect of doping and its percentage on the band gap energy that affects photodegradation efficiency of Amaranth and Brilliant Blue FCF, is discussed in detail in the following sections.
2. Experimental
2.1 Preparation of in situ modified tungsten doped TiO2 hybrid nanoparticles
Tungsten doped TiO2 hybrid nanoparticles were synthesized under mild hydrothermal conditions (T = 150 °C, P = autogeneous). 1 M of reagent grade TiO2 (Loba Chemie, India) was taken as the starting material and the dopant (WO3: 2 mol%; 5 mol%) was added to it. A certain amount of 1M HCl was added as a mineralizer to the precursors. At the same time different concentrations (0.8, 1.0, 1.2 and 1.4 M) of n-butylamine (Sisco Research Lab PVT, Ltd., Mumbai India, Assay (GC)) was added to the above mentioned mixture and was stirred vigorously for a few minutes. The final compound was then transferred to the Teflon liner (Vfill = 10 ml), which was later placed inside a General-Purpose autoclave. Then the assembled autoclave was kept in an oven with a temperature programmer-controller for 8–18 h. The temperature was kept at 150 °C. After the experimental run, the autoclave was cooled to room temperature. The product in the Teflon liner was transferred to a clean beaker, washed with doubly distilled water, and later the product was allowed to settle. The surplus solution was removed using a syringe and the remnants were centrifuged for 20 min at 1500 rpm. The recovered product was dried in a hot air oven at 40–50 °C for a few hours. The dried particles were subjected to a systematic characterization and photocatalytic studies.2.2 Characterization of the in situ modified tungsten doped TiO2 hybrid nanoparticles
The fabricated products were characterized using different analytical techniques. The Fourier Transform Infrared spectra were recorded using FTIR, JASCO-460 PLUS, Japan, at a resolution of 4 cm−1. The Powder X-ray diffraction patterns were recorded using Bruker, D8 Advance, Germany, with Cu-Kα, λ = 1.542 Å radiation, voltage = 40 mV, current = 30 mA, scan speed 1.5° min−1. The data were collected in the 2θ range 5–100°. The optical properties were studied by using UV-Vis spectrophotometer. Particle size and its distribution were measured using dynamic light scattering (DLS) (Horiba particle size analyzer, LB-550, Japan). Zeta potential was measured using Zetasizer 2000 instrument (Malvern instruments). TEM images of the tungsten(VI) oxide doped TiO2 hybrid nanoparticles were recorded using JEM 2000FX II (JOEL. Ltd., Tokyo, Japan).2.3 Photoreactor and experimental procedure
A cylindrical flow photoreactor that was designed and fabricated in our laboratory was used as shown in Fig. 1. At the center of this cylindrical vessel, a 6 W low-pressure mercury lamp placed inside a quartz sleeve with an emission peak at 264 nm (Philips, Netherlands) was positioned. This was surrounded by a circulating water jacket meant to control the temperature during reaction. In the case of visible light-irradiation, sunlight was used. The reaction suspension was prepared by adding different amounts (0.4–2 g) of photocatalyst powder per litre of dye solution. An external aerator was used as an oxygen source and air was continuously pumped into the reactor through air diffusers to fully fluidize the tungsten doped TiO2 hybrid nanoparticles during the photoreaction. For the purpose of the application potential in practice, the initial pH was adjusted to 7.5 using 0.02 M HCl solution and 0.02 M NH3OH solution before reaction. The tungsten doped TiO2 hybrid nanoparticles were mixed with the Brilliant Blue and Amaranth solution respectively and were placed in darkness for 30 min to ensure balance of adsorption and desorption. The suspensions were sampled at specific intervals to monitor the changes of Brilliant Blue and Amaranth concentrations. Sampled suspensions were centrifuged at 1500 rpm for 30 min to remove the tungsten doped TiO2 hybrid nanoparticles and then analyzed by UV-Vis spectrophotometer. | ||
| Fig. 1 Flow photoreactor. | ||
3. Results and discussion
3.1 Characterization of in situ modified tungsten doped TiO2 hybrid nanoparticles
The powder XRD results of the nanoparticles synthesized match well with the I4/amd space group. XRD data reveal that when compared to pure TiO2, there is a slight change in the lattice parameters of tungsten doped TiO2 nanoparticles (TiO2–X, X = 2, 5 mol% WO3) at the a-axis and c-axis. This confirms the existence of tungsten atoms in TiO2 nanoparticles. The radii of W(VI) ion (60 pm) is bigger than that of Ti(VI) (42 pm) and is certain to make the cell parameter, on doping with W, bigger than that of pure TiO2. The powder XRD patterns of (2 mol%; 5 mol%) tungsten doped TiO2 hybrid nanoparticles reveal five primary peaks at 25.32°, 37.82°, 48.06°, 53.9° and 62.7° for 2 mol% dopant (Fig. 2a) and 25.36°, 37.84°, 48.08°, 55.01° and 62.72° for 5 mol% dopant (Fig. 2b). This can be attributed to different diffraction planes of TiO2. The powder XRD data indicate that the cell volume of tungsten doped TiO2 hybrid nanoparticles slightly increases with 2 mol% and 5 mol% tungsten doping (Table 2).20 | ||
| Fig. 2 Powder XRD pattern of tungsten doped TiO2 nanoparticles modified using 1.0 M n-butylamine. | ||
The functional groups present in the modified nanoparticles can be studied using FTIR spectroscopy. Fig. 3 shows the FTIR spectra of the reagent grade TiO2 without modifier, reagent grade TiO2 with modifier and tungsten doped TiO2 nanoparticles modified with n-butylamine (0.8 M and 1.4 M) as a surface modifier. The intensity of the absorption peaks was stronger for 1.4 M surface modified tungsten doped TiO2 nanoparticles compared to 0.8 M surface modified tungsten doped (2 mol% and 5 mol%) TiO2 hybrid nanoparticles. The FTIR spectra of the modified hybrid nanoparticles show the presence of new peaks and this in turn suggests that the reagents were chemically immobilized on the surface of the nanoparticles. Thus, it can be concluded that the tungsten doped TiO2 nanoparticles synthesized with the above mentioned modifier, have organic coverage on their surfaces, which changes the surface properties of the nanoparticles. The major vibrational modes of WO3 are located at 808, 714 and 276 cm−1 and are assigned to the W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode, the W
O stretching mode, the W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending mode and W–O–W deformation mode, respectively, while the peak around 650 cm−1 is representative of TiO2 matrices.21 The peaks around 1503 and 3675 cm−1 correspond to the presence of CH3 and N–H stretching bonds. In addition, the new peaks around 2965 and 3400 cm−1 correspond to O–H and NH4+ stretching bond. The absorption peaks around 1638, 3600 and 3695 cm−1 belong to the C
O bending mode and W–O–W deformation mode, respectively, while the peak around 650 cm−1 is representative of TiO2 matrices.21 The peaks around 1503 and 3675 cm−1 correspond to the presence of CH3 and N–H stretching bonds. In addition, the new peaks around 2965 and 3400 cm−1 correspond to O–H and NH4+ stretching bond. The absorption peaks around 1638, 3600 and 3695 cm−1 belong to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bond.22
O stretching bond.22
 | ||
Fig. 3
FTIR spectra of reagent grade TiO2 ( ), modified undoped TiO2 hybrid nanoparticles ( ), modified undoped TiO2 hybrid nanoparticles ( ), tungsten doped TiO2 hybrid nanoparticles modified with 0.8 M ( ), tungsten doped TiO2 hybrid nanoparticles modified with 0.8 M (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ), and 1.6 M n-butylamine ( ), and 1.6 M n-butylamine ( ): (a) 2 mol% and (b) 5 mol% dopant. ): (a) 2 mol% and (b) 5 mol% dopant. | ||
Fig. 4 shows TEM images of 2 mol% and 5 mol% tungsten doped TiO2 hybrid nanoparticles modified with (0.8 and 1.4 M) n-butylamine. It shows a thin organic coverage of the surface modifier on the synthesized nanoparticles. The agglomeration is less when a higher concentration of the surface modifier is used. The in situ surface modification leads to the controlling of growth direction and particle size, thus, preventing agglomeration. It is found that the surface modifier cannot only affect the dispensability of the synthesized tungsten doped TiO2 hybrid nanoparticles, but also change their growth habit. The morphology attained is quite suitable for the photodegradation purposes; as the tungsten doped TiO2 hybrid nanoparticles are rounded, they can be more active in photodegradation of the organic pollutants present in the industrial effluents.
 | ||
| Fig. 4 TEM image of tungsten doped TiO2 hybrid nanoparticles: (a) 2 mol% tungsten, 0.8 M n-butylamine; (b) 5 mol% tungsten, 0.8 M n-butylamine, (c) 2 mol% tungsten, 1.4 M n-butylamine; (d) 5 mol% tungsten, 1.4 M n-butylamine. | ||
Fig. 5 shows the particle size distribution of the tungsten doped TiO2 hybrid nanoparticles synthesized confirming the nano-range of the particles. The range is narrower in the case of 2 mol% tungsten doped TiO2 hybrid nanoparticles. The particle size in all cases is in the range 30–250 nm, with an average diameter of 112.9 nm in the case of 2 mol% tungsten doped TiO2 hybrid nanoparticles modified with 1.4 M surfactant and 135.7 nm for 5 mol% tungsten doped TiO2 hybrid nanoparticles modified with 1.4 M surfactant. These results are in agreement with powder XRD data and TEM images.
 | ||
| Fig. 5 Particle size distribution using DLS for (a) 2 mol% tungsten doped; and (b) 5 mol% tungsten doped TiO2 hybrid nanoparticles modified using 1.4 M n-butylamine. | ||
3.2 Zeta potential of in situ modified tungsten doped TiO2 hybrid nanoparticles
Zeta (ζ) potential measurements were performed for in situ surface modified TiO2 nanoparticles in order to characterize the surface charge of nanoparticles and Fig. 6 shows the results as a function of pH. The obtained ζ potential of the nanoparticles was found to decrease with increasing pH as is expected for a surface with acid–base groups. The iso-electric point or point of zero charge (PZC) for TiO2 nanoparticles was found to be 4.2. The significance of the ζ potential is that its value can be related to the stability of colloidal dispersions. The ζ potential indicates the degree of repulsion between adjacent, similarly charged particles in dispersion. For small enough nanoparticles, a high ζ potential will confer stability, i.e. the solution or dispersion will resist aggregation. When the potential is low, attraction exceeds repulsion and the dispersion breaks and flocculates.23–25 Therefore, colloids with high ζ potential (negative or positive) are electrically stabilized while colloids with low ζ potentials tend to coagulate or flocculate. As Fig. 6 indicates, ζ potential for the surface modified TiO2 nanoparticles is −40 meV at pH 12, which indicates its stability. This property is quite suitable for photodegradation application of the synthesized nanoparticles. | ||
| Fig. 6 Zeta potential of TiO2 nanoparticles modified with n-buytlamine as surfactant. | ||
3.3 Band gap energy of in situ modified tungsten doped TiO2 hybrid nanoparticles
The effect of doping was studied by UV-Vis spectroscopy in the range of 200–600 nm. Fig. 7 shows that pure TiO2 had no absorption in the visible light region (λ > 400 nm). The in situ modified tungsten doped TiO2 hybrid nanoparticles showed a remarkable absorption band shift toward longer wavelength region, which indicates a decrease of the band gap energy. In addition, according to the band edges of the samples determined from differentiation of the three curves, the band gap of the three samples are calculated according to the equation EG = hc/λ, where EG is the band gap energy (eV), h is Plank's constant, c is the speed of light (m s−1), and λ is the wavelength (nm). The EG of pure TiO2, 2 mol% tungsten doped TiO2 hybrid nanoparticles and 5 mol% tungsten doped TiO2 hybrid nanoparticles are 3.32, 3.20 and 3.03, respectively. | ||
Fig. 7 Effect of doping on band gap energy of the modified tungsten doped TiO2 nanoparticles: reagent grade TiO2 ( ), 2 mol% tungsten doped ( ), 2 mol% tungsten doped ( ) and 5 mol% tungsten doped ( ) and 5 mol% tungsten doped ( ) TiO2 nanoparticles modified with n-butylamine. ) TiO2 nanoparticles modified with n-butylamine. | ||
3.4 Photodegradation of Brilliant Blue FCF and Amaranth dyes
The absorption of Brilliant Blue FCF and Amaranth dyes (Loba Chemie) was measured at 630 nm and 520 nm (λmax) of the solutions after photodegradation, respectively. The absorption was converted to relative concentration of the photodegraded dyes (ln C/C0), where C and C0 represent concentration after reaction and initial concentration, referring to a standard curve and the displayed linear behavior between relative concentration and absorption at these wavelengths, respectively. For comparison, the photocatalysis of Brilliant Blue and Amaranth dyes on pure TiO2 powders from Loba Chemie were conducted under the same operating conditions. | ||
Fig. 8
Photodegradation of dyes using  5 mol% tungsten doped; 5 mol% tungsten doped;  2 mol% tungsten doped TiO2 nanoparticles modified with n-butylamine and 2 mol% tungsten doped TiO2 nanoparticles modified with n-butylamine and ![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) without catalyst. without catalyst. | ||
As shown in the Fig. 8, in the absence of TiO2, the removal percentage of these dyes is almost zero. The addition of nanoparticles enhances the removal of both contaminants with different speeds. The removal rate of these contaminants increases as the concentration of the dyes increases. It should be mentioned that there was no difference in the extent of degradation based on the light source in the case of reagent grade TiO2 but as this figure indicates, the degradation also occurred in visible light, which confirms the effect of doping on photodegradation of these dyes. An optimal result was achieved at a TiO2 dosage of 1.4 g L−1. The removal decreases with a further increase in the catalyst dosage.
In analyzing the kinetic data of photodegradiation, mediated by semiconductors or nanoparticles like TiO2, the data are analyzed to a simple rate expression of Langmuir–Hinshelwood (L–H) form26 as follows:
 | (1) |
 | (2) |
 | (3) |
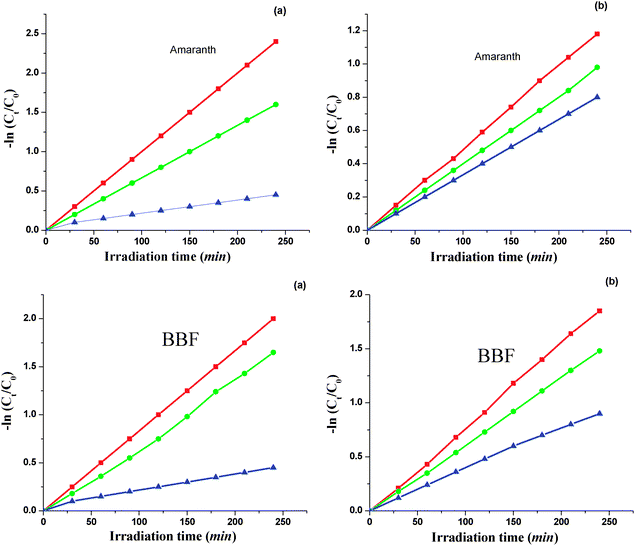 | ||
Fig. 9 Effect of light source: (a) sun light and (b) UV light on photodegradation of dyes using 5 mol% tungsten doped ( ); 2 mol% tungsten doped ( ); 2 mol% tungsten doped ( ) TiO2 nanoparticles modified with n-butylamine and reagent grade TiO2 ( ) TiO2 nanoparticles modified with n-butylamine and reagent grade TiO2 ( ). ). | ||
 | ||
| Fig. 10 Relationship between concentrations of surfactant applied to modify surface of doped TiO2 nanoparticles and photodegradation efficiency. | ||
4. Conclusion
Tungsten doped TiO2 hybrid nanoparticles were successfully modified in situ under hydrothermal conditions. n-Butylamine was used as surface modifier. Surface modification changed the morphology and size of the tungsten doped TiO2 nanoparticles synthesized. In addition, it changed the surface charges and increased the stability of the nanoparticles, which is necessary to achieve higher photodegradation efficiency. The initial concentration and type of pollutant are also important factors, which may affect the photodegradation. Doping the TiO2 nanoparticles with suitable metal oxide such as WO3 can reduce the band gap energy and enhance the feasibility of the photodegradation in the visible light. Simultaneous in situ surface modification and doping TiO2 nanoparticles not only changed the surface morphology, size and surface charges of the nanoparticles synthesized, but also significantly shifted the band gap energy to the visible region, where the photodegradation efficiency is more comparable to the UV region.References
- N. Binitha, Z. Y. Narayanan, K. Ranjana, Ch. Soumini, S. Sankaran, K. S. Femina and M. Vineetha, Photodegradation of Methylorange over Zirconia Doped TiO2 Using Solar Energy, Eur. J. Sci. Res., 2009, 28(4), 566–571 Search PubMed.
- R. Molinari, F. Pirillo, M. Falco, V. Loddo and L. Palsimano, Photocatalytic Degradation of Dyes by Using a Membrane Reactor, Chem. Eng. Process., 2004, 43(9), 1103–1104 CrossRef CAS.
- M. Gonçalves, E. Pinto, P. Nkeonye and C. A. Oliveira, Degradation of C. I. Reactive Orange 4 and its Simulated Dyebath Wastewater by Heterogeneous Photocataysis, Dyes Pigm., 2005, 64, 135–139 CrossRef.
- A. Rezaee, M. T. Ghaneian, A. Khavanin, S. J. Hashemian, Gh. Mousavi, Gh. Ghanizadeh and E. Hajizadeh, Photochemical Oxidation of Reactive Blue 19 Dye (RB19) in Textile Wastewater by UV/K2S2O8 Process, Iran J. Environ. Health Sci. Enh., 2008, 5(2), 95–100 CAS.
- S. Koutaro, K. Hironobu, T. Toshiyuki, B. Koumei, N. Mariko, N. Koji and S. Hiroyuki, Antibacterial Metal Implant with a TiO2-Conferred Photocatalytic Bactericidal Effect Against Staphylococcus aureus, Surface Interface Anal., 2008, 41(1), 17–22 Search PubMed.
- X. Li, H. Liu, L. Cheng and H. Tong, Photocatalytic Oxidation Using a New Catalyst-TiO2 Microsphere-for Water and Wastewater Treatment, Environ. Sci. Technol., 2003, 37(17), 3989–3994 CrossRef CAS.
- A. K. Subramani, K. Byrappa, S. Ananda, K. M. L. Rai, C. Ranganathaiah and M. Yoshimura, Photocatalytic Degradation of Indigo Carmine Dye Using TiO2 Impregnated Activated Carbon, Bull. Mater. Sci., 2008, 30(1), 37–41 CrossRef.
- D. Robert, A. Piscopo and J. V. Weber, First Approach of the Selective Treatment of Water by Heterogeneous Photocatalysis, Environ. Chem. Lett., 2004, 2(1), 5–8 CrossRef CAS.
- F. Li, X. Li and K. Ng, Photocatalytic Degradation of an Odorous Pollutant: 2-Mercaptobenzothiazole in Aqueous Suspension Using Nd3+–TiO2 Catalysts, Ind. Eng. Chem. Res., 2006, 45(1), 1–7 CrossRef CAS.
- W. Hao, S. Zheng, C. Wang and T. Wang, Comparison of the Photocatalytic Activity of TiO2 Powder with Different Particle Size, J. Mater. Sci. Lett., 2002, 21(20), 1627–1629 CrossRef CAS.
- T. Hiroshi, S. Hiromitsu, S. Tomohiko, M. Masanori, G. Yasuhito and I. Junzo, Improvement of Photocatalytic Efficiencies of Rutile TiO2 by Metal Negative Ion Implantation, J. Vac. Soc. Jpn., 2002, 43(5), 177–180 Search PubMed.
- Q. Quan, H. Tan, Q. Zhao and X. Sang, Preparation of lanthanum-doped TiO2 Photocatalysts by Coprecipitation, J. Mater. Sci., 2007, 42(15), 6287–6296 CrossRef.
- V. Subramanian, E. E. Wolf and P. V. Kamat, Influence of Metal/Metal Ion Concentration on the Photocatalytic Activity of TiO2–Au Composite Nanoparticles, J. Am. Chem. Soc., 19(2), 469–474 CAS.
- E. D. Jeong, P. H. Borse, J. S. Jang, J. S. Lee, O. S. Jung, H. Chang, J. S. Jin, M. S. Won and H. G. Kim, Hydrothermal Synthesis of Cr and Fe co-Doped TiO2 Nanoparticle Photocatalyst, Ceramic Proc. Res., 2008, 9(3), 250–253 Search PubMed.
- Y. Choi, T. Umebayashi and M. Yoshikawa, Fabrication and Characterization of C-Doped Anatase TiO2 Photocatalysts, J. Mater. Sci., 2004, 39(5), 1837–1839 CrossRef CAS.
- C. H. Han, Lee, H. S. Han and S. D. Han, Synthesis of Nanocrystalline TiO2 by Sol–Gel Combustion Hybrid Method and Its Application to Dye Solar Cells, Bull. Korean Chem. Soc., 2008, 29(8), 1495–1498 CrossRef CAS.
- C. I. Wu, J. W. Huang, Y.-L. Wen, S. B. Wen and Y. H. Shen, Preparation of TiO2 Nanoparticles by Supercritical Carbon Dioxide, Mater. Lett., 2008, 62(12–13), 1923–1926 CrossRef CAS.
- C. Hu, Y. Tang, Z. Jiang, Z. Hao, H. Tang and P. K. Wong, Characterization and Photocatalytic Activity of Noble-Metal-Supported Surface TiO2/SiO2, Appl. Catal., A, 2003, 253(2), 389–396 CrossRef CAS.
- T. Adschiri and K. Byrappa, Supercritical Hydrothermal Synthesis of Organic–Inorganic Hybrid Nanoparticles, in Nano-hybridization of Organic–Inorganic Materials, ed. A. Muramatsu, Springer-Verlag, Germany, 2008 Search PubMed.
- C. J. Howard, T. M. Sabine and F. Dickson, Structural and Thermal Parameters for Rutile and Anatase, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47(4), 462–468 CrossRef.
- N. Sh. Begum, H. M. Farveez and O. M. Hussain, Characterization and Photocatalytic Activity of Boron-doped TiO2 Thin Films Prepared by Liquid Phase Deposition Technique, Bull. Mater. Sci., 2008, 31(5), 741–745 CrossRef CAS.
- L. P. Donald, M. L. Gary and S. K. George, Infrared Spectroscopy, in Introduction to Spectroscopy, Thomson Learning, USA, 3rd edn, 2001 Search PubMed.
- Zeta Potential of Colloids in Water and Waste Water , ASTM Standard D 4187-82, American Society for Testing and Materials, 1985.
- J. Lyklema, Fundamentals of Interface and Colloid Science, Elsevier, Wageningen, 1995 Search PubMed.
- A. V. Delgado, F. G. Caballero, R. J. Hunter, L. K. Koopal and J. Lyklema, Measurement and Interpretation of Electrokinetic Phenomena, Pure Appl. Chem., 2005, 77(10), 1753–1805 CrossRef CAS.
- S. Y. Kim, T. H. Lim, T. S. Chang and C. H. Shin, Photocatalysis of Methylene Blue on Titanium Dioxide Nanoparticles Synthesized by Modified Sol-Hydrothermal Process of TiCl4, Catal. Lett., 2007, 117(3–4), 112–118 CrossRef CAS.
- J. Wang, R. Li, Z. Zhang, W. Sun, X. Wang, R. Xu, Z. Xing and X. Zhang, Degradation of Hazardous Dyes in Wastewater Using Nanometer Mixed Crystal TiO2 Powders under Visible Light Irradiation, Water, Air, Soil Pollut., 2007, 189(1–4), 225–237 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
