Synthesis, characterization and ethylene (co-)polymerization behavior of half-titanocene 2-(1-(arylimino)ethyl)quinolin-8-olate chlorides†
Wei
Huang
a,
Baixiang
Li
b,
Youhong
Wang
a,
Wenjuan
Zhang
a,
Lin
Wang
a,
Yuesheng
Li
b,
Wen-Hua
Sun
*a and
Carl
Redshaw
*c
aKey Laboratory of Engineering Plastics and Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: whsun@iccas.ac.cn; Fax: +86 10 62618239; Tel: +86 10 62557955
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
cSchool of Chemistry, University of East Anglia, Norwich, NR4 7TJ, UK. E-mail: carl.redshaw@uea.ac.uk; Fax: +44 (0)1603 592003; Tel: +44 (0)1603 593137
First published on 26th July 2011
Abstract
The series of half-titanocene 2-(1-(arylimino)ethyl)quinolin-8-olate dichlorides, Cp′TiCl2L (Cp′ = η5-C5H5 or η5-C5Me5, L = 2-(1-(2,6-R1-4-R2-phenylimino)ethyl)quinolin-8-olate), was synthesized via the stoichiometric reaction of Cp′TiCl3 with the corresponding potassium salt, viz.2-(1-(2,6-R1-4-R2-phenylimino)ethyl)quinolin-8-olate. All compounds were characterized by elemental analysis, 1H and 13C NMR spectroscopy. The molecular structures of complexes C2 and C4 were determined by single-crystal X-ray diffraction. When activated with methylaluminoxane (MAO), all half-titanocene pre-catalysts exhibited high activities for ethylene polymerization and co-polymerization with α-olefins or norbornene.
1. Introduction
Olefin-based polymers, such as polyethylenes (PEs), polypropylenes (PPs), and ethylene/α-olefin co-polymers are the most important to our daily life and thus are by far the most currently produced synthetic polymers. It is widely believed that the design of efficient transition metal catalysts, that precisely control olefin coordination polymerization, can play an important role in the success of this effort, and recent progress of newly designed catalysts offers new possibilities. Since the 1990s, more attention has been devoted to developing pre-catalysts of early-transition1 and late-transition metal complexes2 for olefin polymerization and co-polymerization. Bridged half-metallocene pre-catalysts such as the well-known constrained-geometry complexes (CGC) represent good examples of single-site catalysts, and produce polyolefins with narrow molecular weight distributions as well as exhibiting good incorporation of co-monomers.3 In extending the synthesis of half-metallocene type pre-catalysts, a series of non-bridged half-metallocene pre-catalysts, Cp′M(L)Xn (M = Ti, Zr, Hf; L = anionic ligand; X = halogen or alkyl), have been developed by the Nomura group.4 Other anionic ligands have also been explored including pyridinylalkyloxy,5acetamidinato,6 aryloxy,7iminophenoxy,8ketimide,9 and N-substituted 2-(iminomethyl)pyrrolides.10 Beyond half-metallocene pre-catalysts, the bis(phenoxyimino)titanium complexes reported by the Fujita and Coates groups have been shown to exhibit high activity for the polymerization of both ethylene and propylene.11 Recently, a series of 2-(1-(arylimino)propyl)quinolin-8-ols were successfully prepared in our group, their aluminium complexes showed high activity in the ring-opening polymerization of ε-caprolactone,12 whilst the trichlorotitanium complexes thereof exhibited high activities in ethylene homo-polymerization and co-polymerization.13a Half-titanocene dichloride complexes bearing 2-(1-(arylimino)propyl)quinolin-8-olates also showed high activities towards ethylene polymerization and co-polymerization,13b but they exhibited severe thermo-stability problems at elevated temperatures, which is problematic with regard to industrial considerations. Herein, a series of 2-(1-(arylimino)ethyl)quinolin-8-ols are synthesized and used in preparing the half-titanocene 2-(1-(arylimino)ethyl)quinolin-8-olate chlorides. The newly synthesized half-titanocene pre-catalysts are found to also perform well in the polymerization of ethylene and co-polymerization of ethylene with α-olefins or norbornene, but importantly at higher reaction temperatures.2. Results and discussion
2.1 Synthesis and characterization of half-titanocene complexes (C1–C8)
As for the synthetic procedure used for the 2-(1-(arylimino)propyl)quinolin-8-ol,12 the 2-(1-(arylimino)ethyl)quinolin-8-ols were prepared in good yields via the reaction of 2-acetylquinolin-8-ol with the corresponding anilines.13 As before, a molar equivalent of potassium hydride and the corresponding 2-(1-(arylimino)ethyl)quinolin-8-ol were mixed in toluene at −78 °C and stirred for 4 h, then one equivalent of Cp′TiCl3 (Cp′ = Cp or Cp*) was added at −78 °C and the system was allowed to warm up to room temperature and was stirred for 24 h. The resultant solution was concentrated and extracted with dichloromethane, and the combined filtrate was concentrated and layered with n-heptane to precipitate the formed title complex. The title complexes (Cp′TiLCl2, C1–C8) were obtained in good yields (63.1–94.5%) (Scheme 1). All the 2-(1-(arylimino)ethyl)quinolin-8-ols and their half-titanocene dichlorides were characterized by elemental analysis and NMR measurements. | ||
| Scheme 1 Synthesis of complexes C1–C8. | ||
Single crystals of complexes C2 and C4 suitable for X-ray crystallographic studies were obtained from dichloromethane solutions when layered with n-heptane. The molecular structures are illustrated in Fig. 1 and 2, respectively, and the selected bonds and angles are tabulated in Table 1.
 | ||
| Fig. 1 ORTEP drawing of the molecular structure of C2 (ellipsoids enclose 30% electronic density; H atoms are omitted for clarity). | ||
 | ||
| Fig. 2 ORTEP drawing of the molecular structure of C4 (ellipsoids enclose 30% electronic density; H atoms are omitted for clarity). | ||
| C2 | C4 | |
|---|---|---|
| Bond lengths (Å) | ||
| Ti1–O1 | 1.952(3) | 1.957(2) |
| Ti1–N1 | 2.135(4) | 2.133(3) |
| Ti1–N2 | 2.376(4) | 2.380(3) |
| Ti1–Cl1 | 2.4196(15) | 2.4367(12) |
| Ti1–Cl2 | 2.4551(14) | 2.4383(12) |
| Ti1–Cpcent | 2.078 | 2.071 |
| Bond angles (°) | ||
| O1–Ti1–N1 | 77.40(14) | 77.19(10) |
| O1–Ti1–N2 | 146.37(13) | 146.67(10) |
| N1–Ti1–N2 | 68.98(14) | 69.51(10) |
| O1–Ti1–Cl1 | 89.05(10) | 88.41(8) |
| N1–Ti1–Cl1 | 76.50(11) | 76.04(8) |
| N2–Ti1–Cl1 | 84.32(10) | 82.61(7) |
| O1–Ti1–Cl2 | 87.09(10) | 89.01(8) |
| N1–Ti1–Cl2 | 75.50(11) | 75.40(9) |
| N2–Ti1–Cl2 | 83.63(9) | 83.92(7) |
| Cl1–Ti1–Cl2 | 151.91(5) | 151.18(4) |
As shown in Fig. 1, the complex C2 contains one tridentate 2-(1-(2,6-diethylphenylimino)ethyl)quinolin-8-olate, two chlorides and one Cp ring, which form a pseudo octahedral geometry at the titanium center. Due to their different environments, the Ti–Cl and Ti–N bond lengths are slightly varied with Ti1–Cl1 2.4196(15) Å vs. Ti1–Cl2 2.4551(14) Å and Ti1–N1 2.135(4) Å vs. Ti1–N2 2.376(4) Å. The distance between the η5-Cp centroid and the titanium is 2.078 Å, which is similar to observations for reported half-titanocene complexes,14 though there is a slight difference with ΔTi–CCp at 0.093 Å with the Ti–CCpi.e. bond lengths within the range of 2.353(5) to 2.446(5) Å. The dihedral angle defined by the two chelating planes [ΘTi1–O1–C1–C6–N1 and ΘTi1–N1–C9–C10–N2] is only 1.29°, indicating an almost coplanar feature of the atoms of titanium, coordination oxygen and nitrogen atoms as well as the quinolinyl ligand framework. The dihedral angle of the phenyl plane of the arylamine and the quinolinyl plane is about 86.95°, closer to perpendicular.
The complex C4 (Fig. 2) having an additional p-methyl on the arylamino group possesses similar structural features to C2 (Fig. 1). Slightly different observations within C4 are worthy of mention, specifically the near equal Ti–Cl bond lengths of Ti1–Cl1 2.4367(12) Å and Ti1–Cl2 2.4383(12) Å, and the near coplanarity of the titanium and coordinated atoms of the ligands. Furthermore, the quinolyl plane has the dihedral angle of 0.53° formed by the two chelating planes [ΘTi1–O1–C1–C6–N1 and ΘTi1–N1–C9–C10–N2]. The dihedral angle of the quinolinyl and phenyl of arylamine is 88.11°.
2.2 Catalytic behavior in ethylene polymerization
Various alkylaluminium reagents such as MAO, MMAO, EtAlCl2 and Et2AlCl have been screened for their suitability as co-catalysts, and based on the observations of the catalytic behavior exhibited by the analogs,13MAO was deemed as the optimum co-catalyst herein. Due to its ease of preparation, complex C7 was employed in the optimization of the ethylene polymerization parameters such as the Al/Ti molar ratio, the reaction temperature and the lifetime of active species (Table 2).| Run | Al/Ti | T/°C | t/min | Polym (g) | Act.b | M w c , d | M w/Mnd | Tme/°C |
|---|---|---|---|---|---|---|---|---|
| a Conditions: 2 μmol complex C7, toluene (total volume 100 mL), 10 atm. b 105 g mol−1(Ti) h−1. c 104 g mol−1. d Determined by GPC. e Determined by DSC. | ||||||||
| 1 | 2000 | 60 | 30 | 0.412 | 4.12 | 3.94 | 11.7 | 127.7 |
| 2 | 3000 | 60 | 30 | 1.293 | 12.9 | 4.70 | 13.1 | 128.4 |
| 3 | 4000 | 60 | 30 | 1.128 | 11.3 | 4.62 | 14.5 | 127.7 |
| 4 | 5000 | 60 | 30 | 0.957 | 9.57 | 4.26 | 11.9 | 130.4 |
| 5 | 3000 | 60 | 10 | 0.173 | 5.19 | 3.48 | 9.49 | 128.1 |
| 6 | 3000 | 60 | 20 | 0.458 | 6.87 | 4.76 | 13.9 | 127.8 |
| 7 | 3000 | 60 | 60 | 2.104 | 10.5 | 5.24 | 18.7 | 128.6 |
| 8 | 3000 | 20 | 30 | 0.998 | 9.98 | 5.43 | 5.11 | 128.9 |
| 9 | 3000 | 40 | 30 | 1.05 | 10.5 | 5.07 | 6.25 | 128.2 |
| 10 | 3000 | 80 | 30 | 0.462 | 4.62 | 3.28 | 13.8 | 126.6 |
Varying the Al/Ti molar ratio from 2000 to 5000 at 60 °C over 30 min (Runs 1–4, Table 2), the highest activity of 1.29 × 106 g mol−1(Ti) h−1 was achieved at Al/Ti 3000. Regarding the lifetime of the active species, the ethylene polymerization was terminated at different reaction times of 10, 20, 30, and 60 minutes (Runs 2 and 5–7, Table 2). Comparison of these data indicated the presence of an induction period for forming the active species, and thus the activities obtained increased on prolonging the reaction time (Runs 5, 6, and 2, Table 2). In general, the active species in the current system showed longer lifetime than the previously reported analogues.13 However, slow deactivation also occurred as observed by the relatively lower values for the catalytic activity over 60 cf. 30 min. Considering the thermo-stability of the catalytic system, the temperature parameters were elevated from 20 °C to 80 °C (Runs 2 and 8–10, Table 2), the optimum temperature was found to be 60 °C (Run 2, Table 2). These trends for the reaction parameters were consistent with those observed for their analogs13 and other half-titanocene pre-catalysts.15 The Tm values and molecular weights of the resultant polyethylenes were measured by the DSC and GPC analysis, respectively. In most cases, the broad molecular weight distributions of the polymers indicated the presence of multiply active species in the catalytic system. Generally, the results showed that polyethylenes with higher molecular weights were formed with catalytic systems either at lower reaction temperature or on prolonging the reaction time, whilst the molecular distributions of the polyethylenes increased on either prolonging the reaction time (Fig. 5), increasing the molar ratio of Al/Ti (Fig. 3), or elevating the reaction temperature (Fig. 4).
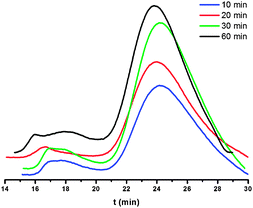 | ||
| Fig. 5 GPC profiles of PEs obtained from Runs 2, and 5–7 in Table 2. | ||
 | ||
| Fig. 3 GPC profiles of PEs obtained from Runs 1–4 in Table 2. | ||
 | ||
| Fig. 4 GPC profiles of PEs obtained from Runs 2, and 8–10 in Table 2. | ||
On comparison with the analog pre-catalysts,13b it can be seen that the current system provided polyethylenes with lower molecular weights.
Using the optimum polymerization conditions of 10 atm ethylene, 60 °C, and Al/Ti 3000 over 30 min, all pre-catalysts C1–C8 were screened for ethylene polymerization (Table 3) in order to further understand the ligand substituent influences on the catalytic activities of these complexes. The most significant influence was observed for the η5-Cp group, whereby the pre-catalysts containing η5-C5Me5 (Cp*) exhibited higher activities than did their analogs bearing η5-C5H5 (Cp), which was thought to be due to the methyl-groups of η5-Cp* enhancing the solubility of the half-titanocene complexes, together with the more electron-donating feature stabilizing the active species.9 Similar influences of the substituents could also be observed in the catalytic tendencies of these groups of half-titanocene pre-catalysts, in which the pre-catalysts containing Cp* exhibited the activity order of C7 (iPr) > C6 (Et) > C5 (Me), whilst the pre-catalysts bearing Cp showed the order of C3 (iPr) > C2 (Et) > C1 (Me). The catalytic performance exhibited by the half-titanocene species presented herein is consistent with that observed for their analogs,13b but the current systems importantly show improvement in thermo-stability. Specifically, the systems herein were optimized at 60 °C, whereas for the analogous systems the preferred temperature was 20 °C.13b However, repeated experimental results still indicated a negative effect for the para-methyl on the arylimino group with the activities observed as C2 (R1 = Et, R2 = H) > C4 (R1 = Et, R2 = Me) and C6 (R1 = Et, R2 = H) > C8 (R1 = Et, R2 = Me). Beyond that, another strange issue arose in that there were two melting points for the polyethylenes produced by C2 and C6, which were partly insoluble in 1,2,4-trichlorobenzene at 135 °C, caused by the presence of the ultrahigh molecular weight polyethylenes with Tm over 140 °C.
| Run | Cats | Polym (g) | Act.b | M w c , d | M w/Mnd | Tme/°C |
|---|---|---|---|---|---|---|
| a Conditions: 2 μmol complex, Al/Ti = 3000, 60 °C, 30 min, toluene (total volume 100 mL), 10 atm. b 105 g mol−1(Ti) h−1. c 104 g mol−1. d Determined by GPC. e Determined by DSC. f n.d.: not determined due to partly insoluble polymers. | ||||||
| 1 | C1 | 0.421 | 4.21 | 4.19 | 12.7 | 127.6 |
| 2 | C2 | 0.637 | 6.37 | n.d.f | n.d.f | 127.7, 141.2 |
| 3 | C3 | 0.889 | 8.89 | 5.33 | 11.2 | 126.9 |
| 4 | C4 | 0.610 | 6.10 | 5.72 | 15.3 | 128.4 |
| 5 | C5 | 0.652 | 6.52 | 4.85 | 12.8 | 127.4 |
| 6 | C6 | 1.07 | 10.7 | n.d.f | n.d.f | 129.2, 142.8 |
| 7 | C7 | 1.293 | 12.9 | 4.70 | 13.1 | 130.4 |
| 8 | C8 | 0.717 | 7.17 | 7.94 | 20.9 | 133.2 |
2.3 Ethylene/1-hexene and ethylene/1-octene co-polymerization
The co-polymerization of ethylene with 1-hexene or 1-octene was also explored using the representative pre-catalyst C7 (Table 4). According to the results of co-polymerizing ethylene with 1-octene (Runs 1–4, Table 4), the catalytic activities decreased along with increasing co-monomer concentration, whilst polymers with higher molecular weights and broader molecular distributions were formed. The broader molecular distribution and lower activity indicated that termination partly happened during the insertion of 1-octene. The DSC analysis showed the potential for higher branches in the polymers for which the Tm values were lowered.| Run | Monomer | Polym (g) | Activityb | M w c , d | M w/Mnd | T m e/°C |
|---|---|---|---|---|---|---|
| a Conditions: 2 μmol complex C7, Al/Ti = 3000, 60 °C, 30 min, toluene (total volume 100 mL), 10 atm. b 105 g mol−1(Ti) h−1. c 104 g mol−1. d Determined by GPC. e Determined by DSC. f n.d.: not determined due to partly insoluble polymers. | ||||||
| 1 | 0.1 M C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 8 8 |
0.397 | 3.97 | 4.44 | 19.5 | 124.4 |
| 2 | 0.4 M C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 8 8 |
0.315 | 3.15 | 5.17 | 19.2 | 123.7 |
| 3 | 0.7 M C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 8 8 |
0.133 | 1.33 | 10.0 | 30.4 | 121.7 |
| 4 | 1 M C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 8 8 |
0.042 | 0.42 | 20.7 | 58.6 | 122.2 |
| 5 | 0.7 M C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 6 6 |
0.283 | 2.83 | n.d.f | n.d.f | 121.4 |
As a comparison, the co-polymerization of ethylene with 1-hexene was conducted and showed relatively better activity results (Run 5 vs. Run 3, Table 4). The Tm values of the co-polymers of ethylene with 1-hexene were lower, however, partly insoluble polymers were also observed, and so further investigations were not carried out.
To determine the degree of incorporation of 1-olefin in the co-polymers, the 13C NMR spectra of the samples of ethylene with 1-octene (Run 3, Table 4) or 1-hexene (Run 5, Table 4) were measured.16 As shown in Fig. 6, there was 4.22 mol% incorporation of 1-octene, whilst the co-polymer of ethylene/1-hexene contained 10.1 mol% incorporation of 1-hexene (Fig. 7).
 | ||
| Fig. 6 13C NMR spectrum of the ethylene/1-octene co-polymer (Run 3, Table 4). | ||
 | ||
| Fig. 7 13C NMR spectrum of the ethylene/1-hexene co-polymer (Run 5, Table 4). | ||
Thus, the current catalytic system produced co-polymers with higher incorporation of 1-hexene than 1-octene under the same reaction conditions, but with a lower incorporation of 1-olefins than the observation by the analogous half-titanocene pre-catalysts bearing the 2-(1-(arylimino)propyl)quinolin-8-olate ligand set.13b
2.4 Co-polymerization of ethylene with norbornene
In addition, the co-polymerization of ethylene with norbornene was investigated in order to understand the incorporation ability of cyclic olefins by the C7/MAO system; the results are collected in Table 5. As for the observations for the co-polymerization of ethylene with 1-octene, the co-polymerization activities of ethylene with norbornene slowly decreased on increasing the concentration of norbornene, consistent with previous reports in the literature.13b Due to the incorporation of the cyclic monomer, the rigidity of the polymer was increased with slightly different values of Tm, though the GPC analysis showed that the copolymers possessed lower molecular weights and broader molecular distributions.| Run | Monomer | Polym (g) | Activityb | M w c , d | M w/Mnd | T m e/°C |
|---|---|---|---|---|---|---|
| a Conditions: 2 μmol Ti, Al/Ti = 3000, 60 °C, 30 min, toluene (total volume 100 mL), 10 atm. b 105 g mol−1(Ti) h−1. c 104 g mol−1. d Determined by GPC. e Determined by DSC. f n.d.: not determined due to partly insoluble polymers. | ||||||
| 1 | 0.1 M | 0.348 | 3.48 | 9.81 | 24.3 | 125.2 |
| 2 | 0.4 M | 0.303 | 3.03 | 5.49 | 15.4 | 124.4 |
| 3 | 0.7 M | 0.256 | 2.56 | 4.42 | 18.0 | 123.7 |
| 4 | 1.0 M | 0.251 | 2.51 | n.d.f | n.d.f | 121.4, 144.2 |
According to Fig. 8, the 13C NMR spectrum of the ethylene/norbornene co-polymer (Run 3, Table 5) indicated 5.87 mol% incorporation of norbornene.17
 | ||
| Fig. 8 13C NMR spectrum of the ethylene/norbornene co-polymer (Run 3, Table 5). | ||
3. Conclusion
The half-titanocene pre-catalysts (C1–C8) were synthesized and fully characterized. All the titanium pre-catalysts performed with high activities towards the polymerization of ethylene when activated with MAO. The pre-catalysts containing Cp* exhibited higher activities than did those pre-catalysts bearing a Cp group. Substituent effects led to the observed catalytic activity order of C3 (iPr) > C2 (Et) > C1 (Me), C7 (iPr) > C6 (Et) > C5 (Me). Appreciable incorporation of 1-olefin or norbornene (a cyclic olefin) was achieved in co-polymerizations by the pre-catalyst C7, suggesting that the systems described herein have potential for use in varied co-polymerization and in the production of advanced co-polymers. Moreover, the title pre-catalysts exhibited enhanced thermo-stability during catalysis and as such are more appropriate for further industrial consideration compared with their ethyl counterparts.13b4. Experimental section
4.1 General procedures
All manipulations of air and/or moisture-sensitive compounds were conducted under a nitrogen atmosphere in a glovebox or using standard Schlenk techniques. Methylaluminoxane (MAO, 1.46 M in toluene) was purchased from Albemarle. Other reagents were purchased from Beijing Reagent Chemicals, and potassium hydride (KH) was washed with hexane before use to remove contained mineral oil. Toluene, n-hexane, n-heptane and norbornene were refluxed over sodium and benzophenone for 4 h, and then distilled and stored under a nitrogen atmosphere. Dichloromethane (CH2Cl2), 1-hexene, and 1-octene were refluxed over calcium hydride, and then collected and stored over activated molecular sieves (4 Å), while CDCl3 was dried over activated 4 Å molecular sieves. IR spectra were recorded on a Perkin Elmer FT-IR 2000 spectrometer using KBr discs in the range of 4000–400 cm−1. Elemental analysis was performed on a Flash EA 1112 microanalyzer. 1H and 13C NMR spectra were recorded on a Bruker DMX 400 MHz instrument at ambient temperature using TMS as an internal standard. DSC traces of polyethylenes were obtained from the second scanning run on a Perkin-Elmer DSC-7 at a heating rate of 10 °C min−1. 13C NMR spectra of the polymers were recorded on a Bruker DMX-300 MHz instrument at 110 °C in deuterated 1,2-dichlorobenzene with TMS as an internal standard. The molecular weights and molecular weight distributions of the polymers were determined by gel permeation chromatography (GPC) using a Waters Alliance GPC 2000 instrument equipped with a refractive index (RI) detector and a set of u-Styragel HT columns of 106, 105,104, and 103pore sizes in series. The measurement was performed at 135 °C with 1,2,4-trichlorobenzene as the eluent at a flow rate of 0.95 mL min−1. Narrow-molecular-weight PS samples were used as standards for calibration.4.2 Synthesis of 2-(1-(arylimino)ethyl)quinolin-8-olate derivatives (L1–L4)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the second part to elute was collected and concentrated to give a yellow green solid in 81% yield (1.50 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.56 (d, J = 8.6 Hz, 1H, quino-H), 8.25 (d, J = 8.7 Hz, 1H, quino-H), 8.17 (s, 1H, OH), 7.53 (t, J = 7.9 Hz, 1H, quino-H), 7.40 (d, J = 8.2 Hz, 1H, quino-H), 7.23 (d, J = 7.6 Hz, 1H, quino-H), 7.10 (d, J = 7.5 Hz, 2H, Ar–H), 6.97 (t, J = 7.5 Hz, 1H, Ar–H), 2.32 (s, 3H, N
1), the second part to elute was collected and concentrated to give a yellow green solid in 81% yield (1.50 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.56 (d, J = 8.6 Hz, 1H, quino-H), 8.25 (d, J = 8.7 Hz, 1H, quino-H), 8.17 (s, 1H, OH), 7.53 (t, J = 7.9 Hz, 1H, quino-H), 7.40 (d, J = 8.2 Hz, 1H, quino-H), 7.23 (d, J = 7.6 Hz, 1H, quino-H), 7.10 (d, J = 7.5 Hz, 2H, Ar–H), 6.97 (t, J = 7.5 Hz, 1H, Ar–H), 2.32 (s, 3H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 2.06 (s, 6H, Ar–CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 166.6, 154.0, 152.5, 148.8, 137.1, 136.6, 129.1, 128.9, 128.1, 125.4, 123.3, 119.6, 118.0, 110.5, 18.1, 16.5. FT-IR (KBr, cm−1): 3398 (w), 2947 (m), 1638 (m), 1503 (m), 1462 (m), 1243 (s), 1191 (m), 1029 (m), 1018 (s), 865 (m), 792 (s), 750 (m), 723 (m), 585 (m). mp: 82–83 °C. Anal. calcd for C19H18N2O: C, 78.59; H, 6.25; N, 9.65%. Found: C, 78.46; H, 6.33; N, 9.54%.
CCH3), 2.06 (s, 6H, Ar–CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 166.6, 154.0, 152.5, 148.8, 137.1, 136.6, 129.1, 128.9, 128.1, 125.4, 123.3, 119.6, 118.0, 110.5, 18.1, 16.5. FT-IR (KBr, cm−1): 3398 (w), 2947 (m), 1638 (m), 1503 (m), 1462 (m), 1243 (s), 1191 (m), 1029 (m), 1018 (s), 865 (m), 792 (s), 750 (m), 723 (m), 585 (m). mp: 82–83 °C. Anal. calcd for C19H18N2O: C, 78.59; H, 6.25; N, 9.65%. Found: C, 78.46; H, 6.33; N, 9.54%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 1.15 (t, J = 7.5 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 166.4, 154.0, 152.6, 147.8, 137.1, 136.6, 131.1, 129.1, 128.9, 126.2, 123.7, 119.6, 118.0, 110.5, 24.8, 16.9, 13.9. FT-IR (KBr, cm−1): 3440 (m), 3053 (w), 2969 (m), 2933 (m), 2862 (m), 1610 (s), 1563 (s), 1506 (s), 1431 (m), 1335 (s), 1309 (m), 1239 (s), 1199 (s), 1163 (s), 1089 (m), 950 (s), 853 (s), 792 (m), 751 (s), 729 (s), 676 (w), 554 (w). mp: 88–89 °C. Anal. calcd for C21H22N2O: C, 79.21; H, 6.96; N, 8.80%. Found: C, 79.14; H, 7.03; N, 8.68%.
CCH3), 1.15 (t, J = 7.5 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 166.4, 154.0, 152.6, 147.8, 137.1, 136.6, 131.1, 129.1, 128.9, 126.2, 123.7, 119.6, 118.0, 110.5, 24.8, 16.9, 13.9. FT-IR (KBr, cm−1): 3440 (m), 3053 (w), 2969 (m), 2933 (m), 2862 (m), 1610 (s), 1563 (s), 1506 (s), 1431 (m), 1335 (s), 1309 (m), 1239 (s), 1199 (s), 1163 (s), 1089 (m), 950 (s), 853 (s), 792 (m), 751 (s), 729 (s), 676 (w), 554 (w). mp: 88–89 °C. Anal. calcd for C21H22N2O: C, 79.21; H, 6.96; N, 8.80%. Found: C, 79.14; H, 7.03; N, 8.68%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 1.19 (d, J = 6.9 Hz, 12H, Ar–CH(CH3)2). 13C NMR (CDCl3, 100 MHz, ppm): δ 166.5, 154.0, 152.6, 146.5, 137.1, 136.6, 135.7, 129.2, 128.9, 123.9, 123.2, 119.6, 118.0, 110.5, 28.5, 23.3, 23.0, 17.2. FT-IR (KBr, cm−1): 3430 (m), 3065 (m), 2969 (w), 2930 (m), 2868 (s), 1631 (s), 1565 (m), 1505 (m), 1455 (m), 1437 (s), 1333 (m), 1309 (s), 1231 (s), 1190 (s), 1166 (m), 1088 (m), 1062 (m), 959 (m), 871 (s), 785 (m), 760 (w), 749 (m), 721 (m), 662 (w), 552 (w). mp: 153–154 °C. Anal. calcd for C23H26N2O: C, 79.73; H, 7.56; N, 8.09%. Found: C, 79.65; H, 7.58; N, 7.97%.
CCH3), 1.19 (d, J = 6.9 Hz, 12H, Ar–CH(CH3)2). 13C NMR (CDCl3, 100 MHz, ppm): δ 166.5, 154.0, 152.6, 146.5, 137.1, 136.6, 135.7, 129.2, 128.9, 123.9, 123.2, 119.6, 118.0, 110.5, 28.5, 23.3, 23.0, 17.2. FT-IR (KBr, cm−1): 3430 (m), 3065 (m), 2969 (w), 2930 (m), 2868 (s), 1631 (s), 1565 (m), 1505 (m), 1455 (m), 1437 (s), 1333 (m), 1309 (s), 1231 (s), 1190 (s), 1166 (m), 1088 (m), 1062 (m), 959 (m), 871 (s), 785 (m), 760 (w), 749 (m), 721 (m), 662 (w), 552 (w). mp: 153–154 °C. Anal. calcd for C23H26N2O: C, 79.73; H, 7.56; N, 8.09%. Found: C, 79.65; H, 7.58; N, 7.97%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 1.14 (t, J = 6.9 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 166.4 (CH
CCH3), 1.14 (t, J = 6.9 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 166.4 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.4, 152.6, 145.1, 137.0, 136.7, 132.6, 130.8, 129.0, 128.8, 126.7, 120.5, 118.0, 110.5, 24.8, 21.4, 16.9, 13.8. FT-IR (KBr, cm−1): 3442 (O–H) (m), 3041 (m), 2966 (m), 2929 (w), 2845 (m), 1630 (CH
N), 153.4, 152.6, 145.1, 137.0, 136.7, 132.6, 130.8, 129.0, 128.8, 126.7, 120.5, 118.0, 110.5, 24.8, 21.4, 16.9, 13.8. FT-IR (KBr, cm−1): 3442 (O–H) (m), 3041 (m), 2966 (m), 2929 (w), 2845 (m), 1630 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) (s), 1577 (m), 1514 (s), 1428 (s), 1357 (m), 1303 (m), 1246 (m), 1190 (m), 1162 (s), 1076 (m), 934 (s), 852 (s), 791 (m), 757 (w), 722 (m), 665 (w), 592 (w). mp: 121 °C. Anal. calcd for C22H24N2O: C, 79.48; H, 7.28; N, 8.43%. Found: C, 79.35; H, 7.36; N, 8.41%.
N) (s), 1577 (m), 1514 (s), 1428 (s), 1357 (m), 1303 (m), 1246 (m), 1190 (m), 1162 (s), 1076 (m), 934 (s), 852 (s), 791 (m), 757 (w), 722 (m), 665 (w), 592 (w). mp: 121 °C. Anal. calcd for C22H24N2O: C, 79.48; H, 7.28; N, 8.43%. Found: C, 79.35; H, 7.36; N, 8.41%.
4.3 Synthesis and characterization of half-titanocene 2-(1-(arylimino)ethyl)quinolin-8-olate dichlorides (C1–C8)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3). 1H NMR (CDCl3, 100 MHz, ppm): δ 170.3 (CH
CCH3). 1H NMR (CDCl3, 100 MHz, ppm): δ 170.3 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 163.4, 145.0, 141.3, 133.7, 131.3, 129.8, 129.5, 129.2, 128.8, 128.4, 125.5, 120.6, 111.8, 19.7, 17.2, 15.9. Anal. calcd for C24H22Cl2N2OTi: C, 60.91; H, 4.69; N, 5.92%. Found: C, 60.85; H, 4.77; N, 5.85%.
N), 163.4, 145.0, 141.3, 133.7, 131.3, 129.8, 129.5, 129.2, 128.8, 128.4, 125.5, 120.6, 111.8, 19.7, 17.2, 15.9. Anal. calcd for C24H22Cl2N2OTi: C, 60.91; H, 4.69; N, 5.92%. Found: C, 60.85; H, 4.77; N, 5.85%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 1.21 (t, J = 7.5 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 170.5 (CH
CCH3), 1.21 (t, J = 7.5 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 170.5 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 154.2, 153.6, 141.4, 138.9, 137.7, 131.3, 129.6, 129.3, 128.9, 124.2, 121.8, 118.8, 111.2, 24.9, 20.3, 18.7. Anal. calcd for C26H26Cl2N2OTi: C, 62.30; H, 5.23; N, 5.59%. Found: C, 62.24; H, 5.26; N, 5.51%.
N), 154.2, 153.6, 141.4, 138.9, 137.7, 131.3, 129.6, 129.3, 128.9, 124.2, 121.8, 118.8, 111.2, 24.9, 20.3, 18.7. Anal. calcd for C26H26Cl2N2OTi: C, 62.30; H, 5.23; N, 5.59%. Found: C, 62.24; H, 5.26; N, 5.51%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 1.45 (d, J = 6.9 Hz, 6H, Ar–CH(CH3)2), 1.02 (d, J = 6.9 Hz, 6H, Ar–CH(CH3)2). 13C NMR (CDCl3, 100 MHz, ppm): δ 173.7, 167.3, 147.1, 142.9, 140.4, 138.9, 138.0, 133.1, 130.6, 128.4, 128.0, 125.3, 125.0, 120.7, 116.3, 111.8, 27.9, 25.4, 24.8, 20.7. Anal. calcd for C28H30Cl2N2OTi: C, 63.53; H, 5.71; N, 5.29%. Found: C, 63.47; H, 5.79; N, 5.26%.
CCH3), 1.45 (d, J = 6.9 Hz, 6H, Ar–CH(CH3)2), 1.02 (d, J = 6.9 Hz, 6H, Ar–CH(CH3)2). 13C NMR (CDCl3, 100 MHz, ppm): δ 173.7, 167.3, 147.1, 142.9, 140.4, 138.9, 138.0, 133.1, 130.6, 128.4, 128.0, 125.3, 125.0, 120.7, 116.3, 111.8, 27.9, 25.4, 24.8, 20.7. Anal. calcd for C28H30Cl2N2OTi: C, 63.53; H, 5.71; N, 5.29%. Found: C, 63.47; H, 5.79; N, 5.26%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 2.32 (s, 3H, Ar–CH3), 1.19 (t, J = 6.9 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 174.1, 167.3, 146.2, 142.9, 138.0, 137.0, 135.0, 133.2, 130.7, 128.4, 127.8, 125.2, 120.5, 116.4, 111.9, 24.6, 21.2, 19.0, 14.8. Anal. calcd for C27H28Cl2N2OTi: C, 62.93; H, 5.48; N, 5.44%. Found: C, 63.04; H, 5.54; N, 5.35%.
CCH3), 2.32 (s, 3H, Ar–CH3), 1.19 (t, J = 6.9 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 174.1, 167.3, 146.2, 142.9, 138.0, 137.0, 135.0, 133.2, 130.7, 128.4, 127.8, 125.2, 120.5, 116.4, 111.9, 24.6, 21.2, 19.0, 14.8. Anal. calcd for C27H28Cl2N2OTi: C, 62.93; H, 5.48; N, 5.44%. Found: C, 63.04; H, 5.54; N, 5.35%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 2.29 (s, 15H, Cp*). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.1, 153.1, 152.4, 148.4, 137.0, 136.7, 129.0, 128.8, 128.0, 125.2, 123.2, 120.3, 118.0, 110.4, 23.1, 19.7, 17.2, 15.9, 12.8. Anal. calcd for C29H32Cl2N2OTi: C, 64.10; H, 5.94; N, 5.16%. Found: C, 64.04; H, 6.01; N, 5.12%.
CCH3), 2.29 (s, 15H, Cp*). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.1, 153.1, 152.4, 148.4, 137.0, 136.7, 129.0, 128.8, 128.0, 125.2, 123.2, 120.3, 118.0, 110.4, 23.1, 19.7, 17.2, 15.9, 12.8. Anal. calcd for C29H32Cl2N2OTi: C, 64.10; H, 5.94; N, 5.16%. Found: C, 64.04; H, 6.01; N, 5.12%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 2.30 (s, 15H, Cp*), 2.17–2.10 (m, 2H, Ar–CH2CH3), 1.22 (t, J = 7.5 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.5, 153.3, 152.5, 147.5, 137.1, 136.7, 130.9, 129.0, 128.9, 125.9, 123.5, 120.4, 118.0, 110.4, 24.9, 20.3, 18.7, 12.9. Anal. calcd for C31H36Cl2N2OTi: C, 65.16; H, 6.35; N, 4.90%. Found: C, 65.13; H, 6.38; N, 4.89%.
CCH3), 2.30 (s, 15H, Cp*), 2.17–2.10 (m, 2H, Ar–CH2CH3), 1.22 (t, J = 7.5 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.5, 153.3, 152.5, 147.5, 137.1, 136.7, 130.9, 129.0, 128.9, 125.9, 123.5, 120.4, 118.0, 110.4, 24.9, 20.3, 18.7, 12.9. Anal. calcd for C31H36Cl2N2OTi: C, 65.16; H, 6.35; N, 4.90%. Found: C, 65.13; H, 6.38; N, 4.89%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 2.28 (s, 15H, Cp*), 1.23 (d, 6H, J = 7.6 Hz, Ar–CH(CH3)2), 1.15 (d, 6H, J = 7.4 Hz, Ar–CH(CH3)2). 13C NMR (CDCl3, 100 MHz, ppm): δ 167.3, 153.3, 152.5, 146.2, 137.1, 136.7, 130.8, 129.0, 128.9, 126.7, 123.0, 120.6, 118.0, 110.4, 27.9, 25.4, 24.8, 20.7, 12.8. Anal. calcd for C33H40Cl2N2OTi: C, 66.12; H, 6.73; N, 4.67%. Found: C, 66.06; H, 6.84; N, 4.59%.
CCH3), 2.28 (s, 15H, Cp*), 1.23 (d, 6H, J = 7.6 Hz, Ar–CH(CH3)2), 1.15 (d, 6H, J = 7.4 Hz, Ar–CH(CH3)2). 13C NMR (CDCl3, 100 MHz, ppm): δ 167.3, 153.3, 152.5, 146.2, 137.1, 136.7, 130.8, 129.0, 128.9, 126.7, 123.0, 120.6, 118.0, 110.4, 27.9, 25.4, 24.8, 20.7, 12.8. Anal. calcd for C33H40Cl2N2OTi: C, 66.12; H, 6.73; N, 4.67%. Found: C, 66.06; H, 6.84; N, 4.59%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), 2.36 (s, 15H, Cp*), 2.34 (s, 3H, Ar–CH3), 2.32–2.26 (m, 2H, Ar–CH2CH3), 1.12 (t, J = 7.5 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.7, 153.5, 152.5, 145.0, 137.1, 136.7, 132.6, 130.8, 129.0, 128.8, 126.7, 120.5, 118.0, 110.4, 24.6, 21.2, 19.0, 14.8, 12.8. Anal. calcd for C32H38Cl2N2OTi: C, 65.65; H, 6.54; N, 4.79%. Found: C, 65.62; H, 6.59; N, 4.71%.
CCH3), 2.36 (s, 15H, Cp*), 2.34 (s, 3H, Ar–CH3), 2.32–2.26 (m, 2H, Ar–CH2CH3), 1.12 (t, J = 7.5 Hz, 6H, Ar–CH2CH3). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.7, 153.5, 152.5, 145.0, 137.1, 136.7, 132.6, 130.8, 129.0, 128.8, 126.7, 120.5, 118.0, 110.4, 24.6, 21.2, 19.0, 14.8, 12.8. Anal. calcd for C32H38Cl2N2OTi: C, 65.65; H, 6.54; N, 4.79%. Found: C, 65.62; H, 6.59; N, 4.71%.
4.4 Procedures for ethylene polymerization and co-polymerization
A 0.25 L stainless steel autoclave equipped with a mechanical stirrer and a temperature controller was heated in a vacuum at 80 °C and recharged with ethylene three times, then cooled to room temperature under an ethylene atmosphere. A toluene solution of the pre-catalyst (with co-monomer) was transferred into the reactor. After the desired reaction temperature was reached, the required amount of the co-catalyst was added with maintaining the total volume at 100 mL, and then the autoclave was immediately pressurized to 10 atm. The ethylene pressure was kept constant throughout the feeding of ethylene during the reaction. After a set period, the feeding of the ethylene was stopped, and then the autoclave was placed in a water–ice bath for 1 h. The resultant mixture was poured into 10% HCl–ethanol solution, and the polymer formed was collected and washed with water and ethanol several times, and then dried under vacuum to a constant weight.4.5 X-Ray structure determinations
Crystals of C2 and C4 suitable for single-crystal X-ray analysis were obtained by laying n-heptane on the dichloromethane solutions. Single-crystal X-ray diffraction for C2 and C4 was performed on a Rigaku RAXIS Rapid IP diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 173(2) K. Cell parameters were obtained by global refinement of the positions of all collected reflections. Intensities were corrected for Lorentz and polarization effects and empirical absorption. The structures were solved by direct methods and refined by full-matrix least-squares on F2. All non-hydrogen atoms were refined anisotropically. Structure solution and refinement were performed by using the SHELXL-97 package.18Crystal data collection and refinement details are given in Table 6.| C2 | C4 | |
|---|---|---|
| Empirical formula | C26H26Cl2N2OTi | C27H28Cl2N2OTi |
| Formula weight | 501.29 | 515.31 |
| Crystal color | Red | Red |
| Temperature/K | 173(2) | 173(2) |
| Wavelength/Å | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P2(1)/c |
| a/Å | 23.852(5) | 17.098(3) |
| b/Å | 13.832(3) | 8.1446(16) |
| c/Å | 16.890(3) | 17.624(4) |
| α/° | 90 | 90 |
| β/° | 118.68(3) | 98.70(3) |
| γ/° | 90 | 90 |
| Volume/Å3 | 4888.6(17) | 2426.0(8) |
| Z | 8 | 4 |
| D calc./mg m−3 | 1.362 | 1.411 |
| μ/mm−1 | 0.590 | 0.596 |
| F(000) | 2080 | 1072 |
| Crystal size/mm | 0.20 × 0.15 × 0.09 | 0.20 × 0.13 × 0.09 |
| θ Range/° | 1.76–25.50 | 1.20–27.47 |
| Limiting indices | −22 ≤ h ≤ 28, −16 ≤ k ≤ 16, −20 ≤ l ≤ 19 | −17 ≤ h ≤ 22, −10 ≤ k ≤ 7, −22 ≤ l ≤ 22 |
| No. of rflns collected | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075 075 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063 063 |
| No. of unique rflns | 4502 | 5545 |
| R int | 0.0486 | 0.0485 |
| Completeness to θ (%) | 98.8(θ = 25.50) | 99.6(θ = 27.47) |
| Goodness-of-fit on F2 | 1.191 | 1.241 |
| Final R indices [I > 2σ (I)] |
R1 = 0.0673,
wR2 = 0.1805 |
R1 = 0.0730,
wR2 = 0.1774 |
| R indices (all data) |
R1 = 0.0780,
wR2 = 0.1862 |
R1 = 0.0789,
wR2 = 0.1850 |
Acknowledgements
This work is supported by NSFC No. 20874105 and 20904059 as well as the MOST 863 program No. 2009AA034601. The EPSRC are thanked for the award of a travel grant (to CR).References
- (a) B. Heurtefeu, C. Bouilhac, É. Cloutet, D. Taton, A. Deffieux and H. Cramail, Prog. Polym. Sci., 2011, 36, 89 CrossRef CAS; (b) H. Makio, H. Terao, A. Iwashita and T. Fujita, Chem. Rev., 2011, 111, 2363 CrossRef CAS; (c) K. Nomura and S. Zhang, Chem. Rev., 2011, 111, 2342 CrossRef CAS; (d) C. Bianchini, G. Giambastiani, L. Luconi and A. Meli, Coord. Chem. Rev., 2010, 254, 431 CrossRef CAS; (e) S. Liu, W. Zuo, S. Zhang, P. Hao, D. Wang and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3411 CrossRef CAS; (f) W. Zuo, W.-H. Sun, S. Zhang, P. Hao and A. Shiga, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3415 CrossRef CAS; (g) P. Hu, F. Wang and G.-X. Jin, Organometallics, 2011, 30, 1008 CrossRef CAS; (h) Y. Long, W. Ye, X. Shi and Y.-S. Li, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6072 CrossRef CAS; (i) C. Wang, Z. Ma, X. Sun, Y. Gao, Y. Guo, Y. Tang and L. Shi, Organometallics, 2006, 25, 3259 CrossRef CAS; (j) H. Makio, N. Kashiwa and T. Fujita, Adv. Synth. Catal., 2002, 344, 477 CrossRef CAS.
- (a) J. Yu, H. Liu, W. Zhang, X. Hao and W.-H. Sun, Chem. Commun., 2011, 47, 3257 RSC; (b) V. C. Gibson, C. Redshaw and G. A. Solan, Chem. Rev., 2007, 107, 1745 CrossRef CAS; (c) V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283 CrossRef CAS; (d) S. D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169 CrossRef CAS; (e) G. J. P. Britovsek, V. C. Gibson and D. F. Wass, Angew. Chem., Int. Ed., 1999, 38, 428 CrossRef CAS.
- (a) J. C. Stevens, F. J. Timmers, D. R. Wilson, G. F. Schmidt, P. N. Nickias, R. K. Rosen, G. W. McKnight and S. Lai, (Dow). Eur. Pat. Appl., 0416815A2, 1991 Search PubMed; (b) J. A. M. Canich, (Exxon). U. S. Patent, 5026798, 1991 Search PubMed; (c) A. L. McKnight and R. M. Waymouth, Chem. Rev., 1998, 98, 2587 CrossRef CAS; (d) P. J. Shapiro, W. D. Cotter, W. P. Schaefer, J. A. Labinger and J. E. Bercaw, J. Am. Chem. Soc., 1994, 116, 4623 CrossRef CAS; (e) P. J. Shapiro, E. Bunel, W. P. Schaefer and J. E. Bercaw, Organometallics, 1990, 9, 867 CrossRef CAS; (f) Y. Zhang, Y. Mu, C. Lü, G. Li, J. Xu, Y. Zhang, D. Zhu and S. Feng, Organometallics, 2004, 23, 540 CrossRef CAS; (g) Y. Zhang, J. Wang, Y. Mu, Z. Shi, C. Lü, Y. Zhang, L. Qiao and S. Feng, Organometallics, 2003, 22, 3877 CrossRef CAS.
- (a) K. Nomura, S. Hasumi, M. Fujiki and K. Itagaki, Dalton Trans., 2009, 9052 RSC; (b) K. Nomura, Dalton Trans., 2009, 8811 RSC; (c) K. Itagaki, M. Fujiki and K. Nomura, Macromolecules, 2007, 40, 6489 CrossRef CAS; (d) H. Zhang, D. J. Byun and K. Nomura, Dalton Trans., 2007, 18, 1802 RSC.
- S. Doherty, R. J. Errington, A. P. Jarvis, S. Collins, W. Clegg and M. R. J. Elsegood, Organometallics, 1998, 17, 3408 CrossRef CAS.
- (a) J. Richter, F. T. Edelmann, M. Noltemeyer, H.-G. Schmidt, M. Schmulinson and M. S. Eisen, J. Mol. Catal. A: Chem., 1998, 130, 149 CrossRef CAS; (b) R. Vollmerhaus, P. Shao, N. J. Taylor and S. Collins, Organometallics, 1999, 18, 2731 CrossRef CAS; (c) L. R. Sita and R. Babcock, Organometallics, 1998, 17, 5228 CrossRef CAS; (d) K. C. Jayaratne, R. J. Keaton, D. A. Henningsen and L. R. Sita, J. Am. Chem. Soc., 2000, 122, 10490 CrossRef CAS.
- (a) A. van der Linden, C. J. Schaverien, N. Meijboom, C. Ganter and A. G. Orpen, J. Am. Chem. Soc., 1995, 117, 3008 CrossRef CAS; (b) S. Fokken, T. P. Spaniol, H.-C. Kang, W. Massa and J. Okuda, Organometallics, 1996, 15, 5069 CrossRef CAS; (c) K. Nomura, N. Naga, M. Miki, K. Yanagi and A. Imai, Organometallics, 1998, 17, 2152 CrossRef CAS; (d) K. Nomura, N. Naga, M. Miki and K. Yanagi, Macromolecules, 1998, 31, 7588 CrossRef CAS.
- (a) R. K. J. Bott, D. L. Hughes, M. Schomann, M. Bochmann and S. J. Lancaster, J. Organomet. Chem., 2003, 665, 135 CrossRef CAS; (b) J. Huang, B. Lian, Y. Qian, W. Zhou, W. Chen and G. Zheng, Macromolecules, 2002, 35, 4871 CrossRef CAS.
- K. Nomura, J. Liu, S. Padmanabhan and B. Kitiyanan, J. Mol. Catal. A: Chem., 2007, 267, 1 CrossRef CAS.
- (a) T. Yasumoto, T. Yamagata and K. Mashima, Organometallics, 2005, 24, 3375 CrossRef CAS; (b) T. Yasumoto, T. Yamagata and K. Mashima, Chem. Lett., 2007, 1030 CrossRef CAS.
- (a) H. Makio, T. Ochiai, H. Tanaka and T. Fujita, Adv. Synth. Catal., 2010, 352, 1635 CrossRef CAS; (b) H. Makio and T. Fujita, Acc. Chem. Res., 2009, 42, 1532 CrossRef CAS; (c) T. Matsugi and T. Fujita, Chem. Soc. Rev., 2008, 37, 1264 RSC; (d) A. Sakuma, M.-S. Weiser and T. Fujita, Polym. J. (Tokyo), 2007, 39, 193 CAS; (e) S. Matsui, Y. Tohi, M. Mitani, J. Saito, H. Makio, H. Tanaka, M. Nitabaru, T. Nakano and T. Fujita, Chem. Lett., 1999, 1065 CrossRef CAS; (f) S. Matsui, M. Mitani, J. Saito, Y. Tohi, H. Makio, N. Matsukawa, Y. Takagi, K. Tsuru, M. Nitabaru, T. Nakano, H. Tanaka, N. Kashiwa and T. Fujita, J. Am. Chem. Soc., 2001, 123, 6847 CrossRef CAS; (g) J. B. Edson, Z. Wang, E. J. Kramer and G. W. Coates, J. Am. Chem. Soc., 2008, 130, 4968 CrossRef CAS; (h) J. Tian, P. D. Hustad and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 5134 CrossRef CAS; (i) J. Tian and G. W. Coates, Angew. Chem., Int. Ed., 2000, 39, 3626 CrossRef CAS.
- W.-H. Sun, M. Shen, W. Zhang, W. Huang, S. Liu and C. Redshaw, Dalton Trans., 2011, 40, 2645 RSC.
- (a) W. Huang, W. Zhang, S. Liu, T. Liang and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1887 CrossRef CAS; (b) W. Huang, W.-H. Sun and C. Redshaw, Dalton Trans., 2011, 40, 6802 RSC.
- (a) W. Zuo, S. Zhang, S. Liu, X. Liu and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3396 CrossRef CAS; (b) W. Zuo, M. Zhang and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 357–372 CrossRef CAS; (c) S. Liu, J. Yi, W. Zuo, K. Wang, D. Wang and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3154 CrossRef CAS; (d) W.-H. Sun, S. Liu, W. Zhang, Y. Zeng, D. Wang and T. Liang, Organometallics, 2010, 29, 732 CrossRef CAS; (e) S. Liu, W.-H. Sun, Y. Zeng, D. Wang, W. Zhang and Y. Li, Organometallics, 2010, 29, 2459 CrossRef CAS.
- (a) E. Y. X. Chen and T. J. Marks, Chem. Rev., 2000, 100, 1391 CrossRef CAS; (b) S. Koltzenburg, J. Mol. Catal. A: Chem., 1997, 116, 355 CrossRef CAS.
- M. R. Seger and G. E. Maciel, Anal. Chem., 2004, 76, 5734 CrossRef CAS.
- (a) D. Ruchatz and G. Fink, Macromolecules, 1998, 31, 4674 CrossRef CAS; (b) I. Tritto, C. Marestin, L. Boggioni, L. Zetta, A. Provasoli and D. R. Ferro, Macromolecules, 2000, 33, 8931 CrossRef CAS; (c) R. A. Wendt, R. Mynott, K. Hauschild, D. Ruchatz and G. Fink, Macromol. Chem. Phys., 1999, 200, 1340 CrossRef CAS; (d) A. Provasoli, D. R. Ferro, I. Tritto and L. Boggioni, Macromolecules, 1999, 32, 6697 CrossRef CAS.
- M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structure, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † CCDC reference numbers 822119 and 822120 for complexes C2 and C4. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c1cy00193k |
| This journal is © The Royal Society of Chemistry 2011 |
