Thiotolerant Ir/SiO2–Al2O3 bifunctional catalysts: effect of support acidity on tetralin hydroconversion
Salim
Nassreddine
a,
Santiago
Casu
a,
José Luiz
Zotin
b,
Christophe
Geantet
a and
Laurent
Piccolo
*a
aInstitut de recherches sur la catalyse et l'environnement de Lyon (IRCELYON), UMR 5256 CNRS & Université Lyon 1, 2 avenue Albert Einstein, F-69626 Villeurbanne, France. E-mail: laurent.piccolo@ircelyon.univ-lyon1.fr; Fax: +33 472445399
bPETROBRAS S.A.-R&D Center-Av. Horácio Macedo, 950-Cidade Universitária-Ilha do Fundão, 21941-915, Rio de Janeiro, RJ, Brazil
First published on 21st February 2011
Abstract
Tetralin hydroconversion over supported iridium catalysts has been investigated under a pressure of 4 MPa in the presence of H2S in a continuous high-pressure gas-phase microreactor. Decalin, naphthalene, ring-contraction bicyclic products and one-ring-opening products are formed. A screening of silica, alumina and amorphous silica–alumina (ASA) supports demonstrates that only ASA provides thiotolerance and ring opening/contraction selectivity to iridium nanoparticles. By testing Ir/ASA catalysts with various silica–alumina ratios but similar Ir particle size (1.5 nm), it is shown that the intermediate concentration of silica (40 wt%) leads to the highest activity and selectivity, in correlation to the Brönsted acidity measured by infrared spectroscopy of adsorbed pyridine.
1. Introduction
Emission criteria and energy consumption require new technologies for internal combustion engines and production of high-quality fuels. Thus, gasoline and diesel regulations are becoming stricter, with low sulfur and aromatic contents. In the case of diesel, cetane number (CN), which measures the fuel combustion efficiency, will probably increase in the next years. This ever growing demand of high-quality diesel, especially in Europe, gave birth to new upgrading technologies such as aromatics saturation (ASAT).1 An additional route, complementary to ASAT, has been proposed, the so-called selective ring opening (SRO) route.2,3 The chemistry of SRO refers to several combined catalytic steps and requires balanced metallic and acidic functions to achieve optimal performances, i.e., reduce the number of rings while retaining the number of carbon atoms of the reactant molecule. In theory, the SRO reaction may lead to very high CN but in practice, it is often limited to the opening of one ring, which in some cases may bring a dismal loss of CN.4 In fact, there has been a controversy on the identification of SRO products when bicyclic hydrocarbons are used,5 that we have recently clarified.6Cracking catalysts like zeolites have been widely studied for SRO but they can generate non-selective cracking, fast deactivation and pore restrictions.7–11 It has been shown that addition of a noble metal like Pt to acidic materials reduces the strength of Brönsted acid sites (BAS) and significantly enhances isomerization and ring opening of decalin.12 Among acidic supports, amorphous silica–aluminas (ASA) are widely used in petroleum refining, where they are important components of hydrocracking catalysts.13 These solids, in which combined Lewis and Brönsted acid properties can be tuned by the silica content, have consequently attracted much interest.14–17 Recent works demonstrate that ASA contain BAS of zeolitic strength, the lower overall acidity of ASA originating only from their much lower BAS concentration with respect to zeolites.18–22 ASA were recently used in combination with Pt for hydrogenation and hydrodenitrogenation purposes.23,24
We have focused on Ir/ASA catalytic properties towards tetralin hydroconversion in the presence of sulfur. In a previous study, we have investigated the effect of Ir dispersion and loading over an ASA support with a fixed Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio.25 In the present study, we investigate the effect of ASA composition and acidity at fixed Ir dispersion and loading.
Al ratio.25 In the present study, we investigate the effect of ASA composition and acidity at fixed Ir dispersion and loading.
2. Experimental
2.1 Materials and catalyst preparation
Amorphous silica–aluminas (ASA, commercial name SIRAL-x) with different compositions (x = 5 to 70 wt% silica) were supplied by Sasol (formerly Condea), following a patented preparation procedure.17,26 The powders received in hydrated form were activated by heating at 550 °C in air for 3 h. It resulted in the dehydration of the powder and the transformation of the alumina part from AlO(OH) (boehmite) to γ-Al2O3. The characteristics of the SIRAL supports are summarized in Table 1. Pure silica (540 m2 g−1) and γ-alumina (260 m2 g−1) were used as references.| Sample | SiO2 content (wt%) nominal/ICP |
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al atomic ratio nominal/ICP Al atomic ratio nominal/ICP |
Loose bulk density/g L−1 | Average particle size/μm | BET surface area/m2 g−1 | Pore volume/mL g−1 | Average pore diameter/nm |
|---|---|---|---|---|---|---|---|
| SIRAL-5 | 5/5 | 0.045/0.045 | 450–650 | 50 | 370 | 0.70 | 6.6 |
| SIRAL-10 | 10/10 | 0.094/0.094 | 400–600 | 50 | 400 | 0.75 | 6.1 |
| SIRAL-30 | 30/23 | 0.36/0.25 | 250–450 | 50 | 470 | 0.80 | 6.3 |
| SIRAL-40 | 40/36 | 0.57/0.48 | 250–450 | 50 | 500 | 0.90 | 6.4 |
| SIRAL-70 | 70/69 | 2.0/1.9 | 570 | 12 | 386 | 0.27 | 3.6 |
The catalysts were prepared by incipient wetness impregnation of the supports with Ir acetylacetonate (Ir(acac)3, Sigma-Aldrich, purity 97%) dissolved in toluene, using the concentration needed to obtain an Ir loading of 1.0 wt%. After impregnation, the samples were dried at 120 °C overnight and reduced in H2 flow at 350 °C for 6 h. Prior to each activity measurement, the samples were reduced in situ in H2 flow for 2 h at 350 °C. For Ir/ASA, we have shown that the catalysts must be prepared by direct H2reduction of Ir(acac)3-impregnated ASA to avoid particle agglomeration.27
2.2 Materials characterization techniques
The metal contents of the catalysts and the Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratios in ASA were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES, Activa-Horiba Jobin Yvon). The size distribution of Ir nanoparticles was determined by high-resolution transmission electron microscopy (HRTEM, Jeol JEM 2010). Size histograms were calculated from the statistical treatment of the TEM micrographs of carbon-replicated samples, by analyzing more than 300 particles.
Al ratios in ASA were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES, Activa-Horiba Jobin Yvon). The size distribution of Ir nanoparticles was determined by high-resolution transmission electron microscopy (HRTEM, Jeol JEM 2010). Size histograms were calculated from the statistical treatment of the TEM micrographs of carbon-replicated samples, by analyzing more than 300 particles.
The acidity of the catalysts was analyzed by FTIR absorption spectroscopy of adsorbed pyridine. The samples were pressed into thin self-supported wafers (ca. 25 mg). They were evacuated in the sample holder down to secondary vacuum while they were heated at 350 °C (1 h). A background spectrum of the evacuated samples was then recorded. Pyridine was introduced at RT for 15 min at saturated vapor pressure. The samples were then evacuated (1 h) to the 10−6 Torr range in order to desorb weakly bonded pyridine. IR spectra were recorded at RT using a Perkin Elmer FTIR 1760 spectrometer (DTGS detector, spectral resolution 2 cm−1, 64 scans, transmission mode). Before spectrum acquisition, the wafers were also subjected to thermal treatments at 150, 250 or 350 °C for 1 h to analyze the acid site strength. Spectral bands at ca. 1545 and 1450 cm−1 (ν19bpyridine vibration mode)15 were used to identify Brönsted and Lewis acid sites, respectively.
2.3 Catalytic testing and product identification
Experiments were carried out in a gas-phase flow fixed bed catalytic micro-reactor described in details elsewhere.25 The standard reaction conditions for tetralin hydroconversion were: H2 4 MPa, tetralin 6 kPa, H2S 100 ppm, 350 °C.The gas composition at the reactor outlet was determined by on-line gas sampling and gas chromatography (GC-FID, HP-1 column). More than 50 isomers of C10 aromatic and saturated compounds were formed and could hardly be separated and identified, except by careful ex situ analysis by GC × GC/MS.5,6 To facilitate the presentation of catalysis results, reaction products issued from one-ring opening (alkyl-benzenes, alkyl-cyclohexanes, alkyl-cyclopentanes) and ring contraction (mainly methyl-indans, bicyclo-nonanes and bicyclo-octanes) were gathered in a same family, so-called ROCP (ring opening and contraction products). The ROCP distribution is analyzed in details elsewhere.6,25 Other products were hydrogenation products (cis and trans decalins) and a dehydrogenation product (naphthalene). Hydrocarbons with more (alkylation products) or less (cracking products) than 10 carbons were formed in negligible amounts. The quasi absence of cracking products results in a high catalyst stability.25
3. Results and discussion
3.1 Catalyst characterization
The characterization data (ICP-OES and TEM) relative to the Ir-based catalysts used in the present study are gathered together in Table 2. Iridium has been deposited on silica, alumina and ASA (SIRAL-x denotes a Sasol's ASA support with x wt% silica, see Table 1 and Section 2.1) by incipient wetness impregnation, using Ir(acac)3 as a precursor. The target metal loading, 1 wt%, has been reached in most cases. Fig. 1 shows a TEM micrograph for Ir/SIRAL-30. A similar mean particle size of ca. 1.4 nm is observed on all alumina-containing substrates. On pure silica, a bimodal size distribution including a large proportion of big particles of ca. 8 nm is obtained. Particle size is an important parameter since we have observed that the catalytic hydroconversion properties of Ir/ASA depend on Ir dispersion.25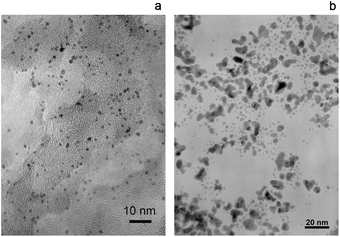 | ||
| Fig. 1 TEM images of Ir nanoparticles from Ir/SIRAL-30 (a) and Ir/SiO2 (b) samples. | ||
3.2 Effect of support identity and sulfur concentration on catalytic properties
Fig. 2 allows one to compare silica, alumina and ASA-supported Ir catalysts in terms of product yields and resistance to sulfur during tetralin hydroconversion. In this experiment, we have tuned the sample masses in order to obtain, for each catalyst, a conversion slightly lower than 100%. This way, the impact of H2S addition to the feed could be followed under optimal conditions.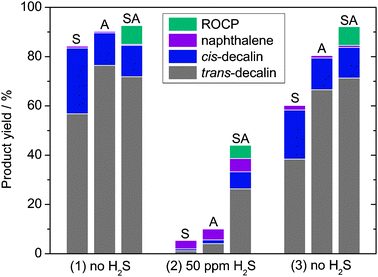 | ||
| Fig. 2 Effect of H2S addition and removal on product yields for tetralin hydroconversion at 350 °C on Ir nanoparticles supported on silica (S, 160 mg), alumina (A, 50 mg) and SIRAL-40 silica–alumina (SA, 16 mg). “ROCP” stands for ring opening/contraction products. | ||
The selectivity to ring opening/contraction products (ROCP) is zero for Ir on silica and alumina. Mostly hydrogenation products (decalins) are formed. When H2S is added to the feed, the conversions vanish from 84% to 5% and from 90 to 10% for Ir on silica and alumina, respectively. Going back to sulfur-free conditions, the conversions increase to 60 and 81% for silica and alumina, respectively. Overall, Ir/Al2O3 appears much more active (4 times less catalyst was used) and a little more resistant to sulfur than Ir/SiO2. The lower activity is due to the lower dispersion observed in the case of silica (Table 2). Note that Ir nanoparticles supported on zirconia or ceria have also been found poorly selective under sulfur-free conditions and inactive in the presence of sulfur.28
It is known that the interaction between metals and acidic supports favors thiotolerance, the electron transfer from the metal to the support weakening the metal–sulfur bond.3 As a matter of fact, in the case of Ir/ASA (SIRAL-40 support), the conversion only decreases from 93 to 44% when H2S is added to the feed. Moreover, the conversion rate is totally recovered when H2S is removed from the feed.
Under the experimental conditions used for Fig. 2, the ROC selectivity for Ir/ASA (SIRAL-40 support) is 8% without H2S and 12% with 50 ppm H2S. ROC selectivity is only slightly sensitive to the presence of sulfur.25 One may observe that in the present case the selectivity remains low.† However, by decreasing the metal loading or increasing the particle size via a sintering procedure, it is possible to reach ROC selectivities of 50%.25 This decrease of the metal/acid site ratio affects the absolute selectivity, but not the selectivity vs. acidity trends, which are reported in the present article (see below) for standard 1 wt% Ir/ASA catalysts. The stability of these catalysts with time-on-stream in the presence of up to 200 ppm H2S is excellent. Their activities and selectivities are stable over days, and no measurable cracking is evidenced at the steady state. Even for the abovementioned more selective catalysts, the steady-state cracking selectivity never exceeded 7%.25
Besides, Fig. 3 shows that the catalytic behavior towards sulfur content is not significantly affected by the Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio in ASA. Indeed, in the 50–200 ppm H2S concentration range, H2S pseudo-orders for the Ir/SIRAL-X series are comprised between −0.50 and −0.43.‡
Al ratio in ASA. Indeed, in the 50–200 ppm H2S concentration range, H2S pseudo-orders for the Ir/SIRAL-X series are comprised between −0.50 and −0.43.‡
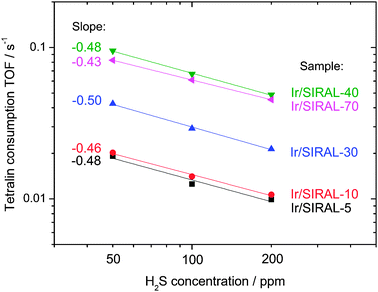 | ||
| Fig. 3 Effect of the H2S content of the reaction feed on the tetralin consumption rate, expressed as a turnover frequency over surface metal atoms (350 °C, 50 mg of catalyst). | ||
One should be aware that ASA alone is very poorly active for tetralin hydroconversion. Indeed, the conversion of tetralin on bare SIRAL-40 was only 7% at 350 °C without H2S (versus 100% for the same mass of Ir/ASA), and the yields for decalins, naphthalene and ROCP were 2%, 3% and 2%, respectively.
3.3 Link between ASA composition, Ir/ASA surface acidity and catalytic properties
FTIR spectroscopy of the SIRAL-5 and SIRAL-10 after evacuation at 350 °C (not shown) evidences a band at 3725 cm−1, assigned to isolated Al–OH groups.17,18 For SIRAL-30, 40 and 70, this band is replaced by a sharper one at 3743–3745 cm−1, associated to isolated Si–OH groups (silanols).16–19 Thus, for 30 wt% silica and above, the surface appears silica-rich.The acidity of the Ir/ASA series has been analyzed by FTIR spectroscopy of adsorbed pyridine. The spectra for Ir/SIRAL-40 after desorption of pyridine at various temperatures are shown in Fig. 4. The total numbers of Lewis acid sites (LAS, electron acceptors) and Brönsted acid sites (BAS, proton donors) are proportional to 1450 cm−1 and 1545 cm−1 peak areas, respectively.15Fig. 5 shows the so-calculated numbers of LAS (top) and BAS (bottom) as a function of the silica content of ASA.§
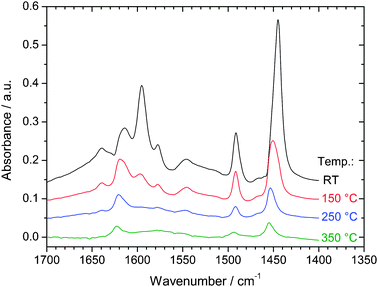 | ||
| Fig. 4 FTIR spectra for Ir/SIRAL-40 after pyridine adsorption and evacuation at various temperatures. | ||
 | ||
| Fig. 5 Relative amounts of Lewis (a) and Brönsted (b) acid sites determined for various support compositions of Ir/ASA after desorption of pyridine at three temperatures. | ||
The number of LAS increases with alumina content, as expected.17 Getting into more details, the IR band at ca. 1620 cm−1 can be decomposed into a contribution at 1622 cm−1 associated to strong tetrahedral LAS and one at 1615 cm−1 associated to medium octahedral LAS.18 Similarly, the frequency shift of the band from 1450 to 1455 cm−1 as pyridine desorption temperature increases from 150 to 350 °C, and the non-disappearance of the 1455 cm−1 band at 350 °C (Fig. 4) imply that both weak and strong LAS are present on SIRAL-40.18
In alumina-rich SIRAL, the LAS are Al3+ coordinately unsaturated sites at surface octahedral lattice positions (medium LAS), generated by the partial dehydroxylation of alumina during the pre-calcination at 550 °C.17 The addition of silica leads to substitution of Si4+ ions by Al3+ ions at tetrahedral lattice positions, giving birth to an aluminosilicate phase of increased acidity at intermediate compositions. However, as the silica content increases, SIRAL would consist of alumina particles gradually encapsulated by pure silica, as shown by XPS,17 and in agreement with Crépeau et al.18 Since the most abundant LAS are located on the alumina phase of SIRAL, their decreasing amount when silica content increases can qualitatively explain the results of Fig. 5a.
The number of BAS increases with silica concentration and reaches a maximum around 40 wt%, in agreement with the results of Daniell et al., who have studied the acidity of the SIRAL product range by CO-FTIR.17 However, in their case, as the silica content further increases (SIRAL-60 and above), the Brönsted acidity vanishes and approximates that of pure silica. In contrast, the Brönsted acidity of our SIRAL-70, as measured after pyridine desorption at 150 °C, is only slightly lower than that of SIRAL-40 (Fig. 5b).
The BAS are generated by introduction of aluminium atoms in the silica lattice. The resulting negative charge is compensated by the formation of protons. The BAS would be of two types over SIRAL: terminal silanol-like groups with moderate acidity on the silica phase and zeolite-like bridged Si–(OH)–Al groups with stronger acidity on the aluminosilicate phase,19,21,22 the amount of which is maximal at the SIRAL-40 surface.17 Strongly acid silanols in the vicinity of aluminium atoms have also been proposed.16,18,20
In Fig. 6, the ASA supports are compared for a wide range of compositions, with respect to tetralin hydroconversion activity and ROC selectivity (350 °C, 100 ppm H2S, 50% tetralin conversion). Both activity and selectivity increase with silica concentration up to 40 wt% of silica, i.e., they are maximal for Ir/SIRAL-40 (14% selectivity), then decrease for Ir/SIRAL-70. In Fig. 6 is also reported the total amount of Brönsted acid sites, as measured after pyridine desorption at 150 °C (extracted from Fig. 5b). A clear correlation between Brönsted acidity and ROC selectivity is evidenced. As isomerization products (RCP) are the most abundant ROCP,25 this suggests that, similar to the results for e.g.isomerization of o-xylene14 and 1-butene16 on silica–alumina, the isomerization processes in tetralin hydroconversion are Brönsted acid-catalyzed by ASA. Following the recent spectroscopic results of Hensen and coworkers,21,22 it is probable that the activity of ASA for isomerizing tetralin derivatives arises from a small concentration of strongly protonating hydroxyl groups located between aluminium and silicon-occupied oxygen tetrahedra. The above results are consistent with our metal dispersion-dependent data, showing that the selectivity to ROCP increases when the number of metal sites decreases.25
 | ||
| Fig. 6 Ring opening/contraction selectivity, total tetralin consumption activity (350 °C, 100 ppm H2S, 50% conversion, 50 mg of catalyst) and Brönsted acidity versus the silica content of Ir-based catalysts. The relative number of BAS has been extracted from Fig. 5b (squares). | ||
4. Conclusion
Ir/SiO2 and Ir/Al2O3 catalyze only hydrogenation and deactivate in the presence of sulfur, whereas Ir supported on amorphous silica–alumina (ASA) promotes aromatics saturation, ring contraction and ring opening, and exhibits stable activity in the presence of sulfur. The thiotolerance of Ir/ASA does not depend on the support's composition. The total activity and the selectivity to ring opening/contraction products scale with the Brönsted acidity of ASA. The combination of Ir and ASA with a Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio of ca. 0.5 leads to the highest acidity and catalytic performances.
Al ratio of ca. 0.5 leads to the highest acidity and catalytic performances.
These results suggest that ring opening and contraction of tetralin are essentially Brönsted-catalyzed processes. Future works will aim at increasing the selectivity to ring-opening products through elucidation of the bifunctional mechanism of tetralin hydroconversion on Ir/ASA.
Acknowledgements
S. Nassreddine thanks the French government for his PhD grant. S. Casu thanks Petrobras and the CNRS for his PhD grant. N. S. Prakash (Pyridine-FTIR), M. Aouine & L. Burel (TEM), and N. Cristin & P. Mascunan (ICP and BET) are greatly acknowledged for their support in catalyst characterization. We thank Sasol Germany GmbH for the supply of SIRAL samples.References
- B. H. Cooper and B. B. L. Donnis, Appl. Catal., A, 1996, 137, 203 CrossRef CAS.
- G. B. McVicker, M. Daage, M. S. Touvelle, C. W. Hudson, D. P. Klein, W. C. Baird, Jr., B. R. Cook, J. G. Chen, S. Hantzer, D. E. W. Vaughan, E. S. Ellis and O. C. Feeley, J. Catal., 2002, 210, 137 CrossRef CAS.
- H. Du, C. Fairbridge, H. Yang and Z. Ring, Appl. Catal., A, 2005, 294, 1 CrossRef CAS.
- R. C. Santana, P. T. Do, M. Santikunaporn, W. E. Alvarez, J. D. Taylor, E. L. Sughrue and D. E. Resasco, Fuel, 2006, 85, 643 CrossRef CAS.
- C. Flego, N. Gigantiello, W. O. Parker Jr. and V. Calemma, J. Chromatogr., A, 2009, 1216, 2891 CrossRef CAS.
- L. Piccolo, S. Nassreddine, G. Toussaint and C. Geantet, J. Chromatogr., A, 2010, 1217, 5872 CrossRef CAS.
- D. Kubicka, N. Kumar, P. Mäki-Arvela, M. Tiitta, V. Niemi, T. Salmi and D. Y. Murzin, J. Catal., 2004, 222, 65 CrossRef CAS.
- A. Corma, V. González-Alfaro and A. V. Orchillés, J. Catal., 2001, 200, 34 CrossRef CAS.
- M. A. Arribas, P. Conceptión and A. Martínez, Appl. Catal., A, 2004, 267, 111 CrossRef CAS.
- H. Ma, X. Yang, G. Wen, G. Tian, L. Wang, Y. Xu, B. Wang, Z. Tian and L. Lin, Catal. Lett., 2007, 116, 149 CrossRef CAS.
- H. Liu, X. Meng, D. Zhao and Y. Li, Chem. Eng. J., 2008, 140, 424 CrossRef CAS.
- D. Kubicka, N. Kumar, P. Mäki-Arvela, M. Tiitta, V. Niemi, H. Karhu, T. Salmi and D. Y. Murzin, J. Catal., 2004, 227, 313 CrossRef CAS.
- G. Busca, Chem. Rev., 2007, 107, 5366 CrossRef CAS.
- J. W. Ward and R. C. Hansford, J. Catal., 1969, 13, 154 CrossRef.
- P. O. Scokart, F. D. Declerck, R. E. Sempels and P. G. Rouxhet, J. Chem. Soc., Faraday Trans. 1, 1977, 73, 359 RSC.
- M. Trombetta, G. Busca, S. Rossini, V. Piccoli, U. Cornaro, A. Guercio, R. Catani and R. J. Willey, J. Catal., 1998, 179, 581 CrossRef CAS.
- W. Daniell, U. Schubert, R. Glöckler, A. Meyer, K. Noweck and H. Knözinger, Appl. Catal., A, 2000, 196, 247 CrossRef CAS.
- G. Crépeau, V. Montouillout, A. Vimont, L. Mariey, T. Cseri and F. Maugé, J. Phys. Chem. B, 2006, 110, 15172 CrossRef CAS.
- B. Xu, C. Sievers, J. A. Lercher, J. A. R. van Veen, P. Giltay, R. Prins and J. A. van Bokhoven, J. Phys. Chem. C, 2007, 111, 12075 CrossRef CAS.
- C. Chizallet and P. Raybaud, Angew. Chem., Int. Ed., 2009, 48, 2891 CrossRef CAS.
- D. G. Poduval, J. A. R. van Veen, M. S. Riguttob and E. J. M. Hensen, Chem. Commun., 2010, 46, 3466 RSC.
- E. J. M. Hensen, D. G. Poduval, D. A. J. M. Ligthart, J. A. R. van Veen and M. S. Rigutto, J. Phys. Chem. C, 2010, 114, 8363 CrossRef CAS.
- M. F. Williams, B. Fonfé, C. Woltz, A. Jentys, J. A. R. van Veen and J. A. Lercher, J. Catal., 2007, 251, 497 CrossRef CAS.
- E. Peeters, M. Cattenot, C. Geantet, M. Breysse and J. L. Zotin, Catal. Today, 2008, 133–135, 299 CrossRef CAS.
- S. Nassreddine, L. Massin, M. Aouine, C. Geantet and L. Piccolo, J. Catal., 2011, 278, 253 CrossRef.
- A. Meyer, Ger. Pat., DE3839580C1, 1990 Search PubMed.
- S. Nassreddine, G. Bergeret, B. Jouguet, C. Geantet and L. Piccolo, Phys. Chem. Chem. Phys., 2010, 12, 7812 RSC.
- S. Casu, PhD thesis, University of Lyon, 2008 Search PubMed.
Footnotes |
| † Ir/ASA catalysts prepared by impregnation with either acetylacetonate (this work) or chloride precursor exhibit similar catalytic properties, even in the presence of sulfur.28 |
| ‡ The pseudo-order n is defined by r = r0cn where r is the tetralin consumption rate (or TOF), r0 a constant and c the H2S concentration in the reactant feed. |
| § The published values for extinction coefficients being somewhat dispersed, we have chosen to report only relative values for LAS and BAS amounts. |
| This journal is © The Royal Society of Chemistry 2011 |
