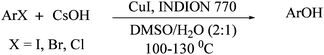Sulfonic acid resin and copper salts: a novel heterogeneous catalytic system for direct hydroxylation of haloarenes†
P. J.
Amal Joseph
,
S.
Priyadarshini
,
M.
Lakshmi Kantam
and
H.
Maheswaran
*
Inorganic and Physical Chemistry Division, Indian Institute of Chemical Technology, Hyderabad, India. E-mail: maheswaran_dr@yahoo.com; Fax: +91 4027160921; Tel: +91 4027193510
First published on 17th May 2011
Abstract
A sulfonic acid resin (INDION-770) in combination with Cu salts is demonstrated to be a versatile heterogeneous catalyst for direct hydroxylation of haloarenes to give corresponding phenols in excellent yields.
Phenols are vital intermediates in chemical, pharmaceutical and material industries.1 The most popular industrial method for preparation of phenol is the Hock process,2 which is limited merely to phenol, and suffers drawbacks such as multistep process and low overall efficiency. Functionalized phenols are prepared through nucleophilic substitution of arylsulfonic acids and activated haloarenes, copper promoted conversion of diazoarenes, and benzyne protocols.3 These methods are seldom used due to harsh reaction conditions and lack of substrate generality. An alternate method for the synthesis of phenols is by oxidative hydroxylation and copper catalyzed hydroxylation of aryl boronic acids.4 A milder method employing an iridium phosphine complexes based catalytic system has also been developed for the preparation of meta-substituted phenols bearing ortho- and para-directing groups, via an aromatic borylation/oxidation sequence.5 Recently, several homogeneous catalytic systems based on both the palladium6 and the copper7 complexes were reported for the cross-coupling of haloarenes with hydroxide salts. Among these systems, palladium based homogeneous catalysts are more reactive but suffer limitations such as high operational cost and toxicity when compared to those of copper based protocols. In both these protocols, appropriate strategies have been implemented to prevent the unwanted side reaction of phenols with unreacted haloarenes to form symmetrical diarylethers.8
In recent years, there has been significant progress in the design and the use of heterogeneous catalysts due to their innate advantages such as ease of product purification, catalyst recoverability and reusability for multiple reaction cycles, and also from the green chemistry point of view. In our previous study we have explored the use of a sulfonic acid resin (INDION-770) exchanged copper (I) catalyst for the direct N-arylation of haloarenes with NH-heterocycles under heterogeneous conditions.9 In continuation of our studies, herein, we report for the first time a copper based heterogeneous catalyst derived from a sulfonic acid resin (INDION-770) for the direct hydroxylation of haloarenes.
Initially, 4-iodooanisole was chosen as a model substrate for screening studies, and it was subjected to various reaction conditions with “INDION-770” resin and copper salts under in situ conditions in a DMSO/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system. The results of these studies are summarized in Table 1. As depicted in Table 1, different copper precursors such as CuI, CuCl, CuBr, Cu2O, Cu(OH)2, Cu(OAc)2 and copper powder promote direct hydroxylation reactions in excellent yields with CsOH as base at 125 °C (Table 1, entries 1–7). Apparently, among the various copper salts screened in the reactions, highest yield (94%) was obtained with CuI. However, when the reaction was carried out in the absence of resin additive, only moderate yield of product was obtained (Table 1, entry 8). Similarly, very poor yield of product was obtained when the reaction was carried out in the absence of both additive and Cu-catalyst (Table 1, entry 9).
1) solvent system. The results of these studies are summarized in Table 1. As depicted in Table 1, different copper precursors such as CuI, CuCl, CuBr, Cu2O, Cu(OH)2, Cu(OAc)2 and copper powder promote direct hydroxylation reactions in excellent yields with CsOH as base at 125 °C (Table 1, entries 1–7). Apparently, among the various copper salts screened in the reactions, highest yield (94%) was obtained with CuI. However, when the reaction was carried out in the absence of resin additive, only moderate yield of product was obtained (Table 1, entry 8). Similarly, very poor yield of product was obtained when the reaction was carried out in the absence of both additive and Cu-catalyst (Table 1, entry 9).
| Entrya | Catalyst | Additive |
Solvent, ratio (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) v) |
Base | Yieldg (%) |
|---|---|---|---|---|---|
| a Reactions (entries 1–7 and 10–21) performed on a 1.0 mmol scale with 4-iodoanisole (1.0 mmol), copper catalyst (0.1 mmol), INDION-770 resin (60 mg; ∼0.25 mmol active sulfonic acid functionality), base (3 mmol) and 0.9 mL of solvent at 125 °C for 12 h. b Reaction (entry 8) was performed without additive. c Reaction (entry 9) performed without catalyst and additive. d Reaction (entry 22) performed with Amberlyst-16 wet resin (60 mg; ∼0.30 mmol active sulfonic acid functionality). e Reaction (entry 23) performed with benzenesulfonic acid (0.30 mmol). f Reaction (entry 24) performed with preformed copper(I)-exchanged INDION-770 (65 mg, ∼0.10 mmol of copper). g Isolated yields after column chromatographic purification. h Determined by GC analysis. | |||||
| 1 | CuI | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 94 |
| 2 | CuCl | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 92 |
| 3 | CuBr | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 87 |
| 4 | Cu2O | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 91 |
| 5 | Cu(OH)2 | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 85 |
| 6 | Cu(OAc)2 | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 90 |
| 7 | Cu | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 92 |
| 8b | CuI | None |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 62 |
| 9c | None | None |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 8 |
| 10 | CuI | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
LiOH | Traceh |
| 11 | CuI | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NaOH | 12 |
| 12 | CuI | IND-770 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 25 |
| 13 | CuI | IND-770 |
Toluene/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | Traceh |
| 14 | CuI | IND-770 |
1,4-Dioxane/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | Traceh |
| 15 | CuI | IND-770 |
DMF/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 5 |
| 16 | CuI | IND-770 |
Glycol/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 35 |
| 17 | CuI | IND-770 | Glycol | CsOH | 30 |
| 18 | CuI | IND-770 | PEG 300/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 28 |
| 19 | CuI | IND-770 | DMSO | CsOH | 35 |
| 20 | CuI | IND-770 | H2O | CsOH | Traceh |
| 21 | CuI | IND-770 |
DMSO/H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 56 |
| 22d | CuI | Amberlyst-16 |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 91 |
| 23e | CuI | PhSO3H |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 70 |
| 24f | Cu–IND |
DMSO/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CsOH | 92 |
Among various bases screened for the reaction, the best result was obtained with CsOH in the presence of CuI at 125 °C (Table 1, entry 1). As can be seen, the reaction showed a linear effect on the basicity of alkali metal hydroxides. For example, no product was detected when the reaction was carried out using LiOH, whereas yield of the desired product increased accordingly in the order of basicity for NaOH < KOH < CsOH (Table 1, entries 1 and 10–12). The screening studies for solvents revealed that they play a vital role in this synthesis protocol. For example, the use of nonpolar solvents like toluene in combination with water resulted in the formation of two phases, in which base is partially soluble in the organic layer, thereby retarding the reaction (Table 1, entry 13). Among polar aprotic solvents used, the reaction proceeded smoothly in DMSO in good yields, and surprisingly, the reaction failed to yield the desired product in solvents such as 1,4-dioxane and DMF (Table 1, entries 1, 14 and 15). Unlike the DMSO/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system, significant amounts of symmetrical diarylether side product were formed when the reaction was carried out in pure DMSO in the absence of water (Table 1, entry 19). In the binary DMSO/H2O (2
1) solvent system, significant amounts of symmetrical diarylether side product were formed when the reaction was carried out in pure DMSO in the absence of water (Table 1, entry 19). In the binary DMSO/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system, it is likely that the phenol formed is converted into caesium phenoxide that was solvated and protected by water molecules, thereby preventing the formation of symmetrical diarylethers. Additional advantage of using water in the solvent system is that it improves the solubility of the base. Notably, the quantity of water is critical in these reactions; for example, the reaction was very slow when the ratio of DMSO/water was less than 1, the best results were obtained when the DMSO/water ratio was 2 (0.6 mL/0.3 mL) (Table 1, entries 1 and 21). These results are reasonable because basicity decreases as the concentration of water increases; indeed CsOH in DMSO is a super base. Another important observation was the use of ethylene glycol as solvent. The base was soluble in glycol and symmetrical diarylethers were not formed even in the absence of water, but an undesirable side product which had been accounted as the coupled product of phenol with glycol was formed in significant amount (55%) (Table 1, entries 16 and 17). Unfortunately, only trace amount of the product was obtained when the reaction was carried out in pure water (Table 1, entry 20). Therefore, among various optimization studies listed in Table 1 for the hydroxylation reaction of 4-iodoanisole, the most promising result was obtained with a CuI/INDION-770 resin catalytic system using 3 mmol of CsOH in 0.9 mL of a 2
1) solvent system, it is likely that the phenol formed is converted into caesium phenoxide that was solvated and protected by water molecules, thereby preventing the formation of symmetrical diarylethers. Additional advantage of using water in the solvent system is that it improves the solubility of the base. Notably, the quantity of water is critical in these reactions; for example, the reaction was very slow when the ratio of DMSO/water was less than 1, the best results were obtained when the DMSO/water ratio was 2 (0.6 mL/0.3 mL) (Table 1, entries 1 and 21). These results are reasonable because basicity decreases as the concentration of water increases; indeed CsOH in DMSO is a super base. Another important observation was the use of ethylene glycol as solvent. The base was soluble in glycol and symmetrical diarylethers were not formed even in the absence of water, but an undesirable side product which had been accounted as the coupled product of phenol with glycol was formed in significant amount (55%) (Table 1, entries 16 and 17). Unfortunately, only trace amount of the product was obtained when the reaction was carried out in pure water (Table 1, entry 20). Therefore, among various optimization studies listed in Table 1 for the hydroxylation reaction of 4-iodoanisole, the most promising result was obtained with a CuI/INDION-770 resin catalytic system using 3 mmol of CsOH in 0.9 mL of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO/water solvent at 125 °C (Table 1, entry 1).
1 DMSO/water solvent at 125 °C (Table 1, entry 1).
The above optimized reaction conditions were further extended to the hydroxylation reactions of various haloarenes, and the results are summarized in Table 2. As described in Table 2, the Cu(I)/INDION-770 resin catalytic system based protocol is rather general in nature, as it is applicable for the reactions of a variety of electron-rich and electron-deficient iodoarenes. The hydroxylation reactions of electro-deficient iodoarenes were rather fast, and furnished excellent yields of the corresponding phenols within 4–8 h at 100–110 °C. Thus 4-iodonitrobenzene, 4-trifluoromethylbenzene and 4-iodoacetophenone react smoothly with CsOH to form the respective phenols in good yields (Table 2, entries 5–7). Similarly, the reactions of electron rich iodoarenes such as 4-iodoanisole, 4-iodotoluene, 1-tert-butyl-4-iodobenzene, 3-iodotoluene, 1-chloro-4-iodobenzene and 2-iodonaphthalene also proceeded smoothly, albeit at slightly higher temperature (125 °C, Table 2, entries 1–3 and 8–10), to afford the corresponding phenols in excellent yields. Notably, this protocol is applicable to sterically hindered iodoarenes, for example, 2-iodoanisole, 2-iodophenol, 2-iodoaniline, 1-iodo-2-nitrobenzene and 1-iodonaphthalene, all undergo hydroxylation reaction smoothly under the optimized reaction conditions to afford the corresponding phenols in excellent yields (Table 2, entries 11–15). Surprisingly, the reaction of highly sterically hindered 2,6-dimethoxyiodobenzene also proceeded in 5 h to afford the product in excellent yield (Table 2, entry 16). The present protocol tolerates a wide variety of functional groups, for example as mentioned above, different groups such as OH, NO2, OMe, CF3, COMe and Me are unaffected in the optimized conditions. However, a complete formation of the hydrolyzed product was observed with 4-iodobenzonitrile, and hence this method is not suitable for haloarenes containing the cyano functionality in the aromatic ring.
| Entrya | ArX | X | Temp/°C | Time/h | Yieldd (%) |
|---|---|---|---|---|---|
a Reactions (entries 1–20) performed on a 1.0 mmol scale with haloarenes (1.0 mmol), CuI (0.1 mmol), INDION-770 resin (60 mg), CsOH (3 mmol) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO/H2O (0.9 mL) at indicated time and temperature.
b Reactions performed using 4 mmol of KOH instead of CsOH.
c Reaction performed using the preformed copper(I)-exchanged INDION-770 resin (65 mg, ∼0.1 mmol of Cu) instead of in situ conditions.
d Isolated yields after column chromatographic purification.
e Yield after the 5th cycle. 1 DMSO/H2O (0.9 mL) at indicated time and temperature.
b Reactions performed using 4 mmol of KOH instead of CsOH.
c Reaction performed using the preformed copper(I)-exchanged INDION-770 resin (65 mg, ∼0.1 mmol of Cu) instead of in situ conditions.
d Isolated yields after column chromatographic purification.
e Yield after the 5th cycle.
|
|||||
| 1 |
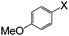
|
I | 125 | 8 | 94, 92c, 88c,e |
| Br | 130 | 48 | 30 | ||
| 2 |
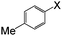
|
I | 125 | 8 | 92 |
| 3 |
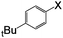
|
I | 125 | 8 | 93 |
| 4 |
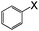
|
I | 125 | 8 | 94 |
| Br | 130 | 48 | 33 | ||
| Cl | 130 | 48 | 14 | ||
| 5 |
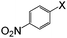
|
I | 100 | 4 | 96 |
| Br | 100 | 5 | 94 | ||
| Cl | 100 | 6 | 92, 90b | ||
| 6 |
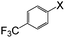
|
I | 110 | 8 | 92 |
| Br | 125 | 6 | 92 | ||
| Cl | 125 | 15 | 88 | ||
| 7 |
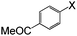
|
I | 110 | 6 | 89 |
| Br | 125 | 5 | 85 | ||
| Cl | 125 | 15 | 78 | ||
| 8 |
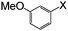
|
I | 125 | 6 | 95 |
| 9 |
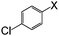
|
I | 125 | 6 | 91 |
| 10 |
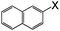
|
I | 125 | 6 | 94 |
| 11 |
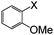
|
I | 125 | 6 | 90 |
| 12 |
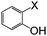
|
I | 125 | 6 | 87 |
| 13 |
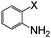
|
I | 125 | 6 | 82 |
| 14 |
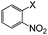
|
I | 100 | 4 | 96 |
| Br | 100 | 6 | 96 | ||
| Cl | 100 | 8 | 94, 91b | ||
| 15 |
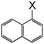
|
I | 125 | 6 | 94 |
| 16 |
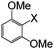
|
I | 125 | 6 | 94 |
| 17 |
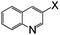
|
Br | 125 | 8 | 82 |
| 18 |
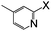
|
Br | 125 | 12 | 78 |
| 19 |
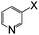
|
Br | 125 | 6 | 81 |
| 20 |
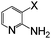
|
Br | 125 | 6 | 78 |
Thereafter, the utility of a CuI/INDION-770 resin based hydroxylation protocol was explored for the reaction of bromoarenes and chloroarenes. As illustrated in Table 2, the reactions of bromoarenes and chloroarenes having electron-withdrawing groups proceeded smoothly under the standard conditions (Table 2, entries 5–7 and 14). It is noteworthy that reaction of 4-chloronitrobenzene and 2-chloronitrobenzene proceeded smoothly even in the presence of KOH as base to afford the corresponding phenols in good yields (Table 2, entries 5 and 14). However, the reactions of chlorobenzene, bromobenzene and deactivated bromoarene such as 4-bromoanisole were sluggish and furnished only low to moderate yields of respective phenols (Table 2, entries 4 and 1). These results are not surprising since most of the copper-based catalysts also show similar activity for such strongly deactivated bromoarenes and chloroarenes in coupling reactions. Finally, the scope of the present protocol was successfully extended to heterocycles such as 3-bromoquinoline, 2-bromo-4-methylpyridine, 3-bromopyridine and 2-amino-3-bromopyridine.10 These substrates also underwent hydroxylation reaction and furnished good yields for the desired coupled product (Table 2, entries 17–20).
At present there are no reports available in the literature regarding the mechanism of copper catalyzed hydroxylation of haloarenes.11 Nevertheless, to better understand the function of sulfonic acid in the hydroxylation reaction, we have carried out an hydroxylation reaction using CuI in combination with benzenesulfonic acid in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO/H2O at 125 °C (Table 1, entry 23). This system also promoted the hydroxylation reaction of 4-iodoanisole, albeit under homogeneous conditions. This observation strongly supports the hypothesis that the active catalytic species is Cu(I)arylsulfonate. Furthermore, we have carried out a reaction between CuI (0.1 mmol), INDION-770 resin (60 mg, ∼0.25 mmol sulfonic acid groups), CsOH (3 mmol) and 2
1 DMSO/H2O at 125 °C (Table 1, entry 23). This system also promoted the hydroxylation reaction of 4-iodoanisole, albeit under homogeneous conditions. This observation strongly supports the hypothesis that the active catalytic species is Cu(I)arylsulfonate. Furthermore, we have carried out a reaction between CuI (0.1 mmol), INDION-770 resin (60 mg, ∼0.25 mmol sulfonic acid groups), CsOH (3 mmol) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO/H2O (0.9 mL) in a sealed tube at 125 °C under stirring for one hour. This reacted resin was filtered and then analyzed by ICP OES, which confirmed the presence of about 1.4 mmol g−1 of copper in the resin. When this exchanged resin was used as catalyst instead of the in situ conditions as described in Table 1, we have obtained similar results for direct hydroxylation reaction of 4-iodoanisole (Table 1, entry 24). These studies clearly show that the complete exchange of copper from cuprous iodide to a sulfonic acid resin takes place and thus the active catalyst for the direct hydroxylation reaction is the heterogeneous copper-exchanged INDION-770 resin. An important advantage of the copper-exchanged INDION-770 resin based protocol is that the spent catalyst can be recovered from the reaction media, and can be reused for several reaction cycles. This aspect is demonstrated for the hydroxylation reaction of 4-iodoanisole. As can be seen in Table 2 (entry 1), the recovered catalyst can be reused successfully for up to five reaction cycles without significant loss in the catalytic activity.
1 DMSO/H2O (0.9 mL) in a sealed tube at 125 °C under stirring for one hour. This reacted resin was filtered and then analyzed by ICP OES, which confirmed the presence of about 1.4 mmol g−1 of copper in the resin. When this exchanged resin was used as catalyst instead of the in situ conditions as described in Table 1, we have obtained similar results for direct hydroxylation reaction of 4-iodoanisole (Table 1, entry 24). These studies clearly show that the complete exchange of copper from cuprous iodide to a sulfonic acid resin takes place and thus the active catalyst for the direct hydroxylation reaction is the heterogeneous copper-exchanged INDION-770 resin. An important advantage of the copper-exchanged INDION-770 resin based protocol is that the spent catalyst can be recovered from the reaction media, and can be reused for several reaction cycles. This aspect is demonstrated for the hydroxylation reaction of 4-iodoanisole. As can be seen in Table 2 (entry 1), the recovered catalyst can be reused successfully for up to five reaction cycles without significant loss in the catalytic activity.
In summary, a novel copper based heterogeneous protocol involving a cheap, commercially available and easy to use sulfonic acid resin “INDION-770” for direct hydroxylation of various haloarenes is presented. This heterogeneous protocol is shown to be very efficient for a wide variety of electron rich as well as electron deficient iodoarenes and activated bromoarenes and chloroarenes. It has also been shown that the spent catalyst can be reused for up to five reaction cycles without significant loss in the activity.
Notes and references
- (a) J. H. P. Tyman, Synthetic and Natural Phenols, Elsevier, New York, 1996 Search PubMed; (b) Z. Rapport, The Chemistry of Phenols, Wiley-VCH, Weinheim, 2003 Search PubMed; (c) M. Weber, M. Weber and M. Kleine-Boymann, in Ullmann's Encyclopedia of Industrial Chemistry, ed. B. Elvers, S. Hawkins and G. Schulz, Wiley, Weinheim, 7th edn, 2004 Search PubMed.
- (a) I. Vlad, M. Mercas, C. Stratula, I. Cheta and F. Oprea, RO 109191, 1994 Search PubMed; (b) O. Gerlich, W. Pompetzki and D. Ahrens, EP 980846, 2000 Search PubMed.
- (a) C. A. Fyfe, In The Chemistry of the Hydroxyl Group, ed. S. Patai, Wiley-Interscience, New York, 1971, vol. 1, pp. 83–127 Search PubMed; (b) T. George, R. Mabon, G. Sweeney, J. B. Sweeney and A. J. Tavassoli, J. Chem. Soc., Perkin Trans. 1, 2000, 2529 RSC; (c) P. Hanson, J. R. Jones, A. B. Taylor, P. H. Walton and A. W. Timms, J. Chem. Soc., Perkin Trans. 2, 2002, 1135 RSC; (d) C. Hoarau and T. R. R. Pettus, Synlett, 2003, 127; (e) D. Tyrer, US 2445500, 1945 Search PubMed; (f) N. J. Stepaniuk and B. J. Lamb, US 4822927, 1989 Search PubMed; (g) M. Matsumoto and J. Kyoshima, JP 06211716, 1994 Search PubMed; (h) J. Oren and M. Adda, WO 9726235, 1997 Search PubMed; (i) Z. Wu and R. Glaser, J. Am. Chem. Soc., 2004, 126, 10632 CrossRef CAS.
- (a) K. S. Webb and D. Levy, Tetrahedron Lett., 1995, 36, 5117 CAS; (b) J. Simon, S. Salzbrunn, G. K. S. Prakash, N. A. Petasis and G. A. Olah, J. Org. Chem., 2001, 66, 633 CrossRef CAS; (c) R. Benjamin, B. R. Travis, B. P. Ciaramitaro and B. Borhan, Eur. J. Org. Chem., 2002, 3429; (d) G. K. S. Prakash, S. Chacko, C. Panja, T. E. Thomas, L. Gurung, G. Rasul, T. Mathew and G. A. Olaha, Adv. Synth. Catal., 2009, 351, 1567 CrossRef CAS; (e) J. Xu, X. Wang, C. Shao, D. Su, G. Cheng and Y. Hu, Org. Lett., 2010, 12, 1964 CrossRef CAS.
- R. E. Maleczka, F. Shi, D. Holmes and M. R. Smith III, J. Am. Chem. Soc., 2003, 125, 7792 CrossRef CAS.
- For examples of palladium catalyzed hydroxylation reaction of haloarenes, see: (a) K. W. Anderson, T. Ikawa, R. E. Tundel and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 10694 CrossRef CAS; (b) M. C. Wills, Angew. Chem., Int. Ed., 2007, 46, 3402 CrossRef CAS; (c) B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., Int. Ed., 2007, 46, 7251 CrossRef CAS; (d) G. Chen, A. S. C. Chan and F. Y. Kuong, Tetrahedron Lett., 2007, 48, 473 CrossRef CAS; (e) T. Schulz, C. Torborg, B. Schaffner, J. Huang, A. Zapf, R. Kadyrou, A. Borner and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 918 CrossRef CAS; (f) A. G. Sergeev, T. Schulz, C. Torborg, A. Spannenberg, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 7595 CrossRef CAS.
- For examples of copper catalyzed hydroxylation reaction of haloarenes, see: (a) A. Tlili, N. Xia, F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 8725 CrossRef CAS; (b) D. Zhao, N. Wu, S. Zhang, P. Xi, X. Su, J. Lan and J. You, Angew. Chem., Int. Ed., 2009, 48, 8729 CrossRef CAS; (c) S. Maurer, W. Liu, X. Zhang, Y. Jiang and D. Ma, Synlett, 2010, 976 CAS; (d) D. Yang and H. Fu, Chem.–Eur. J., 2010, 16, 2366 CrossRef CAS; (e) L. Jing, J. Wei, L. Zhou, Z. Huang, Z. Li and X. Zhou, Chem. Commun., 2010, 46, 4767 RSC.
- For examples of the copper-catalyzed arylation of phenols, see: (a) A. V. Kalinin, J. F. Bower, P. Riebel and V. Snieckus, J. Org. Chem., 1999, 64, 2986 CrossRef CAS; (b) P. J. Fagan, E. Hauptman, R. Shapiro and A. Casalnuovo, J. Am. Chem. Soc., 2000, 122, 5043 CrossRef CAS; (c) R. K. Gujadhur, C. G. Bates and D. Venkataraman, Org. Lett., 2001, 3, 4315 CrossRef CAS; (d) M. Taillefer, H.-J. Cristau, P. P. Cellier and J.-F. Spindler, FR 16547, 2001 Search PubMed; (e) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (f) H.-J. Cristau, P. P. Cellier, S. Hamada, J.-F. Spindler and M. Taillefer, Org. Lett., 2004, 6, 913 CrossRef CAS; (g) B. H. Lipshutz, J. B. Unger and B. R. Taft, Org. Lett., 2007, 9, 1089 CrossRef CAS; (h) H. Zhang, D. Ma and W. Cao, Synlett, 2007, 243 CrossRef CAS; (i) N. Xia and M. Taillefer, Chem.–Eur. J., 2008, 14, 6037 CrossRef CAS; (j) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2008, 47, 3096 CrossRef CAS; (k) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS; (l) D. Maiti and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 17423 CrossRef CAS; (m) P.-F. Larsson, A. Correa, M. Carril, P.-O. Norrby and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5691 CrossRef CAS.
- P. J. Amal Joseph, S. Priyadarshini, M. Lakshmi Kantam and H. Maheswaran, Catal. Sci. Technol., 2011, 1, 234 RSC.
- It is to be noted that a similar reaction of 2-chloropyridine proceeds in the absence of catalyst and ligand, for reference, see: H. W. Orth, B. M. Hassler, B. H. W. Kleffner and N. W. Weiss, US 4942239, 1990 Search PubMed.
- (a) T. Cohen and I. Cristea, J. Am. Chem. Soc., 1976, 98, 748 CrossRef CAS; (b) D. V. Allen Methodology and Mechanism: Reinvestigating the Ullmann reaction, PhD Dissertation Thesis, The Graduate School of University of Massachusetts, Amherst, 2004 Search PubMed; (c) E. R. Strieter, B. Bhayana and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 78 CrossRef CAS; (d) G. O. Jones, P. Liu, K. N. Houk and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 6205 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0cy00090f |
| This journal is © The Royal Society of Chemistry 2011 |