Rhodium-catalyzed asymmetric phenylation of N-phosphinoylarylimines with triphenylborane†‡
Xinyu
Hao
a,
Qian
Chen
b,
Masami
Kuriyama
a,
Ken-ichi
Yamada
a,
Yasutomo
Yamamoto
c and
Kiyoshi
Tomioka
*c
aGraduate School of Pharmaceutical Sciences, Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan. E-mail: yamak@pharm.kyoto-u.ac.jp; Fax: +81 75 753 4604; Tel: +81 75 753 4573
bThe Academy of Fundamental and Interdisciplinary Science, Harbin Institute of Technology, Harbin, Heilongjiang, 150080, P. R. China. E-mail: chenqian1jp@yahoo.co.jp
cFaculty of Pharmaceutical Sciences, Doshisha Women's College of Liberal Arts, Kodo, Kyotanabe 610-0395, Japan. E-mail: tomioka@pharm.kyoto-u.ac.jp; Fax: +81 774 65 8658; Tel: +81 774 65 8676
First published on 4th February 2011
Abstract
Triphenylborane asymmetrically transfers its phenyl group to N-diphenylphosphinoylarylimines to give diarylmethylamines with high ee in high yield without imine hydrolysis under the catalysis of a chiral amidomonophosphane–rhodium(I) complex.
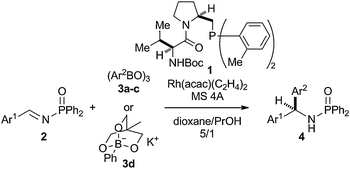
Success in the chiral amidomonophosphane–rhodium-catalyzed asymmetric arylation of N-diphenylphosphinoyl (Dpp) imines with arylboroxines1,2 relied on the use of 4 Å molecular sieves (MS 4Å) as a dehydrating agent to realize almost water-free conditions, giving the corresponding diarylmethylamines with extremely high enantioselectivity in satisfactorily high yield.3,4 For example, the arylation of benzaldehydeN-Dpp-imine 2a with 4-methylphenyl- and 4-methoxyphenylboroxines 3b and 3c in a 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dioxane and propanol in the presence of the 1–Rh(I) catalyst and MS 4Å at 80 °C for 12 h gave the corresponding arylated amines (S)-4b and (S)-4c with 98% ee each in 96% and 92% yields, respectively (Table 1, entries 1 and 2).4 Continuing scope and limitation studies, however, revealed that the reaction of less reactive 4-methyl- and 4-methoxybezaldehyde N-Dpp-imines 2b and 2c with phenylboroxine 3a gave the products (R)-4b and (R)-4c with lower 84% and 83% ee in decreased 79% and 10% yields, respectively, probably due to competitive hydrolysis of the imines (entries 3 and 4). Then, our problem solving research was focused on the survey of arylation conditions that give shorter reaction time to avoid the hydrolysis.
1 mixture of dioxane and propanol in the presence of the 1–Rh(I) catalyst and MS 4Å at 80 °C for 12 h gave the corresponding arylated amines (S)-4b and (S)-4c with 98% ee each in 96% and 92% yields, respectively (Table 1, entries 1 and 2).4 Continuing scope and limitation studies, however, revealed that the reaction of less reactive 4-methyl- and 4-methoxybezaldehyde N-Dpp-imines 2b and 2c with phenylboroxine 3a gave the products (R)-4b and (R)-4c with lower 84% and 83% ee in decreased 79% and 10% yields, respectively, probably due to competitive hydrolysis of the imines (entries 3 and 4). Then, our problem solving research was focused on the survey of arylation conditions that give shorter reaction time to avoid the hydrolysis.
| Entry | 2 | Ar1 | 3 | Ar2 | T (°C) | Time (h) | 4 | Yield (%) | ee (%)b |
|---|---|---|---|---|---|---|---|---|---|
| a The reaction was conducted with 1.67 equiv. of (Ar2BO)3 in the presence of 6.6 mol% of 1, and 6.0 mol% of the Rh(I) except entry 6. b The ee was determined by chiral stationary phase HPLC analysis. c See ref. 4. d Microwave irradiation. e 5 equiv. of triolborate 3d was used. f Without MS 4Å. g Racemic phenyl (4-tolyl)methanol, aldehyde adduct, was obtained in 76% yield. | |||||||||
| 1c | 2a | Ph | 3b | 4-MeC6H4 | 80 | 12 | (S)-4b | 96 | 98 |
| 2c | 2a | Ph | 3c | 4-MeOC6H4 | 80 | 12 | (S)-4c | 92 | 98 |
| 3 | 2b | 4-MeC6H4 | 3a | Ph | 80 | 1 | (R)-4b | 79 | 84 |
| 4 | 2c | 4-MeOC6H4 | 3a | Ph | 80 | 12 | (R)-4c | 10 | 83 |
| 5d | 2c | 4-MeOC6H4 | 3a | Ph | 220 | 0.15 | (R)-4c | 45 | 68 |
| 6e,f | 2b | 4-MeC6H4 | 3d | Ph | 80 | 20 | (R)-4b | 0g | — |
Microwave irradiation of a mixture of 2c and phenylboroxine 3a at 220 °C for 10 min was apparently beneficial but not satisfactory to give (R)-4c with 68% ee in improved 45% yield (entry 5). A cyclic triolborate 3d5 failed to give an imine adduct (R)-4b but gave the corresponding racemic aldehyde adduct, phenyl(4-tolyl)methanol, in 76% yield (entry 6).
Finally, we found triphenylborane as a reactive phenylation reagent to give phenylated amines (R)-4b and (R)-4c with 93% ee each in 92% and 91% yields, respectively, without imine hydrolysis. Herein, we report a catalytic asymmetric phenylation of N-Dpp-arylimines 2 with triphenylborane. In contrast to the widely used boronic reagents, triarylboranes have not been utilized as an aryl source in asymmetric catalysis,6 although a mixture of triphenylborane and diethylzinc has been employed for in situ generation of diphenylzinc.7
When a mixture of 4-tolylaldehyde N-Dpp-imine 2b and triphenylborane (1.67 equiv.) was heated in propan-1-ol at 100 °C for 12 h in the presence of a catalytic amount of 1 (6.6 mol%) and acetylacetonatobis(ethylene)rhodium(I) (6.0 mol%), the phenylated product (R)-4b with 81% ee was obtained in 63% yield (Table 2, entry 1). The reaction was then performed in the presence of KF because promotion of a transmetalation process of organoboron reagents by fluoride has been described.8 The reactions with anhydrous KF and KF on alumina resulted in less satisfactory 39% and 44% yields, and 60% and 81% enantioselectivity, respectively (entries 2 and 3). When the reaction was conducted in the presence of KF on Celite, the reaction more smoothly proceeded to give (R)-4b with 89% ee in increased 72% yield (entry 4). A mixture of dioxane and propanol4 was not suitable solvent for the reaction with triphenylborane, and only a trace amount of the product was produced (entry 5). Finally, tert-butanol was found to be the choice to complete the reaction in only 1 h at 100 °C, giving (R)-4b with 93% ee in 92% yield (entry 6).
| Entry | Solvent | KF source | Time (h) | Yield (%) | ee (%)b |
|---|---|---|---|---|---|
| a The reaction was conducted with 1.67 equiv. of Ph3B in the presence of 2.0 equiv. of the indicated KF source, 6.6 mol% of 1, and 6.0 mol% of the Rh(I). b The ee was determined by chiral stationary phase HPLC analysis. | |||||
| 1 | PrOH | — | 12 | 63 | 81 |
| 2 | PrOH | KF | 6 | 39 | 60 |
| 3 | PrOH | KF/Al2O3 | 6 | 44 | 81 |
| 4 | PrOH | KF/Celite | 6 | 72 | 89 |
| 5 |
Dioxane/PrOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
KF/Celite | 6 | <5 | — |
| 6 | t-BuOH | KF/Celite | 1 | 92 | 93 |
This asymmetric phenylation with triphenylborane was applicable to other N-Dpp-arylimines 2 (Table 3).§Phenylation of 3-tolylimine 2d gave the corresponding 4d with high 96% ee in high 92% yield (entry 2). Electron-deficient 4-chlorobenzaldimine 2f bearing a chlorine atom was converted to 4f with 92% ee in 91% yield (entry 4). Although the reaction of sterically demanding ortho-substituted arylimines 2e and 2g was slower, the reaction proceeded in highly enantioselective manner to give 4e and 4g with 90% ee and 93% ee in 86% and 84% yield, respectively (entries 3 and 5). It is noteworthy that the reaction of 4-methoxybenzaldimine 2c, miserable results of which were the starting point for this study (Table 1, entry 4), also successfully proceeded for 12 h to give 4c with 93% ee in 91% yield (entry 6). Polyaromatic 2-naphthaldimine 2h and heteroaromatic 2-furancarboaldimine 2i were also good substrates to give 4h and 4i with 90% and 91% ee in 94% and 86% yield, respectively (entries 7 and 8).
| Entry | 2 | Ar | Time (h) | 4 | Yield (%) | ee (%)b |
|---|---|---|---|---|---|---|
| a The reaction was conducted with 1.67 equiv. of Ph3B in the presence of 2.0 equiv. of KF/Celite, 6.6 mol% of 1, and 6.0 mol% of the Rh(I). b The ee was determined by chiral stationary phase HPLC analysis. c Entry 6 of Table 2 is presented for comparison. | ||||||
| 1c | 2b | 4-MeC6H4 | 1 | 4b | 92 | 93 |
| 2 | 2d | 3-MeC6H4 | 1 | 4d | 92 | 96 |
| 3 | 2e | 2-MeC6H4 | 10 | 4e | 86 | 90 |
| 4 | 2f | 4-ClC6H4 | 1 | 4f | 91 | 92 |
| 5 | 2g | 2-ClC6H4 | 10 | 4g | 84 | 93 |
| 6 | 2c | 4-MeOC6H4 | 12 | 4c | 91 | 93 |
| 7 | 2h | 2-Naphthyl | 1 | 4h | 94 | 90 |
| 8 | 2i | 2-Furyl | 1 | 4i | 86 | 91 |
In conclusion, we have developed a widely applicable chiral amidomonophosphane–rhodium-catalyzed enantioselective phenylation of aryl-N-Dpp-imines with triphenylborane. The results clearly indicate the utility of triarylborane in avoiding in situwater generation. Because a Dpp group is cleaved under milder acidic conditions than a Boc group,9 this reaction provides a versatile methodology to access a variety type of optically active diarylmethylamines.
This research was partially supported by a Grant- in-Aid for Young Scientist (B) to KY and YY, and a Grant-in-Aid for Scientific Research (A) to KT from the Japan Society for the Promotion of Science (JSPS).
Notes and references
- Palladium-catalyzed cross-coupling reactions of organoboron compounds: N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 Search PubMed.
- Select examples of Rh-catalyzed asymmetric additions of arylboron reagents to imines: (a) N. Tokunaga, Y. Otomaru, K. Okamoto, K. Ueyama, R. Shintani and T. Hayashi, J. Am. Chem. Soc., 2004, 126, 13584–13585 CrossRef CAS; (b) Y. Otomaru, N. Tokunaga, R. Shintani and T. Hayashi, Org. Lett., 2005, 7, 307–310 CrossRef CAS; (c) D. J. Weix, Y. Shi and J. A. Ellman, J. Am. Chem. Soc., 2005, 127, 1092–1093 CrossRef CAS; (d) H.-F. Duan, Y.-X. Jia, L.-X. Wang and Q.-L. Zhou, Org. Lett., 2006, 8, 2567–2569 CrossRef CAS; (e) R. B. C. Jagt, P. Y. Toullec, D. Geerdink, J. G. de Vries, B. L. Feringa and A. J. Minnaard, Angew. Chem., Int. Ed., 2006, 45, 2789–2791 CrossRef CAS; (f) Z.-Q. Wang, C.-G. Feng, M.-H. Xu and G.-Q. Lin, J. Am. Chem. Soc., 2007, 129, 5336–5337 CrossRef CAS; (g) H. Nakagawa, J. C. Rech, R. W. Sindelar and J. A. Ellman, Org. Lett., 2007, 9, 5155–5157 CrossRef CAS; (h) M. Trincado and J. A. Ellman, Angew. Chem., Int. Ed., 2008, 47, 5623–5626 CrossRef CAS; (i) K. Kurihara, Y. Yamamoto and N. Miyaura, Adv. Synth. Catal., 2009, 351, 260–270 CrossRef CAS.
- M. Kuriyama, T. Soeta, X. Hao, Q. Chen and K. Tomioka, J. Am. Chem. Soc., 2004, 126, 8128–8129 CrossRef CAS.
- X. Hao, M. Kuriyama, Q. Chen, Y. Yamamoto, K. Yamada and K. Tomioka, Org. Lett., 2009, 11, 4470–4473 CrossRef CAS.
- Y. Yamamoto, M. Takizawa, X.-Q. Yu and N. Miyaura, Angew. Chem., Int. Ed., 2008, 47, 928–931 CrossRef CAS.
- Direct phenyl transfer reactions from triphenylborane: (a) E. Shirakawa, Y. Yasuhara and T. Hayashi, Chem. Lett., 2006, 35, 768–769 CrossRef CAS; (b) H. Zeng and R. Hua, J. Org. Chem., 2008, 73, 558–562 CrossRef CAS; (c) H. Tsukamoto, T. Uchiyama, T. Suzuki and Y. Kondo, Org. Biomol. Chem., 2008, 6, 3005–3013 RSC.
- (a) J. Rudolph, F. Schmidt and C. Bolm, Adv. Synth. Catal., 2004, 346, 867–872 CrossRef CAS; (b) J. Rudolph, M. Lormann, C. Bolm and S. Dahmen, Adv. Synth. Catal., 2005, 347, 1361–1368 CrossRef CAS; (c) S. Dahmen and M. Lormann, Org. Lett., 2005, 7, 4597–4600 CrossRef CAS; (d) V. J. Forrat, O. Prieto, D. J. Ramón and M. Yus, Chem.–Eur. J., 2006, 12, 4431–4445 CrossRef CAS; (e) C. Bolm, F. Schmidt and L. Zani, Tetrahedron: Asymmetry, 2005, 16, 1367–1376 CrossRef CAS; (f) V. J. Forrat, D. J. Ramón and M. Yus, Tetrahedron: Asymmetry, 2005, 16, 3341–3344 CrossRef CAS; (g) V. J. Forrat, D. J. Ramón and M. Yus, Tetrahedron: Asymmetry, 2008, 19, 537–541 CrossRef CAS; (h) V. J. Forrat, D. J. Ramón and M. Yus, Tetrahedron: Asymmetry, 2009, 20, 65–67 CrossRef CAS; (i) F. Schmidt, R. T. Stemmler, J. Rudolph and C. Bolm, Chem. Soc. Rev., 2006, 35, 454–470 RSC.
- S. W. Wright, D. L. Hageman and L. D. McClure, J. Org. Chem., 1994, 59, 6095–6097 CrossRef CAS.
- R. Ramage, D. Hopton, M. Parrott, G. W. Kenner and G. A. Moore, J. Chem. Soc., Perkin Trans. 1, 1984, 1357–1370 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, analytical, and spectral characterization data of the products. See DOI: 10.1039/c0cy00083c |
| ‡ Dedicated to Prof. Carmen Nájera Domingo in celebration of her 60th birthday. |
| § General procedure of the catalytic asymmetric phenylation: Under argon atmosphere, a round-bottom flask was charged with Rh(acac)(C2H4)2 (3.1 mg, 0.012 mmol), 1 (6.5 mg, 0.013 mmol), 2 (0.200 mmol), triphenylborane (0.334 mmol), and 50% KF on Celite (40 mg). To the flask was added t-BuOH (0.5 mL), and the mixture was stirred at 100 °C. After the indicated reaction time, the mixture was diluted with AcOEt, washed with brine, dried over Na2SO4, and then concentrated. The resulting residue was purified through silica gel column chromatography. |
| This journal is © The Royal Society of Chemistry 2011 |