Activity improvement of gold yolk–shell catalysts for CO oxidation by doping with TiO2†
Robert
Güttel
,
Michael
Paul
and
Ferdi
Schüth
*
Max-Planck-Institut für Kohlenforschung, Department of Heterogeneous Catalysis, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany. E-mail: schueth@mpi-muelheim.mpg.de; Fax: +49 208 306 2995; Tel: +49 208 306 2373
First published on 31st January 2011
Abstract
Au, @ZrO2 yolk–shell catalysts were found to exhibit a surprisingly high activity in CO oxidation even though the gold particle size is about 15 nm. A further enhancement of the activity has been achieved by simply doping these materials with small amounts of TiO2 during synthesis. A comparison of the standard Au, @ZrO2 yolk–shell catalysts with the novel TiO2-doped Au/Ti, @ZrO2 shows significant activity enhancement, even though small amounts of TiO2 are present.
The high catalytic activity of gold in oxidation of CO to CO2 has induced substantial research efforts regarding the influence of the support but also the catalyst's stability against sintering of the active gold nanoparticles.1–4 High activity at low temperature is, for instance, desirable for air purification in long-duration space travel and in CO2 lasers, while a long lifetime at high temperature would be required in automotive exhaust gas catalysts. For improvement of the stability, we already presented a nanostructured yolk–shell Au, @ZrO2 material, which is catalytically stable at temperatures of up to 800°C.5 These materials are shown to reduce the mobility of the gold cores and prevent activity loss due to sintering at high temperatures. The sinter stability is even more surprising, if one considers the high metal loading of about 10 wt% or even more for the yolk–shell material. However, even if the gold yolk–shell catalysts were found to be surprisingly active, their activity is still much smaller than that of the typical Haruta-type catalysts with gold nanoparticles having sizes of around 2–3 nm. This is primarily due to the fact that rather big gold particles with sizes around 15 nm––with accordingly lower catalytic activity––are most suitable for the encapsulation process. It is also well known from the literature that TiO2 outperforms ZrO2 as a support for gold in terms of activity for CO oxidation.6 Thus, two pathways were explored for the improvement of the catalytic activity of encapsulated gold catalysts: the encapsulation of gold nanoparticles inside a titania shell (denoted as Au, @TiO2) and the doping of the standard Au, @ZrO2 yolk–shell material with titania (denoted as Au/TiO2, @ZrO2).
The encapsulation of gold nanoparticles with a titania shell is already discussed in the literature.7–9 McDowell et al. show that titania can directly be attached to a gold nanoparticle surface without the addition of organic linking groups. Li and Zeng demonstrated the preparation and size manipulation of Au@TiO2 nanoreactors for photocatalytic reactions, and Wu et al. prepared Au@TiO2 core–shell material for photocatalytic degradation of acetaldehyde. However, the catalytic properties of the materials were not in the focus of these contributions, and the materials were tested for photocatalytic reactions only.7,9 Furthermore the stability against sintering was not analyzed, and the gold core sizes were at least around 40 nm, which limits the catalytic activity for CO oxidation drastically.
Since already the Au, @ZrO2 catalyst5 was remarkably active considering the gold particle size, the present work focuses on combinations of these gold nanoparticles with titania in yolk–shell materials to increase their activity further. For the Au/TiO2, @ZrO2 material the titania was supposed to act as a promoter, providing low temperature activity, while the zirconia shell is the structural support, providing stability against sintering. Both functionalities should be combined in the titania shell for the Au, @TiO2 material. In this contribution both materials are compared to each other and to the standard Au, @ZrO2 material with respect to their catalytic performance. For comparibility the gold nanoparticles were prepared identically for each material, resulting in a reproducible particle size of ca. 15 nm (for experimental details see ESI†).
The encapsulation of gold nanoparticles inside a titania shell and the characterization will be discussed in more detail elsewhere.10,11 However, the preparation strategy is shown in Scheme 1 for better understanding. Here only the TEM images of typical samples are shown (Fig. 1) in order to prove that full encapsulation of the gold can be achieved. The doping of the standard Au, @ZrO2 material with titania is based on a modification of the synthetic protocol reported earlier and shown in Scheme 1.5,12 Specifically, the doping with titania was achieved by adding titania precursor solution (0.1 mL titanium(IV)-propoxide, Aldrich, in 8 g EtOH) to the gold colloid solution under stirring for 30 min. The titania precursor was intended to adsorb and form a thin layer on the gold nanoparticle surface (step 2b). Subsequently, the Au/TiO2 intermediate colloids were covered by silica according to the standard procedure, which entraps the titania precursor molecules onto the gold surface during the subsequent steps (step 3b). Finally, the encapsulation with zirconia (step 4b) and the removal of the silica core (step 5b) were achieved as previously reported.12 By addition of 0.15 to 3 mL of the titania precursor solution, the amount of titania was varied in the range of up to atomic Ti/Au ratios of 2, equalling about 5.6 wt% TiO2 in the total material.
 | ||
| Scheme 1 Schematic synthesis strategy of Au, @TiO2 (top) and Au/TiO2, @ZrO2 (bottom) material (1: Au colloid; 2a: Au@SiO2; 3a: Au@SiO2@TiO2; 4a: Au, @TiO2; 2b: Au/TiO2; 3b: Au/TiO2@SiO2; 4b: Au/TiO2@SiO2@ZrO2; 5b: Au/TiO2, @ZrO2). | ||
 | ||
| Fig. 1 Bright field TEM images of the Au, @TiO2 material aged for 6 d before (left) and after (right) additional heat treatment (800 °C for 7 h under air, heating rate 2 K min−1); gold nanoparticles are indicated by white arrows. | ||
To investigate whether the titania is forming either a layer around the gold nanoparticle or separated clusters on the gold surface, a modified process was used, since the small amount of titania is not visible through the zirconia shell by TEM (material denoted as Au/TiO2). In this representative process the material was calcined after the encapsulation in silica to form titania crystals. Subsequently, the silica, which prevents proper analysis by electron microscopy, is leached by NaOH in order to allow investigation of this material by TEM. It was observed by TEM-EDX that titanium is present in the sample (see ESI†), and in Fig. 3dtitania nanoparticles (grain size ≈ 8 nm, lattice spacing 0.39 nm) can be seen in the inset. However, the gold seems to be covered by an amorphous phase, which could not be removed despite repeated NaOH treatment. This amorphous layer is most probably titanium silicate, which is known as support for gold catalysts in epoxidation reactions.13 It can be prepared under similar conditions as applied in the present contribution by mixing of TEOS and titanium alkoxide and subsequent calcination at 550 °C.14 The titanium silicate layer thus offers a strong contact between titanium and gold species.
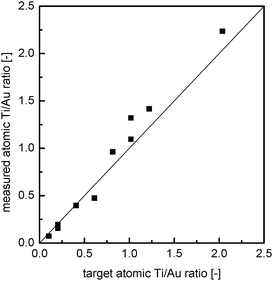 | ||
| Fig. 2 Parity plot for measured (SEM-EDX) versus intended Ti-content in the doped Au/TiO2, @ZrO2 material. | ||
 | ||
| Fig. 3 TEM images of the final Au/TiO2, @ZrO2 material; different atomic Ti/Au ratios (a: 0.19; c: 2.24); b: calcined material from a; d: intermediate Au/TiO2 material (atomic Ti/Au ratio ≈ 0.15). | ||
During preparation it was observed that the addition of more than 2 mL of titania precursor solution (ca. 5.6 wt% TiO2, Ti/Au ratio: 2.24) affects the subsequent coating with a silica layer. In those cases, agglomerates containing gold colloid could be observed by naked eye. TEM images of this material show less defined zirconia spheres not always containing a gold core (compare Fig. 3a and c). Thus, the maximum doping level appears to be in the range of Ti/Au ratios between 1 and 2. The intended titanium mass fraction was found to be in good agreement with the amount measured by SEM-EDX (Fig. 2). This means that the titania offered is mostly incorporated into the material, and only little material is lost during preparation and washing steps.
Some examples of conversion vs. temperature curves are shown in Fig. 4a, a summary of the results for all tested catalysts is given in Fig. 4b (for experimental details see ESI†). The temperatures for half CO conversion as well as the gold mass specific reaction rates are plotted as a function of the titania content (detailed values summarized in ESI†). For this purpose the composition of the material was measured by SEM-EDX. It was observed that the temperature for half conversion decreases with an increasing amount of titania, i.e. the activity increases with doping the standard Au, @ZrO2 material with titania. However, a minimum doping with titania on the order of 0.5 wt% (Ti/Au atomic ratio ca. 0.2) is required to achieve a noticeable and reproducible effect. Further increase of the titania content improves the activity even more, but may lead to reduced quality of the final yolk–shell material as inferred from TEM results.
 | ||
Fig. 4 Results of catalytic oxidation of CO to CO2 for different catalytic materials (titania content obtained by SEM-EDX; catalytic measurement conditions: 1 vol% CO in air, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL h−1 g−1cat); (a) examples of conversion vs. temperature curves for different TiO2 contents, Au, @TiO2 aged for 6 d (see also Fig. 5); (b) temperature for 50% CO conversion (filled symbols) and reaction rate at 60 °C (open symbols) as a function of the titania content. 000 mL h−1 g−1cat); (a) examples of conversion vs. temperature curves for different TiO2 contents, Au, @TiO2 aged for 6 d (see also Fig. 5); (b) temperature for 50% CO conversion (filled symbols) and reaction rate at 60 °C (open symbols) as a function of the titania content. | ||
For comparison, the Au, @TiO2 material was catalytically evaluated under the same conditions. It shows a higher temperature for half conversion compared to the doped Au/TiO2, @ZrO2 material (Fig. 5). This can be explained by the reduced gold content in this material due to the bigger diameter of the titania shell compared to the zirconia system. Taking the gold content into account, the gold mass specific reaction rate of the Au, @TiO2 material is comparable to that of the doped Au/TiO2, @ZrO2. However, the preparation conditions strongly influence the catalytic activity of the Au, @TiO2 material. As discussed in the literature, the aging time during the coating of TiO2 onto the silica is a crucial parameter for the quality of the final titania spheres.10Catalytic measurements confirm this tendency, showing increasing activity for aging times from 0 to 6 d. Since the material obtained without aging shows negligible activity, only results for the materials synthesized with 3 and 6 d aging time are shown in Fig. 5.
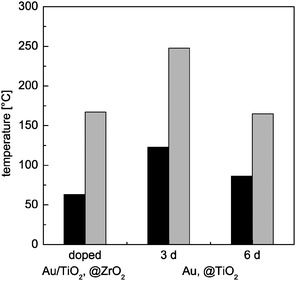 | ||
Fig. 5 Temperature for 50% CO conversion for as made (black) and calcined (grey) material (left: 7.5 wt% Au, 0.6 wt% TiO2, rest ZrO2; middle: 2.2 wt% Au, rest TiO2; right: 1.4 wt% Au, rest TiO2; catalytic measurement conditions: 1 vol% CO in air, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL h−1 g−1cat). 000 mL h−1 g−1cat). | ||
A direct comparison of both materials shows similar gold mass specific reaction rates, which means that the interaction between the doped titania and gold for the doped Au/TiO2, @ZrO2 is comparable to the interaction in the Au, @TiO2 yolk–shell system. From these results it can be concluded that small amounts of titania below 5.6 wt% are sufficient to exert the positive influence on the activity, and the synthetically challenging encapsulation by a titania shell is not necessary. However, the interaction between the doped titania and the gold core needs to be investigated further.
To test the temperature stability, both materials were heated to 800 °C under air with a heating rate of 2 K min−1 and subsequent natural cooling. The treated materials were investigated by TEM and catalytic measurements. For the Au, @TiO2 material the TEM investigations reveal no agglomeration of gold particles outside the titania shell (Fig. 1).10 However, catalytic measurements show a significant loss in activity expressed by an increase in temperature for half conversion of about 80 K for the material which was synthesized by aging for 6 d, and even of 125 K for an aging time of 3 d (Fig. 5). Similarly, TEM does not show any indication for sintering of the doped Au/TiO2, @ZrO2 material (Fig. 3b), while the temperature for half conversion increases by about 100 K after calcination (Fig. 5). Thus the activity after thermal treatment is even lower than for the standard Au, @ZrO2 material. Most probably, the direct contact between titania species and gold has disappeared. This may be induced by a change in the titanosilicate, possibly forming a silica layer around titania particles, as discussed for the interaction between silica and zirconia.15 The systems are thermally stable, though, up to at least 300 °C with respect to their catalytic performance, since this is the highest temperature reached during the catalytic testing of both materials.
The pronounced disagreement with respect to the high-temperature characteristics observed by TEM (stable gold dispersion) and catalytic measurements (significant loss of activity) exists for both types of materials investigated. This discrepancy cannot be explained presently, and requires further investigation. Since no activity loss was detected for the standard Au, @ZrO2 system,5 the reason is most probably correlated with the titania structure and morphology, or the gold–titania interaction. At present the available literature gives no insight into the high-temperature stability of Au/TiO2 catalysts for CO oxidation, which are typically treated at a maximum temperature of 400 °C.6 Standard supported gold catalysts suffer from deactivation induced by agglomeration of the gold nanoparticles at high temperatures, which makes it very difficult to distinguish between deactivation by sintering and by change of the metal–support interaction at high temperatures. The materials presented in this work thus offer the possibility to study metal–support interactions and the effect of high temperature treatment on the catalytic activity without the danger of thermal sintering. In our case, the loss of activity clearly has to be attributed to a change in the interaction between titania and gold, and work is ongoing to determine the nature of this change.
The comparison between the encapsulated and the doped material shows similar activities with respect to the gold mass specific reaction rate. However, the encapsulation of a gold core with a titania shell involves several disadvantages. It was observed during preparation that the titania shells with diameters of less than 200 nm are not sufficiently stable.10 This will limit the achievable gold content in the final material to low values, especially if one considers also the synthesis of smaller gold cores with diameters below 5 nm. In contrast, the encapsulation with zirconia shells allows reduction of the shell diameter down to 50 nm,10 which makes it possible to achieve a reasonable gold content in the catalytic material even for small gold cores. Furthermore, the thermal stability of the titania shell is limited, resulting in a relatively high fraction of broken spheres and also activity loss after thermal treatment. Finally, the titania shell exhibits pores in the size range of 5 to 10 nm. These pores are too big for the stabilization of small gold particles. Further reduction of the core size, which would be essential for higher activity,16 will not be possible, since the gold particles will agglomerate eventually and the effect of encapsulation will be eliminated. In contrast, the doping of the well established Au, @ZrO2 system with small amounts of titania offers the possibility to distinguish between the structural matrix zirconia, providing the stability against sintering, and the catalytic promoter titania for the low temperature activity. A further advantage is the possibility of reducing the pore size in the zirconia shell (see Huang et al.)17 to be able to restrict the mobility of gold cores smaller than 5 nm through the shell.
Based on the presented results it would be highly desirable to reduce the gold core size for further activity improvement. Another interesting possibility is to apply the doping procedure to other materials, such as CeO2 or Fe2O3 as a catalytic promoter and carbon as a structural promoter to extend the yolk–shell approach to other catalytic applications.18–20
Acknowledgements
The authors thank the DFG for financial support through SFB 558 and the MPG, which provided basic funding. For the TEM analysis A. Dreier and B. Spliethoff are gratefully acknowledged. Thanks also go to M. Freimuth for preparation of the samples.Notes and references
- G. C. Bond and D. T. Thompson, Catal. Rev. Sci. Eng., 1999, 41, 319–388 CrossRef CAS.
- M. D. Hughes, Y.-J. Xu, P. Jenkins, P. McMorn, P. Landon, D. I. Enache, A. F. Carley, G. A. Attard, G. J. Hutchings, F. King, E. H. Stitt, P. Johnston, K. Griffin and C. J. Kiely, Nature, 2005, 437, 1132–1135 CrossRef CAS.
- M. Haruta and M. Date, Appl. Catal., A, 2001, 222, 427–437 CrossRef CAS.
- M. Haruta, Nature, 2005, 437, 1098–1099 CrossRef CAS.
- P. M. Arnal, M. Comotti and F. Schüth, Angew. Chem., Int. Ed., 2006, 45, 8224–8227 CrossRef CAS.
- M. Haruta, S. Tsubota, T. Kobayashi, H. Kageyama, M. J. Genet and B. Delmon, J. Catal., 1993, 144, 175–192 CrossRef CAS.
- J. Li and H. C. Zeng, Angew. Chem., Int. Ed., 2005, 44, 4342–4345 CrossRef CAS.
- J. J. McDowell, J. I. McKelvey, L. A. Richard and J. T. Banks, Can. J. Chem., 2008, 86, 703–708 CrossRef CAS.
- X.-F. Wu, H.-Y. Song, J.-M. Yoon, Y.-T. Yu and Y.-F. Chen, Langmuir, 2009, 25, 6438–6447 CrossRef CAS.
- M. Paul, R. Güttel and F. Schüth, 2010, in preparation.
- M. Paul, Dissertation, Ruhr-Universität Bochum, 2009 Search PubMed.
- R. Güttel, M. Paul and F. Schüth, Chem. Commun., 2010, 46, 895–897 RSC.
- A. K. Sinha, S. Seelan, S. Tsubota and M. Haruta, Top. Catal., 2004, 29, 95–102 CrossRef.
- R. B. Khomane, A. A. Kulkarni, A. Paraskar and S. R. Sainkar, Mater. Chem. Phys., 2002, 76, 99–103 CrossRef CAS.
- P. M. Arnal, C. Weidenthaler and F. Schüth, Chem. Mater., 2006, 18, 2733–2739 CrossRef CAS.
- M. Haruta, Catal. Today, 1997, 36, 153–166 CrossRef CAS.
- X. Huang, C. Guo, J. Zuo, N. Zheng and G. D. Stucky, Small, 2009, 5, 361–365 CrossRef CAS.
- N. C. Strandwitz and G. D. Stucky, Chem. Mater., 2009, 21, 4577–4582 CrossRef CAS.
- J. Y. Kim, S. B. Yoon and J. S. Yu, Chem. Commun., 2003, 790–791 RSC.
- C. Galeano, R. Güttel, M. Paul, P. Arnal and F. Schüth, 2010, in preparation.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, composition and catalytic measurement results of the materials, and TEM-EDX results of Au/Ti material. See DOI: 10.1039/c0cy00026d |
| This journal is © The Royal Society of Chemistry 2011 |
