Sulfonic acid containing cation-exchanger resin “INDION-770” and copper(I) salts: a novel reusable catalyst for N-arylation of NH-heterocycles with haloarenes†
P. J.
Amal Joseph
,
S.
Priyadarshini
,
M.
Lakshmi Kantam
and
H.
Maheswaran
*
Inorganic and Physical Chemistry Division, Indian Institute of Chemical Technology, Hyderabad, India. E-mail: maheswaran_dr@yahoo.com; Fax: +91 4027160921; Tel: +91 4027193510
First published on 2nd March 2011
Abstract
Industrial grade sulfonic acid based cation-exchanger resin “INDION-770” in combination with Cu(I) salts is demonstrated to be a versatile heterogeneous catalytic system for direct N-arylation of NH-heterocycles with various haloarenes to give the corresponding N-arylheterocycles in good to excellent yields.
N-Arylheterocycles are prevalent motifs in numerous natural products, pharmaceuticals and agrochemicals.1 The most promising method in the literature for their synthesis is copper-(I) catalyzed Ullmann-type coupling in the presence of nitrogen ligands under homogeneous conditions. Since the initial report by Buchwald et al.,2 substantial progress has been made on the direct copper(I) catalyzed C–N coupling reactions, for the synthesis of N-arylheterocycles.3–7 Indeed, it is very attractive to perform these reactions under heterogeneous conditions due to the innate advantages such as simple work-up, catalyst recoverability, and reusability for multiple reaction cycles. In this direction, we have reported some efficient copper based heterogeneous catalysts for N-arylation reaction of various NH-heterocycles. These reports include heterogeneous catalysts such as Cu-HAP (Cu-Hydroxy-apatite),8Cu-FAP(Cu-Fluoroapatite),8Cu–Al-Hydrotalcite,9 and CuI-Nanoparticles.10
In continuation of these studies, herein, we report a novel copper-based heterogeneous catalyst that is derived from a sulfonic acid containing cation-exchanger resin for direct N-arylation reaction of various structurally divergent haloarenes with NH-heterocycles. For these studies, we have selected a cheap and readily available industrial grade sulfonic acid containing cation-exchanger resin “INDION-770”.11 The potential use of sulfonic acid resins at the conceptual-stage was inspired by a series of intriguing examples that had appeared in the literature. First, it is well-known that acidic carboxylic acid functionalities induce rate accelerations for a variety of reactions under copper-mediated protocols including Ullmann-type reactions.3e,12,13Based on these reports, we have recently demonstrated that Liebeskinds's copper(I)-thiophene-2-carboxylate (CuTC) readily enables activation of haloarenes for the direct N-arylation of NH-heterocycles to the corresponding cross-coupled products in good yields.14 The catalytic activity of CuTC for this reaction is most likely due to unique properties of copper(I)carboxylates in the reaction.15 Therefore, it was conceived that the sulfonic acid based cation-exchanger resin can also be readily employed in combination with Cu(I)-salts in catalytic amounts for effecting direct C–N coupling reactions under heterogeneous conditions, because in situ formation of copper(I)sulfonates could also exhibit rate accelerations similar to those observed for Cu(I)-carboxylates.13d,16 The details of our studies with the INDION-770/Cu(I) system for direct N-arylation reactions of NH-heterocycles with structurally-divergent haloarenes forms the focus of the paper.
First, 4-bromoanisole and imidazole were chosen as model substrates for screening studies, and these substrates were subjected to various reaction conditions with the INDION-770 resin and copper salts under in situ conditions. The results of these studies are summarized in Table 1. As depicted in Table 1, different copper precursors such as CuI, Cu2O, CuBr, and copper powder promote direct C–N coupling reactions in decent yields (73%–87%) with K2CO3 as base in DMSO (Table 1, entries 1–3 and 6). However with CuCl and Cu(OAc)2, the reaction was sluggish, and gave low yields for the desired product (Table 1, entries 4 and 5). Among various bases and solvents that are screened for the reaction, the best results are obtained with K2CO3 and DMSO in the presence of CuI as the catalyst precursor at 125 °C (Table 1, entry 1). Notably, for N-arylation reaction of pyrazole with 4-bromoanisole, the best catalyst–base combination was found to be Cu2O or CuI and Cs2CO3 (Table 1, entries 22 and 24). These results requiring a relatively stronger base for the cross-coupling reactions with pyrazole are not surprising because the leaving N-hydrogen in pyrazole is comparatively less acidic than those in imidazole.17
| Entrya | Catalyst | Solvent | Base | Yieldl/% |
|---|---|---|---|---|
| a Reactions (entries 1–10) performed on a 2.0 mmol scale with 4-bromoanisole (2.0 mmol), imidazole (2.6 mmol), copper salt (0.20 mmol, 10 mol%), INDION-770 resin (200 mg; ∼0.8 mmol of sulfonic acid group), base (4 mmol), and 3 mL of solvent at 125 °C for 24 h. b Reaction performed at 110 °C. c Reactions (entries 11 and 12) performed with 0.20 mmol of p-toluenesulfonic acid (PTSA) instead of the INDION 770 resin. d Reactions (entries 13–14) performed with 0.78 mmol of PTSA. e Reaction (entry 15) performed with 0.95 mmol of benzenesulfonic acid. f Reaction (entry 16) performed with 200 mg of the Amberlyst-16 wet resin. g Reaction (entry 17) performed with 210 mg of the copper exchanged INDION-770 catalyst. h Reaction (entry 18) performed in the absence of the INDION-770 resin. i Reaction (entry 19) performed in the presence of 200 mg of the INDION-770 resin but in the absence of any copper precursor salts. j Reaction (entry 20) performed in the absence of both the copper catalyst and the INDION-770 resin. k Reactions (entries 21–24) performed on a 2.0 mmol scale with 4-bromoanisole (2.0 mmol), pyrazole (2.6 mmol), copper catalyst (0.20 mmol, 10 mol%), INDION-770 resin (200 mg), base (4 mmol), and 3 mL of solvent at 125 °C for 24 h. l Isolated yields after column chromatographic purification. | ||||
| 1 | CuI | DMSO | K2CO3 | 87, 19b |
| 2 | Cu2O | DMSO | K2CO3 | 80 |
| 3 | CuBr | DMSO | K2CO3 | 77 |
| 4 | CuCl | DMSO | K2CO3 | 32 |
| 5 | Cu(OAc)2 | DMSO | K2CO3 | 35 |
| 6 | Cu powder | DMSO | K2CO3 | 73 |
| 7 | CuI | DMSO | Cs2CO3 | 81 |
| 8 | CuI | DMSO | K3PO4 | 75 |
| 9 | CuI | DMF | K2CO3 | 42 |
| 10 | CuI | Xylene | K2CO3 | 05 |
| 11c | Cu2O | DMSO | K2CO3 | 32 |
| 12c | CuI | DMSO | K2CO3 | 35 |
| 13d | Cu2O | DMSO | K2CO3 | 70 |
| 14d | CuI | DMSO | K2CO3 | 71 |
| 15e | CuI | DMSO | K2CO3 | 79 |
| 16f | CuI | DMSO | K2CO3 | 40 |
| 17g | Cu-INDION | DMSO | K2CO3 | 85 |
| 18h | CuI | DMSO | K2CO3 | 19 |
| 19i | DMSO | K2CO3 | ND | |
| 20j | DMSO | K2CO3 | ND | |
| 21k | CuI | DMSO | K2CO3 | 80 |
| 22k | CuI | DMSO | Cs2CO3 | 85 |
| 23k | Cu2O | DMSO | K2CO3 | 12 |
| 24k | Cu2O | DMSO | Cs2CO3 | 88 |
To better understand the role of the sulfonic acid group in the model reactions, we evaluated p-toluenesulfonic acid (PTSA) as the ligand additive in place of the INDION-770 resin. As can be seen, the use of catalytic amounts (10 mol%) of PTSA results in low yield; whilst the use of large amounts (39 mol%) afforded the desired product in good yield (Table 1, entries 11–14). Conversely, the use of the sulfonic acid containing Amberlyst-16 wet resin for the direct N-arylation reaction gave only moderate yield (40%) for the desired product (Table 1, entry 16). In contrast, the yields of the products that are obtained with the INDION-770 resin for the N-arylation reaction are comparable to those obtained with many copper catalyzed reactions in the presence of common nitrogen ligand additives under homogeneous conditions.3–7 These results demonstrate that the sulfonic acid group is an effective additive for copper catalyzed C–N coupling reaction with NH-heterocycles.
Using the optimized reaction conditions in hand, the direct C–N coupling reactions of NH-heterocycles with various haloarenes were studied. As can be seen from Table 2, the Cu(I)/INDION-770 catalyst system based protocol is rather general in nature, as it is applicable for the reactions of a variety of electron-rich and electron-deficient iodoarenes as well as bromoarenes with imidazole, benzimidazole, and pyrazole. The reaction of iodoarenes was rather fast, and their coupling reactions with imidazole gave almost quantitative yields within 10–15 h (Table 2, entries 1–3). Understandably, the reactions of bromobenzene and electron rich bromoarenes such as p-bromoanisole, p-bromotoluene and m-bromoanisole with NH-heterocycles took up 24 h, to afford the corresponding cross-coupled products in good yields (Table 2, entries 4, 10–12, 23, 25, 26, 29, 30, 32 and 33). However, much longer reaction times (36 h) were needed for bromoarenes that contain strongly deactivating groups such as p-NH2 or p-SMe in the aromatic ring (Table 2, entries 15, 16, 36 and 37). Interestingly, the reaction of activated bromoarenes like p-bromonitrobenzene proceeded faster (15 h) to furnish an excellent yield of the desired product (Table 2, entry 5).
| Entry | R | X | NH-heterocycle | Time/h | Yieldd (%) | Entry | R | X | NH-heterocycle | Time/h | Yieldd (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aa | Bc | Aa | ||||||||||
| a Reactions (entries 1–28) performed on a 2.0 mmol scale with haloarenes (2.0 mmol), NH-heterocycle (2.6 mmol), cuprous iodide (0.20 mmol, 10 mol%), INDION-770 resin (200 mg), K2CO3 (4 mmol), and 3 mL of DMSO at 125 °C for the indicated time. b Reactions (entries 29–40) performed on a 2.0 mmol scale with haloarenes (2.0 mmol), pyrazole (2.6 mmol), cuprous oxide (0.20 mmol, 10 mol%), INDION-770 resin (200 mg), Cs2CO3 (4 mmol), and 3 mL of DMSO at 125 °C for the indicated reaction time. c Reactions performed in the absence of both cuprous iodide and INDION-770 resin. d Isolated yields after column chromatographic purification. NR = No reaction. | ||||||||||||
| 1 | H | I | Im | 12 | 94 | 21 | 4-MeO | Cl | Im | 48 | NR | |
| 2 | 4-NO2 | I | Im | 10 | 98 | 38c | 22 | H | Cl | Im | 48 | <8 |
| 3 | 4-MeO | I | Im | 15 | 93 | NR c | 23 | H | Br | BzIm | 24 | 88 |
| 4 | H | Br | Im | 24 | 89 | NR c | 24 | 2-MeO | Br | BzIm | 24 | 82 |
| 5 | 4-NO2 | Br | Im | 15 | 95 | 35c | 25 | 4-MeO | Br | BzIm | 24 | 84 |
| 6 | 2-NO2 | Br | Im | 18 | 95 | 31c | 26 | 3-Me | Br | BzIm | 24 | 85 |
| 7 | 4-CF3 | Br | Im | 20 | 91 | 27 | C4H4 (2-naphth) | Br | BzIm | 86 | ||
| 8 | 4-COMe | Br | Im | 24 | 80 | NR c | 28 | 4-NO2 | Cl | BzIm | 18 | 93 |
| 9 | 4-Cl | Br | Im | 24 | 89 | 29b | H | Br | Py | 24 | 89 | |
| 10 | 4-Me | Br | Im | 24 | 83 | 30b | 4-MeO | Br | Py | 24 | 88 | |
| 11 | 4-MeO | Br | Im | 24 | 87 | NR c | 31b | 2-MeO | Br | Py | 24 | 82 |
| 12 | 3-MeO | Br | Im | 24 | 87 | 32b | 3-MeO | Br | Py | 24 | 88 | |
| 13 | 2-MeO | Br | Im | 24 | 82 | 33b | 4-Me | Br | Py | 24 | 84 | |
| 14 | C4H4 (2-naphth) | Br | Im | 24 | 87 | NR c | 34b | C4H4 (2-naphth) | Br | Py | 24 | 86 |
| 15 | 4-NH2 | Br | Im | 36 | 70 | 35b | 4-Cl | Br | Py | 24 | 85 | |
| 16 | 4-MeS | Br | Im | 36 | 76 | 36b | 4-MeS | Br | Py | 36 | 78 | |
| 17 | 4-CF3 | Cl | Im | 30 | 82 | NR c | 37b | 4-NH2 | Br | Py | 36 | 69 |
| 18 | 4-CN | Cl | Im | 30 | 78 | NR c | 38b | 4-COMe | Br | Py | 36 | 75 |
| 19 | 2-NO2 | Cl | Im | 20 | 92 | 39b | 2-NO2 | Cl | Py | 24 | 92 | |
| 20 | 4-NO2 | Cl | Im | 20 | 94 | 14c | 40b | 4-CF3 | Cl | Py | 36 | 83 |
In the hope of broadening the scope of the N-arylation protocol, we explored the application of our catalyst system to activated chloroarenes and further to sterically hindered haloarenes. As illustrated in Table 2, the reactions of NH-heterocycles with various chloroarenes containing electron-withdrawing groups proceed smoothly, albeit at longer reaction times (20–36 h). Substrates containing p-NO2, p-CN, and p-CF3 groups gave excellent yields (78–94%) for the corresponding N-arylated adducts (Table 2, entries 17, 18, 20, 28, 39 and 40). Small amounts (∼14%) of the hydrolyzed product were obtained in the reactions of imidazole with p-chlorobenzonitrile (Table 2, entry 18). However, chlorobenzene reacted very slowly with imidazole, and even after 48 h of the reaction, very poor yield (∼8%) was obtained (Table 2, entry 22). Similarly, strongly deactivated chloroarenes like p-chloroanisole did not react at all under our experimental conditions (Table 2, entry 21). These results were not surprising because most copper-based catalysts also show a similar activity for such strongly deactivated chloroarene substrates.3–7 The present protocol works efficiently for ortho-substituted haloarenes as the reactions of 2-bromoanisole and 2-chloronitrobenzene with NH heterocycles afforded excellent yields (Table 2, entries 6, 13, 19, 24, 31 and 39).
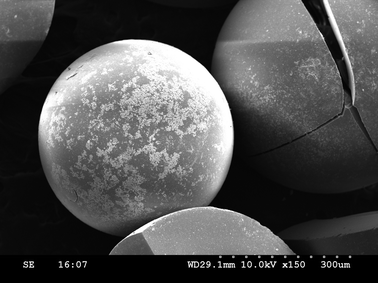
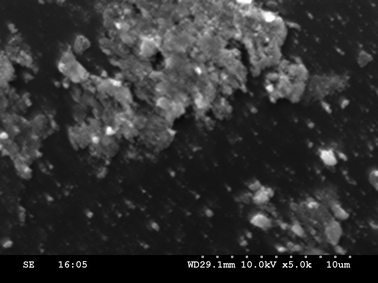
To elucidate the chemistry of the INDION-770/CuI system, we carried out a reaction between CuI (0.2 mmol) and INDION-770 resin (200 mg, ∼0.8 mmol of sulfonic acid groups) in 3 mL of DMSO in a sealed tube at 110 °C under stirring for 15 minutes, to obtain a pale green coloured copper exchanged INDION-770 resin. This was further characterized by ICP-OES, SEM (Fig. 1), and XPS studies.18 When this copper exchanged INDION-770 resin was used as catalyst instead of the general in situ conditions, we obtained similar yields for N-arylation reactions of 4-bromoanisole with imidazole (Table 1, entry 17). These studies proved complete exchange of copper from cuprous iodide to INDION-770 resin, and thus the active catalyst for the N-arylation reaction is the copper-exchanged INDION-770 resin. However, to verify whether any leaching of copper from the copper exchanged INDION-770 resin occurs during the reaction, the spent catalyst was recovered from the reaction mixture and subjected to recycling studies for the N-arylation between imidazole and 4-iodoanisole using optimized reaction conditions (Table 3). Then, after every recycle, both the spent catalyst (Fig. 2) and the reaction solution were analyzed for the copper content using ICP-OES studies. As can be seen in Table 3, the recovered copper exchanged INDION-770 catalyst can be reused successfully for up to four reaction cycles without significant loss in the yield. Notably, the ICP-OES analysis of both the fresh and spent resin based catalysts gave nearly consistent values for the copper content (∼0.90 mmol g−1 {∼56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm}) for all four-recycling studies. Similarly, when the reaction solution was analyzed by ICP-OES studies, only very low (∼0.2–2.2 ppm) amount of copper ions was present for all the four-recycling experiments. These results clearly demonstrate that the leaching of the copper ion from the resin into reaction solution is very negligible; and therefore, the N-arylation protocol described herein is purely heterogeneous in nature.
000 ppm}) for all four-recycling studies. Similarly, when the reaction solution was analyzed by ICP-OES studies, only very low (∼0.2–2.2 ppm) amount of copper ions was present for all the four-recycling experiments. These results clearly demonstrate that the leaching of the copper ion from the resin into reaction solution is very negligible; and therefore, the N-arylation protocol described herein is purely heterogeneous in nature.
| Entry | Cycle | Yieldb (%) | Cu in reaction solution (ppm) | Cu in INDION-770 (ppm) |
|---|---|---|---|---|
| a Reaction performed on a 2.0 mmol scale with 4-iodoanisole (2.0 mmol), imidazole (2.6 mmol), copper exchanged INDION-770 (210 mg, ∼0.2 mmol of Cu), K2CO3 (4 mmol), and 3 mL of DMSO at 125 °C for 24 h. b Isolated yields after column chromatographic purification. | ||||
| 1 | 1 | 91 | 0.2 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 2 | 2 | 90 | 0.2 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 3 | 3 | 87 | 0.8 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 4 | 4 | 84 | 2.2 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
Several mechanisms for copper mediated protocols have been proposed for direct N-arylation reactions involving nitrogen based ligands.3m,19 These catalytic pathways include oxidative addition pathways involving Cu(III) intermediates, the Single Electron Transfer (SET) mechanism, σ-bond metathesis and π-bond metathesis in the literature.3m,19 However, the N-arylation protocol presented herein does not involve nitrogen based ligands, and also the work described herein is heterogeneous in nature, we are not presently clear about the mechanistic aspects involving the copper exchanged INDION-770 resin based system for direct N-arylation reaction. Nevertheless, to verify whether any non-catalyzed mechanistic pathways such as nucleophilic aromatic substitution (SNAr) are involved in the reaction, we carried out several C–N coupling reactions with imidazole and various haloarenes in the absence of the catalyst.20a These studies reveal that with the exception of three specific highly activated halonitroarenes,20b namely, 4-iodonitrobenzene, 4-bromonitrobenzene and 2-bromonitrobenzene (Table 2, entries 2, 5 and 6), such uncatalyzed reactions did not furnish any detectable products for remaining haloarenes that are evaluated in this work (Table 2, entries 3, 4, 8, 11, 14, 17 and 18). Therefore, given the larger scope of the copper exchanged INDION-770 resin based synthesis protocol described herein (Table 2), any significant contributions from uncatalyzed SNAr mechanistic pathways in the reactions can be ruled out as the presence of the copper catalyst is a prerequisite to promote the N-arylation reaction.
In summary, a novel copper based protocol involving a cheap industrial grade sulfonic acid containing cation-exchanger resin “INDION-770” for N-arylation of NH-heterocycles with various haloarenes is presented. This protocol is demonstrated to be heterogeneous in nature with the active catalyst being the copper exchanged INDION-770 resin. It has also been shown that the spent catalyst can be recovered from the reaction mixture by simple filtration, and successfully reused for four-cycles without any significant loss of the catalytic activity and yield of the N-arylated products. Finally, the resin based heterogeneous protocol described herein is very economical, stable to both air and moisture, and applicable for cross-coupling of a variety of both electron-rich as well as electron-deficient iodoarenes, bromoarenes and activated chloroarenes with NH-heterocycles.
Notes and references
- (a) J. Elguero, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, Oxford, 1st edn, 1984, vol. 5, p. 291 Search PubMed; (b) J. Elguero, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon, Elsevier Science Ltd., Oxford, 1st edn, 1996, vol. 3, p. 70 Search PubMed; (c) M. L. Quan, P. Y. S. Lam, Q. Han, D. J. P. Pinto, M. Y. He, R. Li, C. D. Ellis, C. G. Clark, C. A. Teleha, J. H. Sun, R. S. Alexander, S. Bai, J. M. Luettgen, R. M. Knabb, P. C. Wong and P. R. R. Wexler, J. Med. Chem., 2005, 48, 1729 CrossRef CAS; (d) B. Dyck, V. S. Goodfellow, T. Phillips, J. Grey, M. Haddach, M. Rowbottom, G. S. Naeve, B. Brown and Saunders, Bioorg. Med. Chem. Lett., 2004, 14, 1151 CrossRef CAS; (e) P. N. Craig, in Comprehensive Medicinal Chemistry, ed. C. J. Drayton, Pergamon, New York, NY, 1991, vol. 8 Search PubMed; (f) N. R. Candeias, L. C. Branco, P. M. Gois, C. A. M. Afonso and A. F. Trindade, Chem. Rev., 2009, 109, 2703 CrossRef CAS.
- A. Kiyomori, J. F. Marcoux and S. L. Buchwald, Tetrahedron Lett., 1999, 40, 2657 CrossRef CAS.
- (a) J. C. Antilla, A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 11684 CrossRef CAS; (b) J. C. Antilla, J. M. Baskin, T. E. Barder and S. L. Buchwald, J. Org. Chem., 2004, 69, 5578 CrossRef CAS; (c) H.-J. Cristau, P. P. Cellier, J.-F. Spindler and M. Taillefer, Eur. J. Org. Chem., 2004, 695 CrossRef CAS; (d) H.-J. Cristau, P. P. Cellier, J.-F. Spindler and M. Taillefer, Chem.–Eur. J., 2004, 10, 5607 CrossRef CAS; (e) D. Ma, Q. Cai and H. Zhang, J. Org. Chem., 2005, 70, 5164 CrossRef CAS; (f) L. Liu, M. Frohn, N. Xi, D. Celia, H. Randy and P. J. Reider, J. Org. Chem., 2005, 70, 10135 CrossRef CAS; (g) R. A. Altman and S. L. Buchwald, Org. Lett., 2006, 8, 2779 CrossRef CAS; (h) R. A. Altman, E. D. Koval and S. L. Buchwald, J. Org. Chem., 2007, 72, 6190 CrossRef CAS; (i) R. A. Altman and S. L. Buchwald, Nat. Protoc., 2007, 2, 2474 Search PubMed; (j) L. Zhu, L. Cheng, Y. Zhang, R. Xie and J. You, J. Org. Chem., 2007, 72, 2737 CrossRef CAS; (k) L.-V. Xin and W. Bao, J. Org. Chem., 2007, 72, 3863 CrossRef CAS; (l) X. Guo, H. Rao, Y. Fu, Y. Jiang and Y. Zhao, Adv. Synth. Catal., 2006, 348, 2197 CrossRef CAS; (m) H. Maheswaran, G. G. Krishna, K. L. Prasanth, V. Srinivas, G. K. Chaitanya and K. Bhanuprakash, Tetrahedron, 2008, 64, 2471 CrossRef CAS; (n) F. W. Li and T. S. A. Hor, Chem.–Eur. J., 2009, 15, 10585 CrossRef CAS; (o) P. F. Larsson, A. Correa, M. Carril, P. O. Norrby and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5691 CrossRef CAS.
- For copper(II) catalyzed N-arylation of imidazole, see: Y. Wang, Z. Wu, L. Wang, Z. Li and X. Zhou, Chem.–Eur. J., 2009, 15, 8971 Search PubMed.
- For reviews, see: (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (b) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS; (c) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS; (d) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS.
- The palladium based catalysts have also been shown to be useful for N-arylation reactions, for references, see: (a) J. P. Wolfe and S. L. Buchwald, Angew. Chem., Int. Ed., 1999, 38, 2413 CrossRef CAS; (b) A. R. Muci and S. L. Buchwald, Top. Curr. Chem., 2002, 219, 131; (c) J. F. Hartwig, in Modern Arene Chemistry, ed. A. Didier, Wiley-VCH, Weinheim, 2002, p. 107 Search PubMed; (d) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428 CrossRef CAS; (e) B. Schlummer and U. Scholz, Adv. Synth. Catal., 2004, 346, 1599 CrossRef CAS; (f) H. Xu, Mini-Rev. Org. Chem., 2009, 6, 367 Search PubMed; (g) B. P. Fors and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 15914 CrossRef CAS.
- Recently iron based catalysts were used for N-arylation of NH-heterocycles. For references, see: (a) A. Correa and C. Bolm, Angew. Chem., Int. Ed., 2007, 46, 8862 CrossRef CAS; (b) A. Correa and C. Bolm, Adv. Synth. Catal., 2008, 350, 391 CrossRef; (c) Y. C. Teo, Adv. Synth. Catal., 2009, 351, 720 CrossRef CAS.
- B. M. Choudary, C. Sridhar, M. L. Kantam, G. T. Venkanna and B. Sreedhar, J. Am. Chem. Soc., 2005, 127, 9948 CrossRef CAS.
- B. Sreedhar, R. Arundhathi, P. L. Reddy, M. A. Reddy and M. L. Kantam, Synthesis, 2009, 2517 CrossRef CAS.
- B. Sreedhar, R. Arundhathi, P. L. Reddy and M. L. Kantam, J. Org. Chem., 2009, 74, 7951 CrossRef CAS.
- The industrial grade cation exchanger INDION-770 resin is readily available in low price from Ion-Exchange India Limited. More detailed specifications for the INDION-770 resin can be obtained from the website www.ionexchangeglobal.com/pdf/catalyst.pdf.
- For rate enhancements with copper carboxylates in “living” radical polymerization reactions, see: (a) M. van der Sluis, B. Barboiu, N. Pesa and V. Percec, Macromolecules, 1998, 31, 9409 CrossRef CAS; (b) M. Wei, J. Xia, S. G. Gaynor and K. Matyjaszewski, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 1997, 38, 685 CAS.
- In copper(I)-catalyzed Ullmann-type cross-coupling reactions, positive rate enhancement with respect to unfunctionalized substrates has been demonstrated using coupling partners that contain a copper-chelating carboxylic group, such as 2-halogenobenzoic acids, α-amino acids, and β-amino acids, in the reactions, see: (a) C. Couture and A. J. Paine, Can. J. Chem., 1985, 63, 111 CrossRef CAS; (b) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428 CrossRef CAS; (c) Z. Wang, W. Bao and Y. Jiang, Chem. Commun., 2005, 2849 RSC; (d) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS; (e) See also ref. 3e.
- H. Maheswaran, G. G. Krishna, V. Srinivas, K. L. Prasanth and C. V. Rajasekhar, Bull. Chem. Soc. Jpn., 2008, 81, 515 CrossRef CAS.
- (a) G. D. Allred and L. S. Liebeskind, J. Am. Chem. Soc., 1996, 118, 2748 CrossRef CAS; (b) S. Zhang, D. Zhang and L. S. Liebeskind, J. Org. Chem., 1997, 62, 2312 CrossRef CAS; (c) For a review on various uses of CuTC, see: A. Innitzer, Synlett, 2005, 2405 Search PubMed; (d) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2001, 3, 91 CrossRef CAS; (e) L. S. Liebeskind and J. Srogl, J. Am. Chem. Soc., 2000, 122, 11260 CrossRef CAS.
- Recently ‘catechol violet’ was used as an additive in Cu(I) catalyzed direct C–S bond forming reactions of haloarenes. Notably, catechol violet contains the sulfonic acid group, for reference, see: B. Basu, B. Mandal, S. Das and S. Kundu, Tetrahedron Lett., 2009, 50, 5523 Search PubMed.
- The pKa values of NH-hydrogens in benzimidazole, imidazole and pyrazole in DMSO are 16.4, 18.6 and 19.8 respectively. This shows that imidazole NH-hydrogen is more acidic than those in pyrazole. For reference, see: F. G. Bordwell, Acc. Chem. Res., 1988, 21, 456 Search PubMed.
- The copper exchanged INDION-770 resin (both fresh and recycled catalyst) has been characterized by XPS and SEM studies. For more details, see ESI†.
- (a) E. R. Strieter, B. Bhayana and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 78 CrossRef CAS; (b) G. O. Jones, P. Liu, K. N. Houk and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 6205 CrossRef CAS.
- (a) SNAr pathways do not require any catalyst to promote the reaction; for reference to SNAr pathways see: M. B. Smith and J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, John Wiley and Sons, 6th edn, 2007, p. 854 Search PubMed; (b) It is well-known and also our experimental observation that for highly activated nitro groups containing haloarenes such as 4-iodonitrobenzene or 4-bromonitrobenzene, the cross-coupling reactions with nucleophiles proceed in the absence of any catalysts under the likely influence of SNAr pathways.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0cy00074d |
| This journal is © The Royal Society of Chemistry 2011 |