Fe-TAML/hydrogen peroxide degradation of concentrated solutions of the commercial azo dye tartrazine†
Evan S.
Beach‡
,
Ryan T.
Malecky
,
Roberto R.
Gil
,
Colin P.
Horwitz§
* and
Terrence J.
Collins
*
Department of Chemistry, Institute for Green Science, Carnegie Mellon University, Pittsburgh, PA, USA. E-mail: tc1u@andrew.cmu.edu; Fax: +1 412 268-1061; Tel: +1 412 268-6335
First published on 4th March 2011
Abstract
Here we describe a catalytic oxidation process for decomposing concentrated dye solutions, as a model for the treatment of concentrated industrial effluent streams. The prototype Fe-TAML/H2O2 oxidizing system rapidly and extensively degrades the recalcitrant azo dye, tartrazine, in water at ambient temperatures and at dye concentrations that are industrially important. Nearly complete dye removal can be obtained with performance-optimized dosing and pH control. At higher, but still remarkably low catalyst and oxidant concentrations, the dye is removed to below detection limits. 3D surface plots reveal that optimum decolorization at high dye concentration (16.5 g L−1, 30.9 mM) requires 50 times less catalyst and 5 times less oxidant per substrate than at low dye concentration (16.5 mg L−1, 30.9 μM), demonstrating a clear process benefit of higher substrate concentrations. The Fe-TAML/H2O2 system generates environmentally benign and/or biodegradable products from tartrazine: small organic acids, 4-phenolsulfonic acid, and a small amount of 4-nitrobenzenesulfonic acid. The acute toxicity of the Fe-TAML/H2O2/tartrazine reaction mixture toward luminescent bacteria is approximately half that of tartrazine as determined by the Microtox® assay. The results suggest a potential utility of the Fe-TAML/H2O2 technology for treating wastewaters containing high substrate concentrations from the dyeing industry in a straightforward manner to ameliorate environmental impacts, and because of the degradation resistance of tartrazine, that this potential utility might extend to other industries.
Introduction
New methods are needed for degrading recalcitrant pollutants in water to protect aquatic life and water supplies. In the textile industry effective processes are required for degrading dyes in effluent streams in both concentrated and dilute forms. A recent review emphasized the high cost of disposing of the high volumes of dye effluent; 128 tons of dyes are released daily to the global environment.1 Here we describe the destruction of concentrated aqueous solutions of the high volume azo dye, tartrazine, by Fe-TAML/hydrogen peroxide catalytic oxidation. Tartrazine was selected because it is both extensively used and particularly obdurate. It is stable to hydrolysis2 and difficult to remove by adsorption to activated sludge.3 The details of literature methods for biological4,5 and chemical6–9oxidation show that even at low concentrations, tartrazine bleaching requires long reaction times and large excesses of catalysts and oxidants. The enduring nature of tartrazine, together with the difficult decontamination problems associated with dye wastewaters, make it a suitable model target for exploring new approaches to water purification.We have previously reported that Fe-TAML catalysts (Fig. 1a) activate hydrogen peroxide by a non-Fenton mechanism to bleach synthetic dyes in low concentrations with the dye removal being demonstrated by UV/Visible spectroscopy.10–13 In this way, Fe-TAML/H2O2 has been used to bleach both individual dyes and mixtures of dyes from a wide variety of structural classes; phenolic, naphtholic, anthraquinone, fluorescein, stilbene, phthalocyanine, indigoid, aromatic amine, and metallated dyes belonging to the Acid, Basic, Direct, Disperse, and Reactive Dye Colour Index categories.
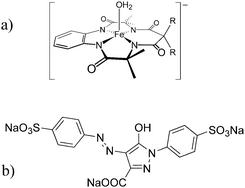 | ||
| Fig. 1 (a) The Fe-TAML catalyst used in this study (R = CH3). (b) Tartrazine. | ||
In this study, the tartrazine concentration was increased 1000-fold to simulate conditions found in textile dyeing baths. Advanced oxidation processes (OH radical-based systems) are typically not practical at pollutant concentrations higher than 5.0 g L−1COD due to excessive reagent use necessitated by the inherently inefficient nature of OH radical reactions.14 The high dye concentrations we used (approx. 18 g L−1COD) approach the level where autothermic wet oxidation is possible. The experiments were designed not only to determine the bleaching and degradation efficacies, but also to gain understanding of the ability of the Fe-TAML/H2O2 process to reduce the environmental impacts of treated dye effluent streams.
Materials and methods
Chemicals
The Fe-TAML catalyst was prepared as the sodium salt by Fisher Scientific U.K., Limited, Loughborough, England. All other chemicals were purchased from ACROS Organics, Sigma-Aldrich Co., TCI America, or Cambridge Isotope Laboratories, and used as received. The tartrazine purity was determined to be 82.3% by 1H NMR spectroscopy.Dosing optimization experiments
For tartrazine, dye concentrations of 3.09 × 10−2 M and 3.09 × 10−5 M were used. At high dye concentration, the Fe-TAML doses ranged from 3.75–75 × 10−6 M and the H2O2 doses ranged from 0.33–2.0 M. At low dye concentration, the Fe-TAML doses ranged from 3.75–75 × 10−7 M and H2O2 doses ranged from 3.125–37.5 × 10−3 M. See ESI† (Tables S1–S6) for exact concentrations used in each experiment. Reactions were in pH 10 buffer (0.1 M carbonate), initiated by hydrogen peroxide addition, and after 15 or 60 min each sample was diluted with additional buffer and immediately analyzed by UV/Visible spectroscopy (at low dye concentration, the dilution step was omitted). Reactions were carried out at room temperature, but not maintained at constant temperature. The “extent of decolorization” was defined as the percent reduction in absorbance at the dyeλmax (400 nm).1H NMR spectroscopy
1H NMR was performed at 300 K with a Bruker Avance DMX-500 at 500.13 MHz equipped with a 5 mm Inverse Broadband (BBI) probe, employing the internal chemical shifts (d) standard TMSP-d4 (sodium trimethylsilylpropionic acid-2,2,3,3-d4). Aqueous samples contained at least 25% D2O for locking and shimming. The built-in pulse program “zgpr” was used for solvent suppression employing a presaturation pulse level of 50 dB. The 90° pulse was calibrated and at least 16 scans were collected for each sample. Processing was carried out using SpinWorks software version 2.5 (Kirk Marat, http://www.umanitoba.ca/chemistry/nmr/spinworks).Ion chromatography, chemical oxidation demand, total carbon, and toxicity
Testing was performed according to standard methods; details are available in the ESI.†Results and discussion
Treatment of tartrazine with sodium hypochlorite
Tartrazine (Fig. 1b) is a monoazopyrazolone dye that is among the top three dyes by total sales. It is primarily employed as a food colorant, but is also widely used in textile and paper applications.1Chlorine (hypochlorite in basic media) provides a well known technology for bleaching dyes.15 Thus the technical and environmental performances of NaOCl in bleaching tartrazine provide a benchmark for Fe-TAML/H2O2.A high concentration of tartrazine reacted with sodium hypochlorite in alkaline conditions and the tartrazine absorbance maximum at 400 nm decreased by 90%. However, after 5 hours reaction time the product mixture still strongly absorbed in the near-UV range (Fig. 2). NMR spectroscopy of the product mixture showed that all 1H signals were in the aromatic region. Furthermore, 2 mM 4-chlorobenzenesulfonic acid (4-CBSA) was produced per 30.9 mM tartrazine. 4-CBSA is among the less biodegradable disubstituted benzene derivatives.16 It has high mobility in the environment, reportedly passing unaltered through biological and physicochemical wastewater treatment systems.17,18 It was not clear whether tartrazine/NaOCl produced other organochlorines as well. By contrast, with the Fe-TAML/H2O2 system, color reduction occurred in both the visible and near-UV ranges and the aromatic rings were extensively degraded. These results highlight that measuring color reduction at the dyeλmax does not provide a sufficient basis for evaluating the environmental compatibility of dye treatment technologies. Knowing the nature of the products is also critical for evaluating both environmental and technical performance.
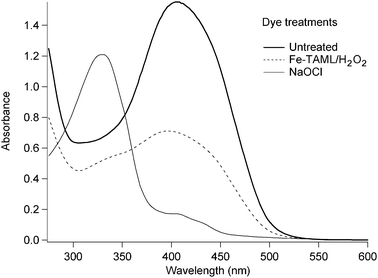 | ||
| Fig. 2 UV/Visible spectra of untreated and treated tartrazine. Conditions: 3.09 × 10−2 M dye, pH 10, 0.309 M (10 equiv.) NaOCl or H2O2, 7.5 × 10−5 M Fe-TAML, 5 hours reaction, room temperature, diluted 500-fold with pH 10 buffer for UV/Visible analysis. | ||
Hydrogen peroxide is one of the most important oxidants in biological transformations. Oxygen, introduced into organic compounds by peroxide oxidations, is among the most familiar elements of biochemistry, in stark contrast with the chlorine that is found in the organic products of hypochlorite chemistry and other chlorine-based oxidants. Designing technologies to produce products comprised of only the common elements of biochemistry is an important strategy for avoiding persistent toxic compounds.19 But tartrazine, like many anthropogenic pollutants, is very resistant to oxidation by hydrogen peroxide—at high dye concentration (3.09 × 10−2 M), in pH 10 buffer (0.1 M carbonate), 20 molar equivalents of H2O2 gave less than 20% bleaching in 1 week. Fe-TAML catalysts are potent peroxide activators—by adding trace Fe-TAML under the same conditions, near complete decolorization can be achieved in a matter of minutes.
Dosage optimization for decolorization of tartrazine by Fe-TAML and H2O2
Green chemical processes have to make economic sense. Because cost often determines whether or not a particular effluent treatment process is chosen, the search for a best possible technology is not necessarily limited by the need to achieve complete removal of a contaminant. Thus, we set out to optimize two sets of conditions—those that allow for maximum dye removal using the least amount of catalyst and oxidant, and those required for complete dye removal.In concentrated dye degradation experiments, a starting pH of 10 was found to be best. At this pH, the rate of activation of H2O2 by Fe-TAML is at a maximum20 and acid-catalyzed demetallation of the catalyst is insignificant.21 This pH is also higher than the tartrazine pyrazolone–OH pKa of 8.9.22 Thus, the “common ion” tautomer of the dye predominates. The common ion is azo rather than hydrazone in character and is the most susceptible to oxidation.23 Commercial dye baths are typically highly alkaline24,25 so this operating pH is representative of industrial process conditions.
Single addition doses of Fe-TAML and H2O2 were optimized for high and low tartrazine concentrations (3.09 × 10−2 M and 3.09 × 10−5 M) with pH 10 carbonate buffer (0.1 M) for 15- or 60-minute reaction times. The relationships of decolorization, Fe-TAML concentration, and H2O2 concentration for 60-minute treatments at the two dye concentrations are shown in a three-dimensional plot (Fig. 3). At the higher dye concentration, maximum color removal was found to occur at a catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate
substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidant ratio of approximately 1
oxidant ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9000 (7.5 × 10−5 M
9000 (7.5 × 10−5 M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.09 × 10−2 M
3.09 × 10−2 M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.6 × 10−1 M). At the lower dye concentration, this ratio was found to be 1
6.6 × 10−1 M). At the lower dye concentration, this ratio was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (3.75 × 10−6 M
1000 (3.75 × 10−6 M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.09 × 10−5 M
3.09 × 10−5 M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.75 × 10−3 M). Thus, treatment of tartrazine is significantly more efficient at higher dye concentrations, as about 50 times less Fe-TAML and 5 times less peroxide are needed per molecule of substrate. The treatment was fast at the high dye concentration; color removal usually reached a constant level within 15 min (see ESI†, Fig. S1 and S2). Similar benefits of high concentration treatment were also seen in experiments with the arylazonaphthol dye, FD&C Red No. 40 (see ESI†), indicating that the effects are not limited to tartrazine alone.
3.75 × 10−3 M). Thus, treatment of tartrazine is significantly more efficient at higher dye concentrations, as about 50 times less Fe-TAML and 5 times less peroxide are needed per molecule of substrate. The treatment was fast at the high dye concentration; color removal usually reached a constant level within 15 min (see ESI†, Fig. S1 and S2). Similar benefits of high concentration treatment were also seen in experiments with the arylazonaphthol dye, FD&C Red No. 40 (see ESI†), indicating that the effects are not limited to tartrazine alone.
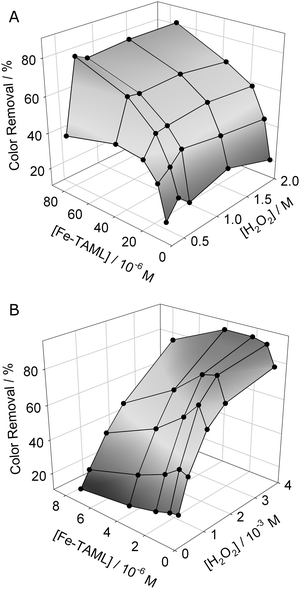 | ||
| Fig. 3 Tartrazine color reduction at 400 nm as functions of Fe-TAML and H2O2 concentrations, 60 min reaction time. Left: 3.09 × 10−2 M dye. Right: 3.09 × 10−5 M dye. | ||
Optimized single-addition doses can achieve 80% dye removal using less H2O2 (21 equivalents) than is needed for mineralization (45 equivalents). Mineralization is calculated as all C converted to CO2, N to HNO3, S to H2SO4, and Na to NaOH, with H2O as the balance. To maximize tartrazine removal, the reaction pH should be controlled and peroxide requirements are higher than 21 equivalents. Table 1 summarizes the effects of pH control and multiple dosing on the extent of tartrazine degradation. At the high dye concentrations, the generation of a large amount of acidic products caused the 2–3 unit pH drop during treatment. At lower pHs, the less reactive hydrazone form of tartrazine dominates22 and the catalytic activity is reduced with the result that the performance of the overall bleaching process deteriorates. By maintaining the pH above 9, achieved by adding a minimum volume of concentrated NaOH solution during the course of the reaction, single additions of Fe-TAML/H2O2 can achieve 92–93% tartrazine removal (Table 1, lines 4 and 5), using 65 equivalents H2O2. The highest level of tartrazine removal (>97%), below detection limits for the 1H NMR quantification technique, requires additional H2O2 (104 equivalents total) and multiple additions of both catalyst and oxidant (Table 1, line 6). The Fe-TAML and H2O2 demands are still remarkably low at >97% removal. Thus, depending on the wastewater remediation goals, a process could be dosed to favor maximum efficiency or maximum dye removal. As more aggressive Fe-TAML activators are developed, it is anticipated that both the rate and depth of dye oxidation processes will increase further.
| No. of Fe-TAML additions | Fe-TAML, 10−5 M/addition | No. of H2O2 additions | [H2O2]/M/addition | Total equiv. H2O2 per dye | pH kept >9 | Total reaction time/min | Tartrazine removal (%) | 4-PSA formeda/mM | Formic acid formedb/mM |
|---|---|---|---|---|---|---|---|---|---|
| Reaction conditions: 3.09 × 10−2 M dye, 0.1 M pH 10 buffer, 15 min reaction time. Tartrazine removal and product formation determined by 1H NMR.a Maximum 4-phenolsulfonic acid production (1 per aromatic ring) is 61.8 mM.b Maximum formic acid production (15 per dye molecule) is 464 mM.c Doses were added at 0 and 15 min.d Concentrations of total added reagents were 1.2 × 10−4 M for Fe-TAML and 3.2 M for H2O2 (the second additions diluted the reaction mixture by 25%). | |||||||||
| 1 | 7.5 | 1 | 0.66 | 21 | N | 15 | 79 | 7.5 | 5.2 |
| 1 | 7.5 | 1 | 0.66 | 21 | Y | 15 | 81 | 6.9 | 5.0 |
| 1 | 7.5 | 1 | 2.0 | 65 | N | 15 | 74 | 7.7 | 6.4 |
| 1 | 7.5 | 1 | 2.0 | 65 | Y | 15 | 92 | 6.3 | 13.1 |
| 1 | 7.5 | 1 | 2.0 | 65 | Y | 60 | 93 | 6.3 | 14.6 |
| 2c | 7.5d | 2c | 2.0d | 129 | Y | 30 | >97 | 1.9 | 18.7 |
| 2c | 7.5d | 2c | 2.0d | 129 | N | 30 | 92 | 1.9 | 7.8 |
4-Phenolsulfonic acid (4-PSA) is major intermediate in the process. As 4-PSA is generated during tartrazine treatment, it competes with the dye for the Fe-TAML/H2O2 reactive intermediate in a reaction that does not contribute to dye decolorization. At higher doses of H2O2, the degradation of 4-PSA was increased and more formic acid was formed. Formic acid is a terminal product (discussed below). Oxygen evolution was consistently observed upon quenching the reactions with catalase suggesting that H2O2 disproportionation is not a process limitation.
Degradation of tartrazine evaluated by COD
We have used the COD test here to differentiate peroxide equivalents that are incorporated into the reaction products from those that are either decomposed or not used at all, thus estimating the efficiency of H2O2 usage. The efficiency-optimized single-dose Fe-TAML/H2O2 treatment led to a COD decrease of 12%. This reduction of 2.2 g L−1COD (2.2 g L−1 O2) corresponds to about 5 equivalents of H2O2 relative to tartrazine. The treated sample received a dose of 22.5 equivalents of H2O2 relative to tartrazine, so >20% of the H2O2 dose was used productively. Efficient use of H2O2 is one of the defining features of Fe-TAML/peroxide chemistry.Product identification
Product identification and toxicity and ecotoxicity determinations are important for evaluating the overall success of wastewater treatment processes. Degradation products may negatively impact the environment and, in principle, the effects may be equal to or greater than those of the starting materials. Thus we have carried out both bulk and specific product identification studies and tested for before and after treatment toxicities.Ion chromatography and total carbon analysis for assessment of degree of mineralization
Ion chromatography and total carbon (TC) results for a high-concentration tartrazine/Fe-TAML/H2O2 reaction mixture are consistent with a relatively low degree of mineralization. For the treatment described in Table 1, line 1, IC did not detect nitrite and nitrate and sulfate production were 0.66% and 6.5% of the theoretical amounts, respectively. The amount of TC (inorganic + organic carbon) in the sample did not change after treatment, indicating that Fe-TAML/H2O2 did not produce substantial CO or CO2 under these conditions.Identification of tartrazine degradation products by NMR spectroscopy
1H NMR spectroscopy with solvent peak suppression allowed for direct analysis of the reaction mixture. The 1H chemical shifts of tartrazine and many of its degradation products are pH-dependent, so reaction mixtures were spiked with standards in order to identify peaks. In D2O/H2O mixtures, only the aromatic protons of tartrazine give observable signals. After treatment with Fe-TAML/H2O2, the spectrum was complex, but at 500 MHz many of the major peaks were resolved (Fig. 4). Among the products identified were 4-PSA, 4-nitrobenzenesulfonic acid, formic acid, maleic acid, malonic acid, fumaric acid, cis-epoxysuccinic acid, and unreacted tartrazine.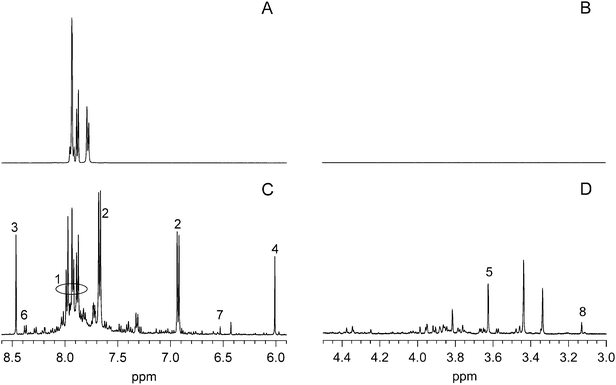 | ||
| Fig. 4 1H NMR spectra of unreacted tartrazine (A and B) and tartrazine degradation products (C and D). Unreacted tartrazine has no 1H signals in the aliphatic region (plot B). The peaks labeled in plots (C & D) are identified in Table 2. Sample composition for plots (A and B): 3.09 × 10−2 M tartrazine, pH 10 buffer (0.1 M carbonate); for plots (C and D): reaction with 7.5 × 10−5 M Fe-TAML and 0.625 M H2O2 (20 equivalents), 15 min reaction. For clarity, plots are shown with different intensity scales. | ||
| Product labela | Product | [Product]b/mM |
|---|---|---|
| *Oxalic acid was determined by ion chromatography.a Labels in NMR spectrum (Fig. 4).b Reaction conditions: 3.09 × 10−2 M tartrazine, 7.5 × 10−5 M Fe-TAML, 0.625 M H2O2, 0.1 M pH 10 carbonate buffer, 15 min reaction time. Concentration of products 1–8 was determined by 1H NMR. | ||
| 1 | Unreacted tartrazine | 6.9 |
| 2 | 4-Phenolsulfonic acid | 7.4 |
| 3 | Formic acid | 5.4 |
| 4 | Maleic acid | 1.9 |
| 5 | cis-Epoxysuccinic acid | 0.7 |
| 6 | 4-Nitrobenzenesulfonic acid | 0.7 |
| 7 | Fumaric acid | 0.3 |
| 8 | Malonic acid | 0.2 |
| * | Oxalic acid | 1.7 |
Quantitative estimates of the reaction products were determined by integrating the peak areas relative to the internal standard (Table 2). The presaturation pulse technique can suppress signals that are close to the water peak, but calibration standards for maleic acid, cis-epoxysuccinic acid, and malonic acid, which have shifts close to the water resonance, gave 85–105% of the theoretical concentrations. There was excellent correlation between the 1H NMR data and UV/Visible spectrophotometry data for tartrazine determination: tartrazine removal was determined to be 78% by the NMR method, compared to 79% by the spectrophotometric method.
The first eight compounds in Table 2 account for just over 50% of the 1H spectrum integrals, and when oxalic acid is included, about 40% of the starting carbon by mass can be accounted for. Most of the remaining unidentified signals fall in the aromatic region of the NMR spectrum, and some of these possibly arise from the products of 4-PSA coupling reactions. The amount of H2O2 needed to form products 1–8 in the amounts detected is consistent with the H2O2 utilization as determined by COD.
The fate of the azo bond is of concern because azo groups give rise to environmental persistence. After a partial degradation process (20 equivalents H2O2) it is plausible that some of the products contain intact azo bonds. We found that Fe-TAML/H2O2 treatment of the water-soluble azo compound 4,4′-diazobenzenedicarboxylic acid resulted in no reaction under either the high- or low-concentration scales under conditions similar to the tartrazine treatments. Thus, a possible weakness in the environmental performance of the process—inertness of the azo bond to direct attack by Fe-TAML/H2O2—became of concern. However, there is evidence that the tartrazine azo bond is indirectly degraded to some extent. First, nearly total decolorization is achieved. Second, 4-PSA was the major identified product. 4-PSA has been previously reported as the product of asymmetric hydrolytic cleavage of the azo bond26,27 of a model compound, 3-carboxy-1-(4′-sulfophenyl)-5-pyrazolone sodium salt, under similar conditions to the tartrazine reaction led to detection of 4-PSA, but as a minor product compared to an as-yet unidentified aromatic compound that also appears in the tartrazine product mixture. This indicates that the tartrazine aromatic ring adjacent to the azo group is the greater contributor to 4-PSA production and that the azo bond is disrupted.
Formic, maleic, fumaric, and malonic acids are well-known oxidation products of phenol oxidative degradations.28 In the tartrazine degradation reaction, these compounds could result from the action of Fe-TAML/H2O2 on the intermediate 4-PSA. This was established by treatment of 4-PSA with Fe-TAML/H2O2, which yielded formic, maleic, malonic, and fumaric acids. Treatment of a mixture of formic, maleic, malonic, and oxalic acids with Fe-TAML/H2O2 under dye treatment conditions resulted in no change in the concentration of those compounds as determined by ion chromatography, indicating that they are terminal products of the tartrazine degradation. It has been shown previously that small molecule diacids are resistant to Fe-TAML/H2O2 degradation under alkaline conditions.29
It is not possible to rule out a role for OH radicals in the Fe-TAML-promoted degradation reaction. Earlier studies of Fe-TAML reactivity suggest that the chemistry mimics peroxidase enzymes, most likely through a reactive high-valent iron oxo species.20,30–32 The relatively low levels of catalyst needed and high efficiency distinguish the Fe-TAML system from Fenton reagents. In the tartrazine reaction, evidence for 2-electron oxidation processes was seen using cyclobutanol as a probe. It is reported that different products result from cyclobutanol if the oxidation is a 1- or 2-electron process.33–35γ-Butyrolactone, which was detected by 1H NMR spectroscopy in tartrazine + cyclobutanol/Fe-TAML/H2O2 reaction mixtures, results from cyclobutanone which has only been reported as a product of 2-electron oxidizing agents. Fe-TAML/H2O2 does not react with cyclobutanol in isolation, so evidently the dye or its intermediate breakdown products mediate the process. 4-PSA also showed this mediating effect, suggesting that aromatic hydroperoxides may be generated. These have been previously implicated in degradation of azo dyes by lignin peroxidase and H2O2.36
Toxicity and biodegradability
Aquatic toxicity was assessed using the Microtox assay (luminescent bacteria, Vibrio fischeri). Assays were determined for untreated tartrazine and for the tartrazine reaction mixture after 15-minute treatment with optimized single-addition doses of Fe-TAML and H2O2 for high dye concentration (7.5 × 10−5 M catalyst, 0.625 M H2O2) at pH 10. The results show that the products of Fe-TAML/H2O2 treatment (15 min EC50, 5.80%) are less toxic than the starting material (15 min EC50, 2.53%); it takes about 2.3 times as much treated water to achieve the same toxicity as the untreated water.Of the major identified reaction products, 4-PSA, formic acid, maleic acid, 4-nitrobenzenesulfonic acid, fumaric acid, malonic acid, and oxalic acid are known to be biodegradable under various conditions. Production of biodegradable chemicals from resistant ones is an important “green” attribute of this technology. Nevertheless, further toxicity examination coupled with biological treatment of degraded textile effluent streams may be needed prior to deployment of Fe-TAML/H2O2. Given the complex chemical mixtures that typify dyeing industry effluents, such an approach may be needed to meet the goal of a completely nontoxic effluent stream.
Conclusions
The Fe-TAML/H2O2 process for removal of concentrated dyes offers advantages over traditional decolorization methods. The very low catalyst concentrations that the Fe-TAML/H2O2 process always employs would translate to an insignificant amount of iron being added to a waste stream—under the described operating parameters for maximum dye removal, the undiluted effluent stream would contain about 6 ppm iron. Allowing for solvents of crystallization, one mole of the sodium salt of the catalyst used in this study weighs approximately 0.7 kg. For tartrazine, this means one kilogram of commercial grade Fe-TAML catalyst will treat approximately thirteen tonnes of the concentrated tartrazine solution to achieve >97% decolorization. Overall, the considerable degradation resistance of tartrazine and the other results described herein indicate that the performance of Fe-TAML/H2O2 is on target to meet the requirements for real-world treatment of dye-contaminated effluents. This utility might be extended to the decontamination of the effluent streams of other chemically based industries. Investigating dye oxidation by Fe-TAML/H2O2 in the presence of dyeing process auxiliaries, humic substances, and other contaminants commonly present in waste streams will be the next step in developing this technology.Acknowledgements
We thank the Heinz Endowments for support to T.J.C. E.S.B. is a National Science Foundation Graduate Research Fellow and is grateful to the AATCC Foundation Student Research Support Grant Program for additional funding. R.T.M. received support from the Howard Hughes Medical Institute and the Arnold and Mabel Beckman Foundation. The NMR spectrometers of the Department of Chemistry NMR Facility at Carnegie Mellon University were purchased in part with funds from the National Science Foundation (CHE-0130903).References
- H. Zollinger, Color Chemistry: Syntheses, Properties and Applications of Organic Dyes and Pigments, VHCA, Zurich, 3rd, revised edn, 2003 Search PubMed.
- D. M. Marmion, Handbook of U.S. Colorants: Foods, Drugs, Cosmetics, and Medical Devices, Wiley-Interscience, New York, NY, 3rd edn, 1991 Search PubMed.
- G. M. Shaul, T. J. Holdsworth, C. R. Dempsey and K. A. Dostal, Chemosphere, 1991, 22, 107–119 CrossRef CAS.
- A. Kandelbauer, O. Maute, R. W. Kessler, A. Erlacher and G. M. Gübitz, Biotechnol. Bioeng., 2004, 87, 552–563 CrossRef CAS.
- A. Paszczynski and R. L. Crawford, Biochem. Biophys. Res. Commun., 1991, 178, 1056–1063 CAS.
- E. P. Chagas and L. R. Durrant, Enzyme Microb. Technol., 2001, 29, 473–477 CrossRef CAS.
- K. V. S. Rao, B. Lavédrine and P. Boule, J. Photochem. Photobiol., A, 2003, 154, 189–193 CrossRef CAS.
- M. A. Salem and A. H. Gemeay, Monatsh. Chem., 2000, 131, 117–129 CrossRef CAS.
- K. Tanaka, K. Padermpole and T. Hisanaga, Water Res., 2000, 34, 327–333 CrossRef CAS.
- N. Chahbane, D. Lenoir, T. Collins, A. Kettrup and K.-W. Schramm, Chem. Ing. Tech., 2004, 76, 1284 CrossRef CAS.
- N. Chahbane, D.-L. Popescu, D. A. Mitchell, A. Chanda, D. Lenoir, A. D. Ryabov, K.-W. Schramm and T. J. Collins, Green Chem., 2007, 9, 49–57 RSC.
- C. P. Horwitz, D. R. Fooksman, L. D. Vuocolo, S. W. Gordon-Wylie, N. J. Cox and T. J. Collins, J. Am. Chem. Soc., 1998, 120, 4867–4868 CrossRef CAS.
- C. P. Horwitz, J. Spatz, T. J. Collins, P. Richard and A. Ogunbameru, 226th ACS National Meeting, New York, NY, 2003.
- R. Andreozzi, V. Caprio, A. Insola and R. Marotta, Catal. Today, 1999, 53, 51–59 CrossRef CAS.
- J. Oakes and P. Gratton, J. Chem. Soc., Perkin Trans. 2, 1998, 2201–2206 RSC.
- H. Wellens, Z. Wasser- Abwasser-Forsch., 1990, 23, 85–98 Search PubMed.
- M. C. Alonso, L. Tirapu, A. Ginebreda and D. Barcelo, Environ. Pollut., 2005, 137, 253–262 CrossRef CAS.
- J. A. Leenheer, J. Hsu and L. B. Barber, J. Contam. Hydrol., 2001, 51, 163–178 CrossRef CAS.
- T. Collins, Science, 2001, 291, 48–49 CrossRef CAS.
- A. Ghosh, D. A. Mitchell, A. Chanda, A. D. Ryabov, D. L. Popescu, E. C. Upham, G. J. Collins and T. J. Collins, J. Am. Chem. Soc., 2008, 130, 15116–15126 CrossRef CAS.
- A. Ghosh, A. D. Ryabov, S. M. Mayer, D. C. Horner, D. E. Prasuhn Jr., S. Sen Gupta, L. D. Vuocolo, C. Culver, M. P. Hendrich, C. E. F. Rickard, R. E. Norman, C. P. Horwitz and T. J. Collins, J. Am. Chem. Soc., 2003, 125, 12378–12379 CrossRef CAS.
- S. J. Bell and E. P. Mazzola, Dyes Pigm., 1989, 11, 93–99 CrossRef CAS.
- J. Oakes, Review of Progress in Coloration and Related Topics, 2002, 32, 63–79 Search PubMed.
- I. Arslan-Alaton, Rev. Prog. Color. Relat. Top., 2003, 119, 345–353 Search PubMed.
- C. M. Carliell, S. J. Barclay and C. A. Buckley, Water SA, 1996, 22, 225–233 Search PubMed.
- S. Goszczynski, A. Paszczynski, M. B. Pasti-Grigsby, R. L. Crawford and D. L. Crawford, J. Bacteriol., 1994, 176, 1339–1347.
- C. López, A.-G. Valade, B. Combourieu, I. Mielgo, B. Bouchon and J. M. Lema, Anal. Biochem., 2004, 335, 135–149 CrossRef CAS.
- H. R. Devlin and I. J. Harris, Ind. Eng. Chem. Fundam., 1984, 23, 387–392 CrossRef CAS.
- S. Sen Gupta, M. Stadler, C. A. Noser, A. Ghosh, B. Steinhoff, D. Lenoir, C. P. Horwitz, K.-W. Schramm and T. J. Collins, Science, 2002, 296, 326–328 CrossRef.
- D.-L. Popescu, M. Vrabel, A. Brausam, P. Madsen, G. Lente, I. Fabian, A. D. Ryabov, E. R. van and T. J. Collins, Inorg. Chem., 2010, 49, 11439–11448 CrossRef CAS.
- A. D. Ryabov and T. J. Collins, Adv. Inorg. Chem., 61, 471–521 Search PubMed.
- A. Chanda, X. Shan, M. Chakrabarti, W. C. Ellis, D. L. Popescu, d. O. F. Tiago, D. Wang, L. Que, T. J. Collins, E. Münck and E. L. Bominaar, Inorg. Chem., 2008, 47, 3669–3678 CrossRef CAS.
- K. Meyer and J. Rocek, J. Am. Chem. Soc., 1972, 94, 1209–1201 CrossRef CAS.
- O. Pestovsky and A. Bakac, J. Am. Chem. Soc., 2004, 126, 13757–13764 CrossRef CAS.
- J. Rocek and A. E. Radkowsky, J. Am. Chem. Soc., 1973, 95, 7123–7132 CrossRef CAS.
- M. Chivukula, J. T. Spadaro and V. Renganathan, Biochemistry, 1995, 34, 7765–7772 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Tables and plots of color reduction measurements for tartrazine and FD&C Red No. 40 at various catalyst and oxidant doses, for 15 or 60 min treatments; discussion of FD&C Red No. 40 treatment; details of ion chromatography and COD experiments; 1H NMR of 4-phenolsulfonic acid/Fe-TAML/H2O2 product mixture. See DOI: 10.1039/c0cy00070a |
| ‡ Present address: Center for Green Chemistry & Green Engineering at Yale, Yale University, New Haven, CT, USA. E-mail: evan.beach@yale.edu; Tel: +1 203 432 5215. |
| § Present address: GreenOx Catalysts, Inc., Pittsburgh, PA, USA. E-mail: chorwitz@greenoxcatalysts.com; Tel: +1 412-268-3439. |
| This journal is © The Royal Society of Chemistry 2011 |
