Application of mobilized Cu-nanoparticles as heterogeneous catalyst for the synthesis of α-amino phosphonatesviaA2-P coupling†
Mazaahir
Kidwai
*a,
Saurav
Bhardwaj
a,
Neeraj Kumar
Mishra
a,
Arti
Jain
a,
Ajeet
Kumar
b and
Subho
Mozzumdar
b
aGreen Chemistry Research Laboratory, Department of Chemistry, University of Delhi, Delhi 110007, India. E-mail: kidwai.chemistry@gmail.com; Fax: +91 11 27666235
bLaboratory for Nanobiotechnology, Department of Chemistry, University of Delhi, Delhi 110007, India
First published on 22nd March 2011
Abstract
Cu-nanoparticles were synthesized and utilized as an efficient, novel and recyclable catalyst for a multi-component coupling reaction using aldehyde, amine and diethyl phosphite. This method provides a wide range of substrate applicability and devoid of co-catalyst/heavy metals with an excellent yield of bioactive α-amino phosphonates. Besides this, catalyst could be recovered and reused up to four runs with almost consistent activity.
Introduction
Nanochemistry is an exponentially growing research field in modern sciences that involves the synthesis and application of nanoparticles of different sizes and shapes. Nanoparticles are different from their bulk counterparts and exhibit unique properties. The synthesis of α-amino phosphonates have attracted much interest because of their biological activity and structural analogy to α-amino acids.1 Its resemblance with amino acids, means they can act as peptide mimics, enzyme inhibitors, antibiotics, insecticide, fungicides and pharmacological agents.2Various synthetic methods have been used for the synthesis of α-amino phosphonates.3 Most of the synthetic routes involve the addition of diethyl phosphite to imines.4 Three-component reactions of aldehydes, amines and diethyl phosphite are efficiently promoted by the presence of catalytic amounts of Lewis acids such as BF3OEt2, ZnCl2 and MgBr2.5
However, all these methods suffer from several limitations such as the use of toxic reagents, long reaction time, non-recyclability of the catalyst6 and the hygroscopic nature of imines as well as the decomposition of Lewis acids due to the formation of water.7
In view of the above, it is necessary that an efficient and convenient method should be developed to synthesize α-amino phosphonates. In continuation of our progressive work for the development of new synthetic methods for the organic compounds8 we report herein the synthesis of α-amino phosphonates using Cu-nanoparticles as selective and efficient catalyst.
In recent years, transition metal nanoparticles have been used as excellent catalysts in various synthetic organic transformations due to their high surface area to volume ratio and presence of coordination sites, which are mainly responsible for their catalytic activity.9 In the present study, the Cu-nanoparticles were used as catalyst since these are cost effective and afford a high yield of the products even at mild reaction conditions.10
Results and discussion
We chose the synthesis of (3a) as the model reaction in which benzaldehyde, aniline and diethyl phosphite were taken in acetonitrile under the nitrogen atmosphere (Scheme 1). To the reaction mixture Cu-nanoparticles (10 mol%) were added through a septum. The reaction mixture was heated with stirring at 50 °C for 3 h and 3a was obtained in 94% yield. A blank reaction was also carried out using benzaldehyde (1 mol), diethyl phosphite (1 mol) and aniline (1.1 mol). The reaction did not proceed even after prolonged reaction time and no such product formed support the catalytic activity of nanoparticles.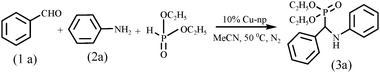 | ||
| Scheme 1 Cu-nanoparticles catalyzed A2-P coupling reaction of benzaldehyde, aniline and diethyl phosphite. | ||
To compare the catalytic efficiency of Cu-nanoparticles with some other Lewis acids, the same reaction was also performed with Mg(OTf)2, AlCl3,Sc(dodecyl sulfate)3 and Yb(OTf)3. As follows from the Table 1, Cu-nanoparticles afforded the better result rather than the Lewis acids.
| Entry | Catalyst | Condition | t (h) | Yielda (%) |
|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), aniline (1 mmol) and diethyl phosphite (1 mmol). b US = ultrasound. | ||||
| 1 | Mg(OTf)3 | 80 °C | 6 | 50 |
| 2 | AlCl3 | US/refluxingb | 3 | 26 |
| 3 | Sc(dodecyl sulfate)3 | 30 °C | 12 | Trace |
| 4 | Yb(OTf)3 | 120 °C | 2 | 74 |
| 5 | Cu-nanoparticle | 50 °C | 3 | 94 |
| 6 | Cu-turning | 50 °C | 3 | 72 |
Besides this, we observed that the concentration of the catalyst played a major role in catalyzing the condensation reaction for the synthesis of α-amino phosphonate derivatives. Using the model reaction as described above, and varying just the concentration of the Cu-nanoparticles, it was observed that 10 mol% of the Cu-nanoparticles afforded the optimum reaction rate and yield (Table 2). The surface area for adsorption decreases with an increase in the particle size which reduced the catalytic efficiency of nanoparticles.11
| Entry | Cu-np (18 ± 2 nm)/mol (%) | t (h) | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), aniline (1.1 mmol), diethyl phosphite (1 mmol) and Cu-nanoparticles (18 ± 2) nm; solvent CH3CN; temperature 50 °C; N2. b Isolated yields. | |||
| 1 | 5 | 6 | 92 |
| 2 | 10 | 3 | 94 |
| 3 | 30 | 3.5 | 95 |
| 4 | 40 | 3 | 95 |
It was also observed that the catalytic activity of Cu-nanoparticles depends on the nanoparticle size. The maximum reaction rate was observed for the particles having a diameter of ∼20 nm (Table 3). It is postulated that in the case of particles with a size <20 nm, a downward shift of the Fermi level takes place, with a consequent increase of band gap energy. On the other hand, for nanoparticles with diameters above 20 nm, the change of the Fermi level is not significant. As these particles exhibit less surface area for adsorption with increased particle size, a decrease in catalytic efficiency results.12
| Entry | Particle size/nm | t (h) | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), aniline (1.1 mmol), diethyl phosphite (1 mmol) and Cu-nanoparticles (x ± 2) nm; solvent CH3CN; temperature 50 °C; N2; 1 atm. b Isolated yields. | |||
| 1 | 10 | 5 | 90 |
| 2 | 20 | 3 | 94 |
| 3 | 30 | 4 | 92 |
| 4 | 40 | 6 | 89 |
After all the standardization, we chose a variety of structurally different aldehydes, and amines possessing a wide range of the functional group to understand the scope and efficiency of the Cu-nanoparticles promoted A2-P coupling reaction. It was observed that aldehydes possessing electron withdrawing group (Table 4, entry 2) and amines possessing electron donating group (Table 4, entries 4, 5) exhibited good reactivity and afforded the higher yield. Heterocyclic aldehydes (Table 4, entry 6) also displayed high reactivity. The functional groups such as NO2 or OCH3 were unaffected during the course of reaction.
| Entry | R1 | R2 | Product | t (h) | Yield (%)c |
|---|---|---|---|---|---|
| a Reaction conditions: 1.0 equiv. of aldehyde , 1.1 equiv. of amine, 1.0 equiv. of diethyl phoshite, 10 mol% Cu-np (18 ± 2 nm); solvent: CH3CN; temperature 50 °C; N2; 1 atm. b Isolated yields. | |||||
| 1 | C6H5– | C6H5– | 3a | 3 | 94 |
| 2 | 4-NO2C6H4– | C6H5– | 3b | 1.5 | 96 |
| 3 | 4-MeOC6H4– | C6H5– | 3c | 3 | 92 |
| 4 | C6H5– | CH3C6H4 | 3d | 2.45 | 95 |
| 5 | C6H5– | CH3OC6H4 | 3e | 2.30 | 97 |
| 6 | C7H5O2– | C6H5– | 3f | 2 | 95 |
| 7 | C6H5– | Aminobenzoxazole | 3g | 3 | 94 |
High purity of products was also confirmed by performing single XRD of crystalline compound 3f as shown in Fig. 1 and 2.
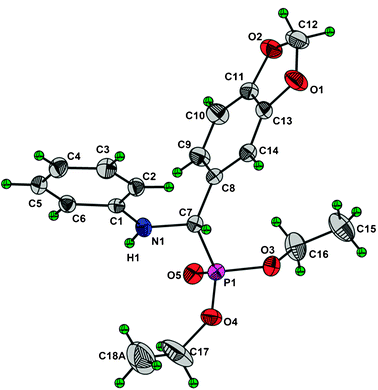 | ||
| Fig. 1 ORTEP diagram of the title compound drawn in 30% probability ellipsoids showing atomic numbering scheme. Only of the disordered methyl group (C18A) has shown. | ||
The refinements showed large thermal parameters and anomalous bond distances for the two ethyl groups. Therefore disorder was applied using PART command on one of methyl carbon and refined with fix bond distance [C–C 1.490(3) Å]. The other ethyl group was refined only with fix bond distance [C–C 1.490(3) Å] and no disorder was applied on this. The similarity restraints SIMU and DELU make the thermal displacement parameters more reasonable and improve the residual index and model itself. The final residual index are; R = 0.0559, Rw = 0.1791 for the observed and R = 0.0728, Rw = 0.1892 for all reflections using 232 parameters. Details of the crystallographic data are given in the ESI.†
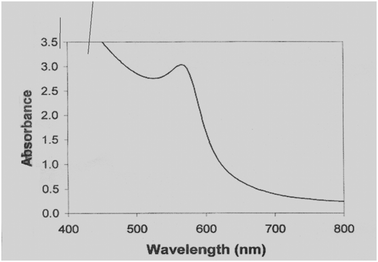
To check the leaching of Cu-nanoparticles and progress of reaction, we took equal amount from model reaction mixture [benzaldehyde (1 mmol), aniline (1.1 mmol), diethyl phosphite (1 mmol) and Cu-nanoparticles (18 ± 2) nm; solvent CH3CN] during the reaction process at same intervals of time (20 min.). After separating the product from this reaction mixture we have taken UV spectra of each sample. We observed that the intensity of product was continuously increased. Besides this, peak of CuO and Cu complex was not observed along with the product. This shows that Cu-nanoparticles do not leach into the reaction mixture (Fig. 3).
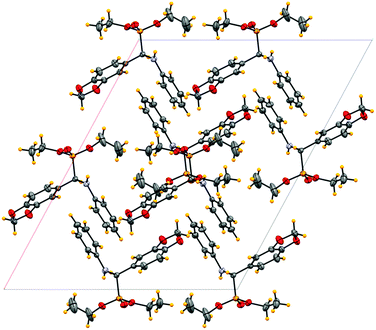 | ||
| Fig. 2 Unit cell packing of the title compound viewing along b-axis. Only one of the disordered methyl group has shown. | ||
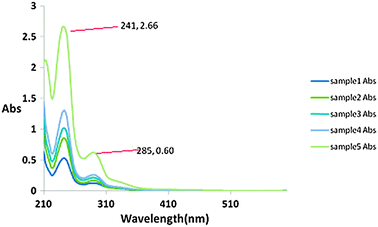 | ||
| Fig. 3 UV spectra of five samples. | ||
In general, the formation of α-amino phosphonatesvia three component coupling reaction proceeded smoothly to afford the corresponding α-amino phosphonates. A mechanism was proposed involving the activation of the P–H bond of diethyl phosphite by Cu-nanoparticles. Resulted diethyl phosphate–Cu intermediate that reacted with the imine generated in situ from aldehyde and amine to yield the corresponding α-amino phosphonates and regenerated the Cu-nanoparticles for further reaction (Scheme 2).13
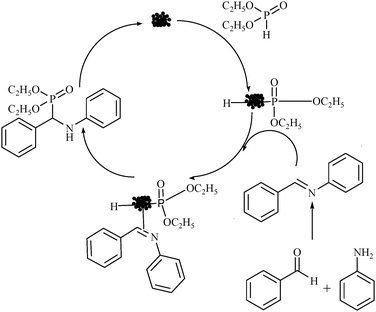 | ||
| Scheme 2 A proposed mechanism of the synthesis of α-amino phosphonates. | ||
Cu-nanoparticles can be recovered by the centrifugation of the reaction mixture and washing nanoparticles with ethyl acetate several times. We performed four reactions by reusing the same nanoparticles. Results are summarized in the Fig. 4. With an increase in the number of reusability runs, the catalytic activity of Cu-nanoparticles decreased. The reason for the same is attributed to the aggregation of nanoparticles and consequent increase in the particle size (Fig. 5).
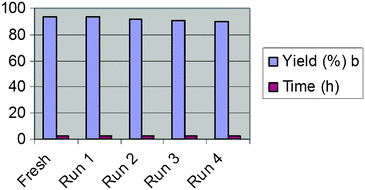 | ||
| Fig. 4 Recycling and reusability of Cu-nanoparticles. (a) Reaction conditions: 1.0 equiv. of aldehyde, 1.1 equiv. of aniline, 1.0 equiv. of diethyl phosphite, 10 mol% Cu-np (18 ± 2 nm); solvent: CH3CN; temperature 50 °C; N2; 1 atm. (b) Isolated yields. | ||
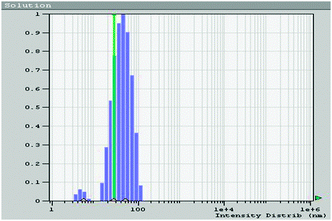 | ||
| Fig. 5 QELS data of Cu-nanoparticles shows aggregation of nanoparticles: plot of population distribution (%) vs. size distribution in nm. | ||
Experimental
Preparation of Cu-nanoparticles
Cu-nanoparticles were prepared by the reduction of Cu2+ ions to Cu0 in a reverse micellar system. Pol(oxyethylene)(tetramethyl)-phenyl ether, commercially known as Tritonx-100 (TX-100), was used as surfactant in the process. To a reverse micellar solution of CuSO4 aqueous solution, another reverse micellar solution of N2H4 aqueous solution was added with constant stirring. The resulting solution was further stirred for 3 h under N2 atmosphere to allow complete Ostwald ripening (particle growth). The Cu-nanoparticles were extracted using absolute ethanol followed by centrifugation. The size of nanoparticles was controlled by varying the water content parameter, Wo (defined as the molar ratio of water to surfactant concentration, Wo = [H2O]/[surfactant]. The prepared nanoparticles were of spherical shape, with an average size of 10–18 nm, as confirmed by transmission electron microscopy (TEM) (Fig. 6), quasi elastic light scattering (QELS) data (Fig. 7) and UV-vis spectra for characterization of Cu-nanoparticles is also given in Fig. 8.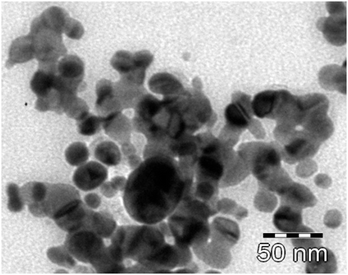 | ||
| Fig. 6 TEM image of Cu-nanoparticles. The scale bar corresponds to 50 nm in the TEM image. | ||
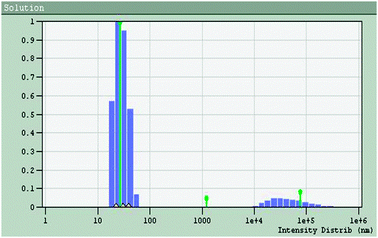 | ||
| Fig. 7 QELS data of Cu-nanoparticles: plot of population distribution (%) vs. size distribution in nm. | ||
Typical procedure for the synthesis of α-amino phosphonates
In a 50 mL round bottom flask, the mixture of aldehyde (1 mmol), amine (1.1 mmol) and diethyl phosphite (1 mmol) in acetonitrile (2 mL) were stirred at 50 °C under the nitrogen atmosphere. Cu-nanoparticles (10 mol%) were added to the resultant reaction mixture. The progress of reaction was continuously monitored through TLC. On completion of reaction, as indicated by TLC examination, excess of water was added to the reaction mixture. The crude product was extracted with ethyl acetate and dried over anhydrous Na2SO4. Solvent was removed under vacuum. Subsequently, the reaction mixture was purified by silica gel column chromatography using hexane (90)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (10) as an eluent to get pure α-amino phosphonates (Table 4, entry 1–7). All the products were unambiguously established on the basis of their spectral analysis (IR, 1H NMR, 13CNMR, 31P NMR and mass spectra).
ethyl acetate (10) as an eluent to get pure α-amino phosphonates (Table 4, entry 1–7). All the products were unambiguously established on the basis of their spectral analysis (IR, 1H NMR, 13CNMR, 31P NMR and mass spectra).
Compound 3aIRvmax (KBr) 3295 (s, NH), 1236 (s, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (300 MHz, TMS, CDCl3): δ 1.09 (3 H, t, J = 7.0 Hz, –OCH2CH3), 1.24 (3 H, t, J = 7.0 Hz –OCH2CH3), 3.64–3.70 (1 H, t, J = 6.9 Hz, OCH2CH3), 3.90–3.93 (1 H, t, J = 6.9 Hz, –OCH2CH3), 4.09–4.13 (2 H, t, J = 6.9 Hz, –OCH2CH3), 4.70 (1 H, d, J = 23.7 Hz, CHP), 6.57–7.48 (10 H, m, φ). 13C NMR (75 MHz, TMS, CDCl3): 16.15 (dd, 3Jc,p = 5.8 Hz, OCH2CH3), 56.10 (d, 1Jc,p = 150.4 Hz, CH), 63.05 (d, 2Jc,p = 5.8 Hz, OCH2CH3), 113.67, 118.18, 127.73–128.42, 135.77, 146.08–146.27 (C6H6). 31P NMR (242 MHz, TMS, CDCl3): 22.51. m/z (GC-MS, HRMS): 318.92 (M+).
O). 1H NMR (300 MHz, TMS, CDCl3): δ 1.09 (3 H, t, J = 7.0 Hz, –OCH2CH3), 1.24 (3 H, t, J = 7.0 Hz –OCH2CH3), 3.64–3.70 (1 H, t, J = 6.9 Hz, OCH2CH3), 3.90–3.93 (1 H, t, J = 6.9 Hz, –OCH2CH3), 4.09–4.13 (2 H, t, J = 6.9 Hz, –OCH2CH3), 4.70 (1 H, d, J = 23.7 Hz, CHP), 6.57–7.48 (10 H, m, φ). 13C NMR (75 MHz, TMS, CDCl3): 16.15 (dd, 3Jc,p = 5.8 Hz, OCH2CH3), 56.10 (d, 1Jc,p = 150.4 Hz, CH), 63.05 (d, 2Jc,p = 5.8 Hz, OCH2CH3), 113.67, 118.18, 127.73–128.42, 135.77, 146.08–146.27 (C6H6). 31P NMR (242 MHz, TMS, CDCl3): 22.51. m/z (GC-MS, HRMS): 318.92 (M+).
Compound 3f IRvmax (KBr) 3295 (s, NH), 1236 (s, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (300 MHz, TMS, CDCl3): δ 1.18 (3 H, t, J = 7.0 Hz, –OCH2CH3), 1.25 (3 H, t, J = 7.0 Hz, –OCH2CH3), 3.71–4.18 (4 H, m, –OCH2CH3), 4.73(1 H, d, J = 23.7 Hz, CHP), 4.83 (1 H, br, NH), 5.87 (2 H, s, CH2), 6.57–6.91 (5 H, m, φ), 6.97 (1 H, s, φ), 7.09 (1 H, t, J = 6.9 Hz, φ). 13C NMR (75 MHz, TMS, CDCl3): 16.06–16.28 (dd, 3Jc,p = 5.8 Hz, OCH2CH3), 54.52–56.53 (d, 1Jc,p = 154.04 Hz, CH), 63.01–63.10 (d, 2Jc,p = 5.8 Hz, OCH2CH3), 100.92 (s, CH2), 108.00–108.08, 113.67, 118.21, 121.14–121.22, 128.95–129.54, 146.01–146.20, 147.15–147.80 (C6H6). 31P NMR (242 MHz, TMS, CDCl3): 22.40. m/z (GC-MS, HRMS): 360.74 (M+).
O). 1H NMR (300 MHz, TMS, CDCl3): δ 1.18 (3 H, t, J = 7.0 Hz, –OCH2CH3), 1.25 (3 H, t, J = 7.0 Hz, –OCH2CH3), 3.71–4.18 (4 H, m, –OCH2CH3), 4.73(1 H, d, J = 23.7 Hz, CHP), 4.83 (1 H, br, NH), 5.87 (2 H, s, CH2), 6.57–6.91 (5 H, m, φ), 6.97 (1 H, s, φ), 7.09 (1 H, t, J = 6.9 Hz, φ). 13C NMR (75 MHz, TMS, CDCl3): 16.06–16.28 (dd, 3Jc,p = 5.8 Hz, OCH2CH3), 54.52–56.53 (d, 1Jc,p = 154.04 Hz, CH), 63.01–63.10 (d, 2Jc,p = 5.8 Hz, OCH2CH3), 100.92 (s, CH2), 108.00–108.08, 113.67, 118.21, 121.14–121.22, 128.95–129.54, 146.01–146.20, 147.15–147.80 (C6H6). 31P NMR (242 MHz, TMS, CDCl3): 22.40. m/z (GC-MS, HRMS): 360.74 (M+).
X-Ray crystallography
Compounds were crystallized from their solution in chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (6
ethanol (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). The unit cell parameters were refined by the least-squares fit of 32.45 high angle (2.97° ≤ θ ≤ 32.45°) reflections. These reflections were centered individually on the diffract meter. The Lorentz and polarization corrections were applied. Multi-scan absorption correction was applied. The structure was determined with direct methods using the program SHELXS-97. The coordinates of non-hydrogen atoms were refined anisotropically using program SHELXL-97. The positions of hydrogen atoms were obtained from difference Fourier maps and were included in the final cycles of refinement using isotropic temperature factors of non-hydrogen atoms to which they were attached. For the molecular graphics, the program Diamond-2 and Mercury were used. The final R-factor for 2702 observed reflections [I ≥ 2σ(I)] was 0.0559.
4). The unit cell parameters were refined by the least-squares fit of 32.45 high angle (2.97° ≤ θ ≤ 32.45°) reflections. These reflections were centered individually on the diffract meter. The Lorentz and polarization corrections were applied. Multi-scan absorption correction was applied. The structure was determined with direct methods using the program SHELXS-97. The coordinates of non-hydrogen atoms were refined anisotropically using program SHELXL-97. The positions of hydrogen atoms were obtained from difference Fourier maps and were included in the final cycles of refinement using isotropic temperature factors of non-hydrogen atoms to which they were attached. For the molecular graphics, the program Diamond-2 and Mercury were used. The final R-factor for 2702 observed reflections [I ≥ 2σ(I)] was 0.0559.
Conclusion
Overall this methodology offers the competitive advantages of recyclability of the catalyst without significant loss of catalytic activity; it can be used or reused without further purification, and without using additives or co-factor; it requires lower catalyst loading, has broad substrate applicability, gives high yields in short reaction times, and is simple and easy to carry out. Here we have successfully developed a novel, facile, economic and practical method for the synthesis of α-amino phosphonates, hence making this procedure more environmentally acceptable and no catalyst has widespread use in organic synthesis for preparation of α-amino phosphonates.Acknowledgements
The authors Saurav Bhardwaj and Ajeet Kumar are thankful to CSIR, New Delhi for their senior research fellowships. The authors also want to acknowledge Kuldeep Mahiya (Department of Chemistry, University of Delhi, Delhi) for solving and refinement of the crystal data.References
- S. C. Fields, Tetrahedron, 1999, 55, 12237 CrossRef CAS; E. K. Fields, J. Am. Chem. Soc., 1952, 74, 1528 CrossRef CAS.
- P. Kafarski and B. Lejcza, Phosphorus, Sulfur Silicon Relat. Elem., 1991, 63, 193 CrossRef CAS; M. C. Allen, W. Fuhrer, B. Tuck, R. Wade and J. M. Wood, J. Med. Chem., 1989, 32, 1652 CrossRef CAS; F. R. Atherton, C. H. Hassall and R. W. Lambert, J. Med. Chem., 1986, 29, 29 CrossRef CAS; J. Emsley and D. Hall, The Chemistry of Phosphorus, Harper and Row, London, 1976, p. 494 Search PubMed; L. Maier and H. Sporri, Phosphorus, Sulfur Silicon Relat. Elem., 1991, 61, 69 Search PubMed; I. A. Natchev and Liebigs, Ann. Chem., 1988, 861 Search PubMed.
- S. Bhagat and A. K. Chakraborti, J. Org. Chem., 2007, 72, 1263 CrossRef CAS; H. Firouzabadi, N. Iranpoor and S. Sobhani, Synthesis, 2004, 2692 CrossRef CAS; R. G. Macias and K. Nakayama, Synthesis, 2010, 57; S. Sobhani and A. Vafaee, Synthesis, 2009, 1909 CrossRef CAS; M. Z. Kassaee, F. Movahedi and H. Masrouri, Synlett, 2009, 1326 CrossRef CAS; S. Bhagat and A. K. Chakraborti, J. Org. Chem., 2008, 73, 6029 CrossRef CAS; S. Sobhanij, E. Safaei, M. Asadic and F. Jalili, J. Organomet. Chem., 2008, 693, 3313 CrossRef; M. H. Sarvari, Tetrahedron, 2008, 64, 5459 CrossRef.
- J. S. Yadav, B. V. S. Reddy, K. S. Raj, K. Reddy, A. Bhaskar and R. Prasad, Synthesis, 2001, 2277 CrossRef CAS; L. Sabine and K. Horst, Synthesis, 1992, 1–2, 90.
- H.-J. Ha and G.-S. Nam, Synth. Commun., 1992, 22, 1143 CAS; J. Zon, Pol. J. Chem., 1981, 55, 643 CAS; K. Manabe and S. Kobayashi, Chem. Commun., 2000, 669 RSC; J. Wu, W. Sun, H.-G. Xia and X. Sun, Org. Biomol. Chem., 2006, 4, 1663–1666 RSC.
- C. Qian and T. Huang, J. Org. Chem., 1998, 63, 4125 CrossRef CAS; S. Chandrasekhar, S. J. Prakash, V. Jagadeshwar and C. Narsihmulu, Tetrahedron Lett., 2001, 42, 5561 CrossRef CAS; R. Ghosh, S. Maiti, A. Chakraborty and D. K. Maiti, J. Mol. Catal. A: Chem., 2004, 210, 53 CrossRef CAS; A. Heydari, H. Hamadi and M. Pourayoubi, Catal. Commun., 2007, 8, 1224 CrossRef CAS; S. Iimura, D. Nobutou, K. Manabe and S. Kobayashi, Chem. Commun., 2003, 1644 RSC.
- S. Sara, E. Safaei, M. Asadi and F. Jalili, J. Organomet. Chem., 2008, 693, 3313 CrossRef; B. C. Ranu, A. Hajra and U. Jana, Org. Lett., 1999, 1, 1141 CrossRef.
- M. Kidwai, R. Poddar, S. Bhardwaj, S. Singh and P. M. Luthra, Eur. J. Med. Chem., 2010, 45, 5031–5038 CrossRef CAS; M. Kidwai, R. Venkataramanan and B. Dave, Green Chem., 2001, 3, 278 RSC; M. Kidwai, V. Bansal and P. Mothsra, J. Mol. Catal. A: Chem., 2007, 266, 43 CrossRef CAS; M. Kidwai and R. Poddar, Catal. Lett., 2008, 124, 311 CrossRef CAS.
- M. Kidwai and S. Bhardwaj, Appl. Catal., A, 2010, 387, 1–4 CrossRef CAS; M. Kidwai, S. Bhardwaj and R. Poddar, Beil. J. Org. Chem., 2010, 6(No. 35) Search PubMed; M. Kidwai, A. Jain and S. Bhardwaj, Catal. Lett., 2010, 141, 183.
- M. Kidwai, S. Bhardwaj, N. K. Mishra, V. Bansal, A. Kumar and S. Mozumdar, Catal. Commun., 2009, 10, 1514–1517 CrossRef CAS; M. Kidwai, N. K. Mishra, V. Bansal, A. Kumar and S. Mozumdar, Tetrahedron Lett., 2007, 48, 8883 CrossRef CAS.
- P. Sharma, S. Brown, G. Walter, S. Santra and B. Moudgil, Adv. Colloid. Interface Sci., 2006, 471, 123; M. Kidwai, N. K. Mishra, V. Bansal, A. Kumar and S. Mozumdar, Tetrahedron Lett., 2009, 50, 1355 CrossRef CAS.
- M. Samim, N. K. Kaushik and A. N. Maitra, Bull. Mater. Sci., 2007, 30, 535 Search PubMed; R. K. Sharma, P. Sharma and A. N. Maitra, J. Colloid Interface Sci., 2003, 265, 134 CrossRef CAS.
- M. Kidwai, V. Bansal, N. K. Mishra, A. Kumar and S. Mozumdar, Synlett, 2007, 1581 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC reference number 761136. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cy00060d |
| This journal is © The Royal Society of Chemistry 2011 |