Aluminium atomic layer deposition applied to mesoporous zeolites for acid catalytic activity enhancement
Sreeprasanth Pulinthanathu
Sree
a,
Jolien
Dendooven
b,
Tamás I.
Korányi
a,
Gina
Vanbutsele
a,
Kristof
Houthoofd
a,
Davy
Deduytsche
b,
Christophe
Detavernier
b and
Johan A.
Martens
*a
aCentre for Surface Chemistry and Catalysis, KU Leuven, Kasteelpark Arenberg 23, Leuven B-3001, Belgium. E-mail: Johan.Martens@biw.kuleuven.be; Fax: (+32)16321998; Tel: (+32)16321637
bDepartment of Solid State Sciences, Ghent University, Krijgslaan 281–S1, Gent B-9000, Belgium. E-mail: christophe.detavernier@ugent.be; Fax: (+32)92644996; Tel: (+32)92644354
First published on 10th February 2011
Abstract
Atomic Layer Deposition (ALD) of aluminium is a new method for enhancing acidity and acid catalytic activity in mesoporous zeolites and hierarchical materials.
ALD originally developed in semiconductor engineering is a thin film deposition technique involving chemisorption of precursor molecules followed by reaction in a two-phase deposition cycle.1 Pretreated support is alternately exposed to metal compound vapour, e.g.Al(CH3)3 and water vapour to obtain the desired metal content.1ALD has been applied for tuning the pore size of inorganic membranes,2 to prepare supported vanadium oxide catalysts for alkane dehydrogenation,3 and cobalt based toluene hydrogenation catalyst.4 Introduction of iridium clusters into the interstitial spaces of zeolite beta polycrystalline material has been demonstrated.5
Ultrastable Y (US-Y) zeolite is a popular catalyst.6 The improved catalytic behaviour after ultrastabilization has been ascribed to the formation of mesopores, lifting intracrystalline diffusional limitations, and to strengthening of the Si–OH–Al acid sites.7 The presence of distorted Al tetrahedra and the synergetic effects of bridging hydroxyls and extra-framework Al debris causes additional acidity enhancement.6b Controlled mesoporosity development and enhancement of the Al content relative to Si in zeolites has previously been attempted by desilication8 and realumination but exceptional acidity enhancement has not been reached.6a Here we report ALD of aluminium (ALD-Al) to be an elegant technique for introducing aluminium in nanoporous materials and demonstrate an enhancement of the acidity and acid catalytic activity of US-Y zeolite and Zeotile-4 hierarchical material. Practical advantages of ALD compared to other methods are the automation and speed of the treatment.†
The application of 10 ALD-Al cycles on US-Y zeolite (CBV-712, PQ corp.) involving sequential exposure to Al(CH3)3 and water resulted in an increase of the Al content from 2428 to 3015 mmol kg−1 (Table 1), corresponding to a change of bulk Al/(Al + Si) molar ratio from 0.146 to 0.175. The Al/(Al + Si) molar ratio at the surface of the zeolite particles probed by XPS showed a modest increase from 0.167 to 0.270 indicating that the aluminium introduced viaALD penetrated into the zeolite crystals and did not accumulate at the surface. The supercages of zeolite Y have free diameters of ca. 1.2 nm and are accessible via 4 apertures measuring 0.74 nm.9 Apparently, the Al(CH3)3 molecule with kinetic diameter of ca. 0.55 nm10 penetrated into the zeolite.
In the three-dimensional pore system of a Y zeolite with 4 windows per cage, pore plugging is unlikely. In this particular US-Y zeolite sample, the mesopores are mainly cylindrical pores accessible from the outside improving accessibility.7
In parent and ALD modified US-Y zeolites mainly three 27Al MAS NMR signals were observed (Fig. 1, Table 1) with intensity maxima around 54 ppm, 30 ppm and 0 ppm assigned to tetrahedral framework Al (AlT), distorted tetrahedral framework and pentacoordinated Al (AlP), and octahedral extra framework Al (AlO), respectively.11 The octahedral region of the 27Al MAS NMR spectrum presented more than one signal ascribed to monomeric and oligomeric species.11a,12Al introduced viaALD ended up mostly in AlO and AlP coordination (Table 1).
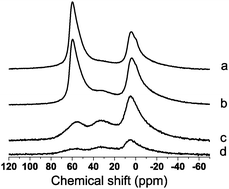 | ||
| Fig. 1 27Al MAS NMR spectra of parent (a) and ALD-Al modified (b) US-Y zeolite, ALD-Al modified Zeotile-4 (c) and ALD-Al modified Silicalite-1 (d). | ||
ALD-Al modified US-Y retained its crystallinity as verified with XRD. No new crystalline phases were detected. In the N2 adsorption isotherm (Fig. 2) strong N2 uptake at low relative pressures was due to micropore filling. The hysteresis loop at higher relative pressures was ascribed to capillary condensation in mesopores. Quantification (Table 2) revealed both the mesopore and the micropore volumes to be reduced by 0.03–0.04 cm3 g−1 through ALD-Al. This reduction of porosity was ascribed to the deposition of extra-framework Al species in the pores.
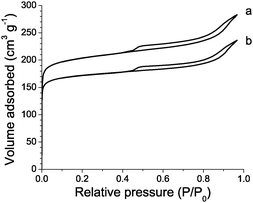 | ||
| Fig. 2 N2 adsorption isotherms at 77 K on parent (a) and ALD-Al modified (b) US-Y zeolite. | ||
| Sample | SBET | VMesoa | Vμb | Br c | Lc | Br c |
|---|---|---|---|---|---|---|
| Unit | [m2 g−1] | [cm3 g−1] | [cm3 g−1] | 423 K | 423 K | 523 K |
| a Mesopore volume. b Micropore volume. c Brønsted (Br) and Lewis (L) acid site concentration (mmol kg−1) determined by FTIR of adsorbed pyridine at the indicated temperature. n.d.: not determined. | ||||||
| US-Y | 636 | 0.147 | 0.290 | 488 | 151 | 351 |
| ALD-Al US-Y | 533 | 0.116 | 0.249 | 570 | 156 | 401 |
| Zeotile-4 | 873 | 0.889 | 0.090 | n.d. | n.d. | n.d. |
| ALD-Al Zeotile-4 | 754 | 0.783 | 0.076 | 8 | 14 | 3 |
| Silicalite-1 | 353 | 0.000 | 0.155 | n.d. | n.d. | n.d. |
The acidity was characterized using pyridine and FTIR spectroscopy (Table 2). The Brønsted acidity was quantified via the concentration of protonated pyridine retained at 423 K. Strong acid sites retained pyridine at 523 K.13a The total concentration of Brønsted and Lewis acid sites of 488 and 151 mmol kg−1, respectively, of this US-Y zeolite was in excellent agreement with the data reported by Morinet al. on a similar sample.13b While there was hardly any influence of ALD-Al on the Lewis acid site content (increase from 151 mmol kg−1 to 156 mmol kg−1), there was a significant increase in the total number of Brønsted acid sites from 488 mmol kg−1 initially to 570 mmol kg−1 after ALD-Al (Table 2). A similar increasing trend appeared for the strongest Brønsted acid sites retaining pyridine at 523 K (increase from 351 to 401 mmol kg−1). An increase in strong acid site concentration by 50 mmol kg−1 is significant. For comparison, a mesoporous SBA-15 material with Si/Al ratio of 15 has a total concentration of Brønsted acid sites of 143 mmol kg−1 none of which are strong.13c,d An enhancement of the aluminium content of ZSM-5 sample by desilication corresponding to a decrease of Si/Al ratio from 49.6 to 37.1 caused a decrease rather than an increase of the concentration of strong Brønsted acid sites from 256 mmol kg−1 to 179 mmol kg−1 measured with the same pyridine adsorption technique as applied here.13e Increasing the aluminium content through desilication improves the accessibility of the acid sites via formation of secondary porosity but tends to lower acidity in contrast to the present ALD-Al technique which enhances acidity.13e,f A combination of both modifications seems challenging as it may lead to an improved accessibility as well as enhanced acidity.
This increase of acidity occurred at almost constant AlT concentration (Table 1) and must have been linked with the enhanced concentration of AlP or AlO type Al atoms (Table 1). It is unlikely that the AlO assumed to be present in cationic species compensating framework charges was responsible for the increased number of strong Brønsted acid sites. Bridging hydroxyls bonded to distorted Al tetrahedra with links to oxygen atoms of the framework were more likely responsible for the strong acid sites introduced viaALD-Al.
A small amount of dispersed platinum (0.5 wt%) was introduced into the US-Y zeolite to obtain a bifunctional catalyst. In decane hydroconversion the ALD-Al modification rendered the US-Y zeolite active at lower temperatures (Fig. 3). Mechanistically, in bifunctional catalysis decane is dehydrogenated on the metal. Protonation by the Brønsted acid sites leads to the formation of an aliphatic alkylcarbenium ion, which is isomerized and eventually cracked.7a After hydrogenation on the noble metal sites saturated reaction products are obtained. The selectivity of the US-Y zeolite with and without ALD-Al was very similar. On parent and modified US-Y zeolite, isomerization and hydrocracking were consecutive reactions.
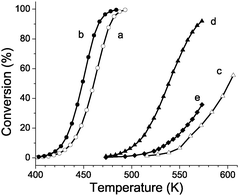 | ||
| Fig. 3 Decane conversion over parent (a) and ALD-Al modified (b) US-Y zeolite, parent (c) and ALD-Al modified (d) Zeotile-4 material, and ALD-Al modified Silicalite-1 (e). The reaction temperature was increased stepwise. | ||
Both catalysts exhibited high isomerization yields, and primary cracking which are signatures of well-balanced bifunctional catalysts14 on which the turnovers on acid sites are rate limiting.15 Thus the catalytic activity enhancement after ALD-Al was ascribed to acidity enhancement. From the decane conversion at the same reaction temperature (Fig. 3), on a weight basis the catalytic activity of ALD-Al treated US-Y zeolite was about twice that of the parent zeolite (Fig. 3). This activity gain was larger than expected from the moderate increase in concentration of strong acid sites from 351 to 401 mmol kg−1 (Table 2) and must be linked with the creation of special sites.16 Between successive reaction product samplings at increasing reaction temperature (Fig. 3), there was a time lapse of several hours on-stream. After the last data point at high temperature, the temperature was decreased again and the activity verified. There was no deactivation. ALD treated samples were stored under ambient conditions for 1 year. Fresh and stored catalysts displayed a very similar catalytic activity. These observations showed that the activity enhancement of US-Y zeolite was durable.
Zeotile-4 is a hierarchical material with siliceous framework presenting 3-dimensional mesoporosity between nanoslabs and microporosity inside nanoslabs.17 In the Zeotile-4 material straight mesopores with ca. 7.5 nm diameter are sideways interconnected via slits. Zeotile-4 was rendered catalytically active viaALD-Al (Fig. 3). The Al loading corresponded to 1815 mmol kg−1 (Table 1). A significant amount of the Al was present in tetrahedral coordination, and incorporated in the silicate framework (Fig. 1c). The micro- and mesoporosity were decreased after ALD revealing the penetration of the aluminium species into the Zeotile-4 particles (Fig. 4). The enhanced concentration of acid sites determined viapyridine adsorption (Table 2) gave rise to significant catalytic activity enhancement (Fig. 3d). ALD-Al modified Zeotile-4 was a well balanced bifunctional catalyst. Finally, activation of Silicalite-1 zeolite was attempted. There was little Al uptake in the ALD-Al treatment (Table 1, Fig. 1d), hardly any change of porosity (Table 2, Fig. 4) and low catalytic activity (Fig. 3e). The pore width of Silicalite-1 zeolite seems to be insufficient for adsorbing the Al(CH3)3 precursor molecule.
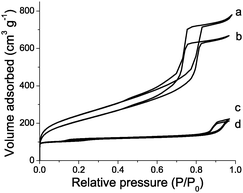 | ||
| Fig. 4 N2 adsorption isotherms at 77 K on parent (a) and ALD-Al modified (b) Zeotile-4 material, and parent (c) and ALD-Al modified (d) Silicalite-1. | ||
In conclusion we demonstrated the potential of ALD for introduction of acid sites and enhancement of catalytic activity in large pore zeolites and hierarchical materials. In future work the ALD-Al tool will be handled to prepare model catalysts for characterization of zeolite acidity. For large pore zeolites with potentially very attractive pore architectures in which the introduction of trivalent elements and generation of acidity is problematic18ALD may offer a solution. Introduction of other chemical elements relevant to catalysis such as Fe and Ga can be envisaged.
This work is part of strategic basic research (SBO), supported by the Flemish IWT. Long-term structural funding by the Flemish Government (Methusalem) is highly appreciated. The work is conducted in the framework of Interuniversity Attraction Poles (IAP-PAI). J.D. acknowledges the Flemish FWO for a PhD grant.
Notes and references
- (a) T. Hibino, M. Niwa and Y. Murakami, Zeolites, 1993, 13, 518–523 CrossRef CAS; (b) E. L. Lakomaa, Appl. Surf. Sci., 1994, 75, 185–196 CrossRef CAS; (c) R. L. Puurunen, A. Root, P. Sarv, M. V. Viitanen, H. H. Brongersma, M. Lindblad and A. O. I. Krause, Chem. Mater., 2002, 14, 720–729 CrossRef CAS; (d) R. L. Puurunen, J. Appl. Phys., 2005, 97, 121301 CrossRef; (e) S. M. George, Chem. Rev., 2010, 110, 111–131 CrossRef CAS.
- (a) M. A. Cameron, I. P. Gartland, J. A. Smith, S. F. Diaz and S. M. George, Langmuir, 2000, 16, 7435–7444 CrossRef CAS; (b) C. A. Cooper and Y. S. Lin, J. Membr. Sci., 2002, 195, 35–50 CrossRef CAS; (c) B. A. McCool and W. J. DeSisto, Chem. Vap. Deposition, 2004, 10, 190–194 CrossRef CAS; (d) P. C. Stair, C. Marshall, G. Xiong, H. Feng, M. J. Pellin, J. W. Elam, L. Curtiss, L. Iton, H. Kung, M. Kung and H. H. Wang, Top. Catal., 2006, 39, 181–186 CrossRef CAS; (e) L. Velleman, G. Triani, P. J. Evans, J. G. Shapter and D. Losic, Microporous Mesoporous Mater., 2009, 126, 87–94 CrossRef CAS.
- (a) J. Keränen, A. Auroux, S. Ek and L. Niinistö, Appl. Catal., A, 2002, 228, 213–225 CrossRef CAS; (b) H. Poelman, G. Silversmit, D. Poelman, G. B. Marin and B. S. Sels, Catal. Today, 2009, 142, 125–131 CrossRef CAS.
- L. B. Backman, A. Rautiainen, M. Lindblad and A. O. I. Krause, Appl. Catal., A, 2009, 360, 183–191 CrossRef CAS.
- H. Vuori, R. J. Silvennoinen, M. Lindblad, H. Osterholm and A. O. I. Krause, Catal. Lett., 2009, 131, 7–15 CrossRef CAS.
- (a) J. Klinowski, Stud. Surf. Sci. Catal., 1989, 52, 39–71 CrossRef; (b) R. Szostak, Stud. Surf. Sci. Catal., 2001, 137, 261–297 CrossRef CAS; (c) J. A. Martens and P. A. Jacobs, Stud. Surf. Sci. Catal., 2001, 137, 633–671 CAS.
- (a) J. A. Martens, W. Souverijns, W. Van Rhijn and P. A. Jacobs, in Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knozinger and J. Weitkamp, Wiley, Weinheim, 1997, pp. 324–365 Search PubMed; (b) A. H. Janssen, A. J. Koster and K. P. de Jong, Angew. Chem., Int. Ed., 2001, 40, 1102–1104 CrossRef CAS; (c) A. H. Janssen, A. J. Koster and K. P. de Jong, J. Phys. Chem. B, 2002, 106, 11905–11909 CrossRef CAS.
- (a) J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. Perez-Ramirez, Chem.–Eur. J., 2005, 11, 4983–4994 CrossRef CAS; (b) J. C. Groen, W. Zhu, S. Brouwer, S. J. Huynink, F. Kapteijn, J. A. Moulijn and J. Pérez-Ramírez, J. Am. Chem. Soc., 2007, 129, 355–360 CrossRef CAS.
- From Database of Zeolite Structures in 〈http://www.iza-structure.org/databases/〉.
- C. Cavallotti, I. Lengyel, M. Nemirovskaya and K. F. Jensen, J. Cryst. Growth, 2004, 268, 76–95 CrossRef CAS.
- (a) G. Engelhardt and D. Michel, in High-Resolution Solid-State NMR of Silicates and Zeolites, Wiley, Chichester, 1987 Search PubMed; (b) J.-P. Gilson, G. C. Edwards, A. W. Peters, K. Rajagopalan, R. F. Wormsbecher, T. J. Roberie and M. P. Shatlock, J. Chem. Soc., Chem. Commun., 1987, 91–92 RSC; (c) D. Coster, A. L. Blumenfeld and J. J. Fripiat, J. Phys. Chem., 1994, 98, 6201–6211 CrossRef CAS.
- N. Malicki, G. Mali, A.-A. Quoineaud, P. Bourges, L. J. Simon, F. Thibault-Starzyk and C. Fernandez, Microporous Mesoporous Mater., 2010, 129, 100–105 CrossRef CAS.
- (a) J. A. Lercher and A. Jentys, Stud. Surf. Sci. Catal., 2007, 168, 435–476 CrossRef CAS; (b) S. Morin, P. Ayrault, N. S. Gnep and M. Guisnet, Appl. Catal., A, 1998, 166, 281–292 CrossRef CAS; (c) J. M. R. Gallo, C. Bisio, G. Gatti, L. Marchese and H. O. Pastore, Langmuir, 2010, 26(8), 5791–5800 CrossRef CAS; (d) M. Gomez-Cazalilla, J. M. Merida-Robles, A. Gurbani, E. Rodriguez-Castellon and A. Jimenez-Lopez, J. Solid State Chem., 2007, 180, 1130–1140 CrossRef CAS; (e) B. Gil, L. Mokrzycki, B. Sulikowski, Z. Olejniczak and S. Walas, Catal. Today, 2010, 152, 24–32 CrossRef CAS; (f) F. Thibault-Starzyk, I. Stan, S. Abello, A. Bonilla, K. Thomas, C. Fernandez, J.-P. Gilson and J. Perez-Ramirez, J. Catal., 2009, 264, 11–24 CrossRef CAS.
- J. A. Martens, P. A. Jacobs and J. Weitkamp, Appl. Catal., 1986, 20, 283–303 Search PubMed.
- F. Alvarez, F. R. Ribeiro, G. Perot, C. Thomazeau and M. Guisnet, J. Catal., 1996, 162, 179–189 CrossRef CAS.
- (a) A. Corma and H. Garcia, Catal. Today, 1997, 38, 257–308 CrossRef CAS; (b) A. Cimino and F. S. Stone, Adv. Catal., 2002, 47, 141–306 CAS; (c) S. Gopal and P. G. Smirniotis, J. Catal., 2004, 225, 278–287 CrossRef CAS.
- (a) S. P. B. Kremer, C. E. A. Kirschhock, A. Aerts, K. Villani, J. A. Martens, O. I. Lebedev and G. Van Tendeloo, Adv. Mater., 2003, 15, 1705–1707 CrossRef CAS; (b) S. Bals, K. J. Batenburg, D. Liang, O. Lebedev, G. Van Tendeloo, A. Aerts, J. A. Martens and C. E. A. Kirschhock, J. Am. Chem. Soc., 2009, 131, 4769–4773 CrossRef CAS.
- (a) J. Martinez-Triguero, M. J. Diaz-Cabañas, M. A. Camblor, V. Fornés, Th. L. M. Maesen and A. Corma, J. Catal., 1999, 182, 463–469 CrossRef CAS; (b) A. Corma, M. J. Díaz-Cabañas and V. Fornés, Angew. Chem., Int. Ed., 2000, 39, 2346–2349 CrossRef CAS; (c) S. I. Zones and S. J. Hwang, Microporous Mesoporous Mater., 2003, 58, 263–277 CrossRef CAS; (d) S. I. Zones, C. Y. Chen, A. Corma, M. T. Cheng, C. L. Kibby, I. Y. Chan and A. W. Burton, J. Catal., 2007, 250, 41–54 CrossRef CAS.
- M. J. Remy, D. Stanica, G. Poncelet, E. J. P. Feijen, P. J. Grobet, J. A. Martens and P. A. Jacobs, J. Phys. Chem., 1996, 100, 12440–12447 CrossRef CAS.
- R. Ravishankar, C. Kirschhock, B. J. Schoeman, P. Vanoppen, P. J. Grobet, S. Storck, W. F. Maier, J. A. Martens, F. C. De Schryver and P. A. Jacobs, J. Phys. Chem. B, 1998, 102, 2633–2639 CrossRef CAS.
- J. Musschoot, Q. Xie, D. Deduytsche, S. Van den Berghe, R. L. Van Meirhaeghe and C. Detavernier, Microelectron. Eng., 2009, 86, 72–77 CrossRef CAS.
- C. A. Emeis, J. Catal., 1993, 141, 347–354 CrossRef CAS.
Footnote |
| † Experimental details:Materials: CBV-712 is a commercially available (PQ Corp.) US-Y-type zeolite with a Si/Al ratio of 5.8.19 It was used as received in the ALD treatments as parent zeolite (notated as US-Y hereafter). Zeotile-417b and Silicalite-120 materials were prepared according to the literature. In ALD-Al the sample was evacuated in a tube furnace in a flow of N2 at 473 K for 6 h and transferred to the ALD instrument.21 The ALD cycle involved Al(CH3)3 introduction, evacuation, water vapour introduction and evacuation (for 30 s each) at 0.2 Pa base pressure and 473 K temperature.Measurements: Nitrogen adsorption at 77 K was done on a Micromeritics Tristar3000. The 27Al MAS NMR spectra were recorded on a Bruker Avance DSX400 spectrometer (9.4 T). FTIR was done on a Nicolet 6700 spectrometer equipped with a vacuum cell. The molar extinction coefficients used for quantification of adsorbed pyridine concentrations were taken from Emeis.22 Samples were impregnated with an aqueous solution of Pt(NH4)3Cl2 to obtain a Pt loading of 0.5 wt%. The crushed and sieved catalyst pellets were loaded in microreactor tubes. Hydroconversion of decane was carried out over the pretreated catalysts (oxidation and reduction at 673 K) at 0.45 MPa total pressure, H2/decane molar ratio of 124 and a contact time of 1400 kg s mol−1. Conversion was increased by increasing reaction temperature. |
| This journal is © The Royal Society of Chemistry 2011 |
