CoAPO-5-type molecular sieve membrane: synthesis, characterization and catalytic performance
Received
8th November 2010
, Accepted 28th December 2010
First published on 10th February 2011
Abstract
The coating of cobalt containing aluminophosphate molecular sieve crystals with the AFI structure has been investigated over a tubular as well as disc shaped α-alumina support. Characterization by SEM and nitrogen gas permeation study suggests a defect-free continuous intergrown CoAPO-5 membrane with a thickness of about 20 μm. The membranes with different cobalt contents were characterized by SEM, EDX, XRD, UV-Vis, and nitrogen permeation. The CoAPO-5 membranes exhibit good activity in the partial oxidation of propane to acetone and 2-propanol under vapor-phase conditions using molecular O2.
1. Introduction
Inorganic membranes, particularly those made of microporous zeolites,1 find widespread applications for the separation of gases under conditions where polymeric membranes cannot be used. Microporous materials with high specific surface area and pore volume provide a unique environment for chemical reaction as well as simultaneous separation of compounds through selective adsorption or ion-exchange.2 The majority of inorganic molecular sieving membranes reported to date are composed of LTA, MFI, MOR, and FAU.3–5 In recent years, the synthesis of framework modified microporous crystals containing titanium and vanadium such as TS-1 (Ti-MFI), VS-1 (V-MFI) and ETS-4 (small-pore titanium silicate) was reported for use in the separation of small gaseous molecules.6–8 However, the instability of silica materials limits their applications such as in high temperature membrane reactors, especially in the presence of steam. Densification of silica membranes is often encountered on prolonged exposure of these materials to water vapor at an elevated temperature. For example, Sea et al. have reported that the H2 permeance of a silica membrane prepared on a porous γ-alumina support by chemical vapor deposition (CVD) underwent a reduction of about 90% from 3.5 × 10−7 to 4.0 × 10−8 mol m−2Pa−1 s−1 after an exposure to 50 mol% water vapor at 673 K for 100 h.9 Considerable efforts have been made to improve the stability of silica microstructures by including methyl groups in the silica microstructures and combination of SiO2 with other oxides such as ZrO2, Co3O4, and Nb2O5.10,11
Alternatively, similar to aluminosilicates, microporous phosphate based molecular sieves also possess unique properties.12 However, aluminophosphates have received much less attention despite the closeness of their structure to those of zeolites. The framework structure of AlPO4 is composed of AlO2− and PO2+ tetrahedral units and shows essentially no ion-exchange capacity. As a result, thermal stability for this class of materials is higher than that of zeolite based aluminosilicates. A few studies have been reported in which attempts have been made to fabricate aluminophosphate membranes. For example, a siliconaluminophosphate molecular sieves SAPO-34 membrane over an alumina support has been demonstrated for selective permeation of CO2 from a mixture of gases.13–15 Very recently, layered aluminophosphates were incorporated into the polymer matrix and the resulting membrane showed high permeability for H2 from a mixture of H2/CO.15 In addition, AlPO4-5/polymer incorporated membranes also exhibit good activity in the pervaporation and dehydration of aqueous–organic mixtures near their azeotropic compositions.16–18 Though the aluminophosphate membranes were widely investigated for their potential applications, other metallophosphates have not been studied. One of the interesting aluminophosphate structures is AlPO-5 with AFI topology containing a 12-ring one-dimensional hexagonal framework structure with a pore size of 0.73 × 0.73 nm and also readily crystallizes with many different templates. In particular, introduction of Co(II/III) in the porous framework has been extensively investigated since it imparts catalytic functions to the material.19 Here we describe the fabrication of the CoAPO4-5 molecular sieving membrane over a porous α-alumina tube under hydrothermal conditions. The membranes with varying amount of cobalt contents were characterized by XRD, SEM, EDX, UV-Vis spectroscopy and gas permeability.
2. Experimental
2.1 Fabrication of CoAPO-5 membranes
Porous α-alumina tube and disc (PM tube, Nikkatto-Tokyo, outer diameter = 2.8 mm, inner diameter = 1.9 mm and mean pore diameter = 1–10 μm) were used as support for fabrication of CoAPO-5-type membranes. Phosphoric acid [TCI, 85%], Co(NO3)2·6H2O (TCI, 99%), and triethylamine (TCI, 98%) were commercially obtained and used without further purification. Pseudoboehmite was used as the aluminium source (Sigma-Aldrich, 70% Al2O3). The synthesis of CoAPO-5 was carried out following the molar gel composition Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2O5
P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCo2+
xCo2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75TEA
0.75TEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2O where x = 0.01–0.03. In a typical synthesis, phosphoric acid was mixed with water and cooled to 0 °C and pseudoboehmite was added. Then the desired amount of Co(NO3)2·6H2O was added under vigorous stirring and finally triethylamine was added and the stirring was continued for additional one hour. Prior to coating of the α-alumina tube with CoAPO-5 crystals, both the ends of the alumina tube were wrapped with a Teflon tape in order to prevent deposition of CoAPO-5 in the inner core surface of the alumina tube. Then the alumina tube was sonicated for 15 min in the presence of gel in order to penetrate the gel inside the pores of the alumina tube and then it was mounted vertically using a Teflon base and placed vertically in an autoclave. Then the synthesis gel was filled to desired level and crystallization of CoAPO-5 was carried out at 200 °C for 24 h under static conditions. In order to get the continuous intergrown and completely packed defect-free membrane, the synthesis was repeated two times over the same alumina tube but with different synthesis gel. After the synthesis, the membrane was washed thoroughly with deionized water and dried at 100 °C for 6 h. The templates occluded in the pores/channels of the molecular sieve were removed by calcinations and the heating was done very slowly with a ramping rate of 0.7 °C min−1 until it reaches 500 °C and cooled to room temperature in a period of 3 h. By adapting similar synthesis procedure, the alumina disc with the CoAPO-5 membrane was also fabricated.
20H2O where x = 0.01–0.03. In a typical synthesis, phosphoric acid was mixed with water and cooled to 0 °C and pseudoboehmite was added. Then the desired amount of Co(NO3)2·6H2O was added under vigorous stirring and finally triethylamine was added and the stirring was continued for additional one hour. Prior to coating of the α-alumina tube with CoAPO-5 crystals, both the ends of the alumina tube were wrapped with a Teflon tape in order to prevent deposition of CoAPO-5 in the inner core surface of the alumina tube. Then the alumina tube was sonicated for 15 min in the presence of gel in order to penetrate the gel inside the pores of the alumina tube and then it was mounted vertically using a Teflon base and placed vertically in an autoclave. Then the synthesis gel was filled to desired level and crystallization of CoAPO-5 was carried out at 200 °C for 24 h under static conditions. In order to get the continuous intergrown and completely packed defect-free membrane, the synthesis was repeated two times over the same alumina tube but with different synthesis gel. After the synthesis, the membrane was washed thoroughly with deionized water and dried at 100 °C for 6 h. The templates occluded in the pores/channels of the molecular sieve were removed by calcinations and the heating was done very slowly with a ramping rate of 0.7 °C min−1 until it reaches 500 °C and cooled to room temperature in a period of 3 h. By adapting similar synthesis procedure, the alumina disc with the CoAPO-5 membrane was also fabricated.
2.2 Characterization of CoAPO-5 membrane
The crystallinity and phase purity of the CoAPO-5 membrane was determined from the powder samples collected from the bottom of the autoclave as well as coated membranes using MAC Science X-ray diffractometer equipped with CuKα radiation. The gas permeation tests were carried out using a home-made apparatus after sealing both ends of the alumina tube to confine the permeation zones. The nitrogen permeation was carried out at room temperature (24 °C) using a flow meter. The pressure on the feed side was 1.5 atm. The morphology and membrane thickness of the sample was determined from scanning electron microscope (SEM) with a Hitachi S-800 microscope. Energy dispersive X-ray analysis was performed to estimate the amount of cobalt present in the membrane. UV-Visible diffuse reflectance spectroscopic analyses were performed to identify the nature of cobalt species in the molecular sieve framework.
2.3
Catalytic reaction
The oxidation was carried out using a home-made modified cylindrical tubular reactor as shown in Fig. 1. The membrane of the desired length (10 cm) was connected in the inner core part of the reactor using connectors and a mixture of propane and helium was fed through the inner core of the α-alumina membrane. Here the helium was used for diluting the reactants. The O2 diluted with helium was passed over the outer surface of the alumina membrane as shown in Fig. 1. The temperature of the reactor was controlled through an in-built electrical coil and the temperature was monitored using a thermocouple. Reaction products were analyzed online using gas chromatograph equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD) using an FFAP capillary column (0.32 mm × 60 m) and a Porapak Q packed column (3 mm × 2 m).
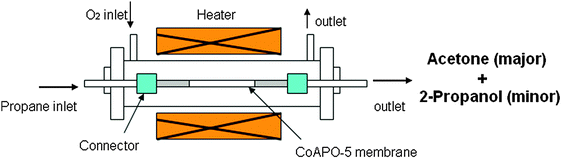 |
| | Fig. 1 Diagrammatic representation of tubular reactor set-up for vapor-phase reactions. | |
3. Results and discussion
3.1 Preparation of CoAPO-5 membranes and characterization
The XRD pattern of the α-alumina/CoAPO-5 composite membrane is shown in Fig. 2. It is seen from the figure that it has the characteristics of a pure AFI phase although the intensity is lower than the CoAPO-5 samples collected at the bottom of the autoclave.16Fig. 3A shows the SEM surface-image of the α-alumina tube (PM-tube, Nikkato). Pores of varying size are clearly visible. The composition of the α-alumina tube was Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 65
SiO2 = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 wt% with a density of 1.6 g cm−3 and porosity of about 47%; the average pore size on the surface of the α-alumina tube ranges from 0.2 to 1.1 μm. CoAPO-5 is a 12-membered ring, wide-pore aluminophosphate with a pore size of 0.73 × 0.73 nm. Table 1 exhibits the cobalt contents in CoAPO-5 composite membranes with various ratios of Co
33 wt% with a density of 1.6 g cm−3 and porosity of about 47%; the average pore size on the surface of the α-alumina tube ranges from 0.2 to 1.1 μm. CoAPO-5 is a 12-membered ring, wide-pore aluminophosphate with a pore size of 0.73 × 0.73 nm. Table 1 exhibits the cobalt contents in CoAPO-5 composite membranes with various ratios of Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P in the mother liquor. As can be seen, the cobalt content of the membranes increases with the amount of cobalt added in the mother liquor but the increase is rather irregular. The CoAPO-5 crystals were coated through sedimentation deposition onto the alumina tube under autogeneous pressure; the SEM picture (Fig. 3B) shows that nearly most of the external surface has been covered with CoAPO-5 crystals after first coating. All the SEM pictures were obtained from calcined alumina/CoAPO-5 membranes. Bundled hexagonal CoAPO-5 crystals with sizes between 2 and 3 μm were observed from the SEM image. Furthermore, in order to obtain a continuous intergrown membrane of CoAPO-5, it was necessary to repeat the coating process over the same α-alumina tube and therefore, the synthesis was carried out two times successively with different synthesis gel. Fig. 3C shows the SEM picture of the alumina tube coated twice; though the surface coverage is improved by second coating still there are a few uncovered patches. In order to maintain the phase purity of CoAPO-5 and avoid microstructure cracking at high film thickness and/or higher loadings, the coating was stopped after two repeated synthesis. The final thickness of the membrane was found to be approximately 20 μm as estimated from the broken structure of the SEM image (Fig. 3D).
P in the mother liquor. As can be seen, the cobalt content of the membranes increases with the amount of cobalt added in the mother liquor but the increase is rather irregular. The CoAPO-5 crystals were coated through sedimentation deposition onto the alumina tube under autogeneous pressure; the SEM picture (Fig. 3B) shows that nearly most of the external surface has been covered with CoAPO-5 crystals after first coating. All the SEM pictures were obtained from calcined alumina/CoAPO-5 membranes. Bundled hexagonal CoAPO-5 crystals with sizes between 2 and 3 μm were observed from the SEM image. Furthermore, in order to obtain a continuous intergrown membrane of CoAPO-5, it was necessary to repeat the coating process over the same α-alumina tube and therefore, the synthesis was carried out two times successively with different synthesis gel. Fig. 3C shows the SEM picture of the alumina tube coated twice; though the surface coverage is improved by second coating still there are a few uncovered patches. In order to maintain the phase purity of CoAPO-5 and avoid microstructure cracking at high film thickness and/or higher loadings, the coating was stopped after two repeated synthesis. The final thickness of the membrane was found to be approximately 20 μm as estimated from the broken structure of the SEM image (Fig. 3D).
 |
| | Fig. 2
XRD pattern of the α-alumina/CoAPO-5 membrane and the peaks marked with asterisks indicate α-alumina diffraction peaks. | |
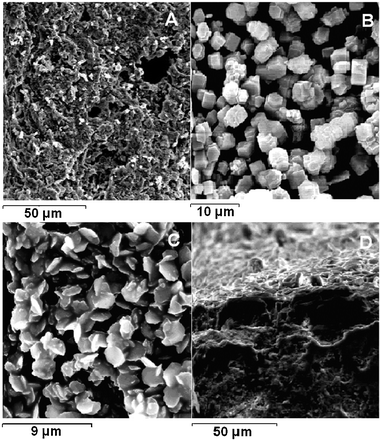 |
| | Fig. 3
SEM images of the α-alumina tube (A); CoAPO-5 after first coating (B); CoAPO-5 after second coating (C) and edge-broken finished tubular membrane (D). | |
Table 1 Physical characteristics of CoAPO-5 membranes with different cobalt content
| Entry |
Gel composition, Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
Product composition,aCo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, wt% P, wt% |
Membrane thicknessa/μm |
Permeabilityb/mol m−2 s−1Pa−1 |
|
Estimated by EDX analysis and thickness of the membrane was obtained from SEM.
Nitrogen permeability at room temperature.
|
|
Alumina tube |
— |
Al = 65 wt%, Si = 33 wt% |
Uncoated |
5.2 × 10−5 |
| M1 |
0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49.3 49.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.0 50.0 |
20–22 |
9.6 × 10−7 |
| M2 |
0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.4 48.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.0 50.0 |
20–22 |
8.4 × 10−7 |
| M3 |
0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47.7 47.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.0 50.0 |
20–22 |
7.3 × 10−7 |
UV-Vis
diffuse reflectance spectrum of the CoAPO-5 membrane showed absorption bands between 500 and 650 nm typical of high spin Co(II) species in a tetrahedral environment.20 Furthermore, the color of the CoAPO-5 membrane was blue if it contained more Co(II) species, but the color became green-yellow if it had more Co(III) species.20 It is clearly seen from Fig. 4 that the as-synthesized tubular and disc membranes are bluish in color; for comparison the uncoated disc membrane is also shown in Fig. 4. After being calcined and maintained at the calcination temperature, the color of CoAPO-5 was green-yellow, revealing that it contained more Co(III) species. When the calcined CoAPO-5 was cooled to room temperature, its color again became blue indicating that Co(III) has been reduced to Co(II). The amounts of different elements present in the CoAPO-5 were estimated from EDX analysis and listed in Table 1. Furthermore, it is important to mention that all the membranes before calcinations did not show any nitrogen permeability at room temperature indicating that both intercrystalline and pores/channels were completely occupied with triethylamine templates. However, after calcinations all the membranes exhibit permeation and the permeation values range between 7.3 × 10−7 and 9.6 × 10−7 mol m−2Pa−1 s−1. In addition, the nitrogen permeation on the α-alumina tube before coating was found to be 5.2 × 10−5 mol m−2Pa−1 s−1. Thus the calcined membranes exhibited nitrogen permeation two orders of magnitude lower than the uncoated membranes. Comparison of nitrogen permeation values of uncoated and calcined membranes indicates the compact packing of CoAPO-5 crystals although one cannot neglect the diffusion of gas through intercrystalline voids. The nitrogen permeability of α-alumina/CoAPO-5 membranes was also comparable to the value of reported γ-alumina/silica membranes indicating the compactness of the tubular membranes.9 Thus the combination of different analytical and spectroscopic techniques like XRD, SEM, EDX, UV-Visible and gas permeability over the calcined membranes clearly indicates the successful fabrication of continuous intergrown defect-free CoAPO-5 membranes under hydrothermal conditions.
 |
| | Fig. 4
CoAPO-5
membranes: (A) tubular membranes; (B) disc membrane; (C) uncoated disc alumina support. | |
3.2
Oxidation of propane over CoAPO-5 tubular membrane
The catalytic performances of CoAPO-5 membranes were tested for the partial oxidation of propane to acetone and 2-propanol. The tubular reactor for carrying out reactions using the CoAPO-5 membrane at high temperature is shown in Fig. 1. About 10 cm of a calcined defect-free membrane is mounted in the center by connecting both the ends of the alumina tube using connectors. The propane gas mixed with helium was passed through the inner side of the alumina tube, whereas O2 mixed with helium was passed over the CoAPO-5 membrane layer directly as shown in Fig. 1. Table 2 shows the observed conversion of propane and selectivity for acetone and 2-propanol. The conversion of propane increased marginally with the cobalt content and acetone was detected as the major product. The selectivity of acetone ranged from 79.0 to 84.0%, whereas the selectivity of 2-propanaol was found to be between 14.0 and 18.0%. Recently, Oyama et al. investigated the selective oxidation of propane using titanosilicate TS-1/Au with a mixture of H2 and O2 as oxidants under fixed bed reaction conditions.21 In the present study, though the catalyst and reactor set-up are different, the CoAPO-5 membrane exhibits comparable activity and selectivity for the oxidized products. Furthermore, the tubular membrane reactor avoids the mixing of O2 and organics (or) O2 and H2 in one pot, which often leads to potential explosion. Therefore the membrane reactors are advantageous and can be employed even for continuous operation provided the membranes are stable enough. Mechanistically, a free-radical pathway has been proposed for heterogeneous catalysis over CoAPO-5.22 At the onset of reaction, since most of the cobalt species in the CoAPO-5 catalyst was Co(II) species, the reaction might be initiated via both autoxidation and catalysis by the CoAPO-5 catalyst. In the course of the reaction, however, the concentration of Co(II) species decreases due to oxidation by oxygen to Co(III) species. The Co(III) then predominately reacts with propane and produces free-radicals and itself reduced to Co(II). The organic propyl radicals then combine with molecular O2 to give the peroxy-intermediate, which undergoes decomposition to give acetone as the major product. The above catalytic reaction reveals that coating of a thin layer of metal containing aluminophosphate molecular sieves can be effectively used for oxidation reactions. In particular, membranes with redox metals in the aluminophosphate framework could serve as a viable alternate strategy for continuous production as well as separation of organic fine chemicals through partial oxidation using molecular oxygen.
Table 2
Oxidation of propane over a CoAPO-5 membrane reactor under vapor-phase conditionsa
| Entry |
C3H8 conv. (%)b |
Selectivity (%) |
TON, min−1 |
|
Acetone
|
2-Propanol
|
CO2 |
Reaction conditions: ratio of propane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He = 5 He = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mL and the feed rate is 10 mL min−1. Ratio of O2 5 mL and the feed rate is 10 mL min−1. Ratio of O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He = 5 He = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mL and the feed rate is 10 mL min−1.
Conversion is based on the propane consumed. 5 mL and the feed rate is 10 mL min−1.
Conversion is based on the propane consumed.
|
| M1 |
0.8 |
81.0 |
17.0 |
2.0 |
1.54 |
| M2 |
1.3 |
79.0 |
18.0 |
3.0 |
1.1 |
| M3 |
1.6 |
84.0 |
14.0 |
2.0 |
0.93 |
4. Conclusions
CoAPO-5
membranes with thickness about 20 μm have been successfully fabricated over tubular as well as disc shaped α-alumina supports under hydrothermal conditions. The tubular membranes after calcinations exhibited nitrogen gas permeability in the order of 10−7. Various analytical and spectroscopic characterizations such as XRD, SEM, EDX, and UV-Vis confirmed the presence of Co(II) species in the AFI aluminophosphate structure. The CoAPO-5 membrane exhibited promising activity in the partial oxidation of propane to acetone and 2-propanol under vapor-phase conditions.
References
- J. Caro, M. Noach, P. Kölsch and R. Schäfer, Microporous Mesoporous Mater., 2000, 38, 3–24 CrossRef CAS; Z. P. Lai, G. Bonilla, I. Diaz, J. G. Nery, K. Sujaoti, M. A. Amat, E. Kokkoli, O. Terasaki, R. W. Thompson, M. Tsapatsis and D. G. Vlachos, Science, 2003, 300, 456–460 CrossRef CAS; J. Coronas and J. Santamaria, Top. Catal., 2004, 29, 29–44 CrossRef CAS.
- A. Bhaumik and T. Tatsumi, Microporous Mesoporous Mater., 2000, 34, 1–7 CrossRef CAS; M. Paul, N. Pal, P. R. Rajamohanan, B. S. Rana, A. K. Sinha and A. Bhaumik, Phys. Chem. Chem. Phys., 2010, 12, 9389–9394 RSC.
- Y. J. Wang and F. Caruso, Adv. Funct. Mater., 2004, 14, 1012–1018 CrossRef CAS; E. E. McLeary, J. C. Jansen and F. Kapteijn, Microporous Mesoporous Mater., 2006, 90, 198–220 CrossRef CAS; S. M. Kwan and K. L. Yeung, Chem. Commun., 2008, 3631–3633 RSC.
- C. L. Flandders, V. A. Tuan, R. D. Noble and J. L. Falconer, J. Membr. Sci., 2000, 176, 43 CrossRef CAS; A. A. Kittur, S. S. Kulkarni, M. I. Aralaguppi and M. Y. Kariduraganavar, J. Membr. Sci., 2005, 247, 75–86 CrossRef CAS.
- K. Kusakabe, T. Kuroda, A. Murata and S. Morooka, Ind. Eng. Chem. Res., 1997, 36, 649 CrossRef CAS; H. Kita, T. Inoue, H. Asamura, K. Tanaka and K. Okamoto, Chem. Commun., 1997, 45–46 RSC; K. Kusakabe, T. Kuroda, K. Uchino, Y. Hasegawa and S. Morooka, AIChE J., 1999, 45, 1220 CAS.
- L. Y. Y. Au, J. L. H. Chau, C. T. Ariso and K. L. Yeung, J. Membr. Sci., 2001, 183, 269 CrossRef CAS.
- G. Guan, K. Kusakabe and S. Morooka, Microporous Mesoporous Mater., 2001, 50, 109 CrossRef CAS.
- G. Guan, K. Kusakabe and S. Morooka, Sep. Sci. Technol., 2002, 37, 1031 CrossRef CAS.
- B. K. Sea, E. Soewito, M. Watanabe, K. Kusakabe, S. Morooka and S. S. Kim, Ind. Eng. Chem. Res., 1998, 37, 2502 CrossRef CAS.
- K. Yosida, Y. Hirano, H. Fujii, T. Tsuru and M. Asaed, J. Chem. Eng. Jpn., 2001, 34, 523 CrossRef CAS.
- V. Boffa, D. H. A. Blank and J. E. ten Elshof, J. Membr. Sci., 2008, 319, 256 CrossRef CAS; S. Battersby, S. Smart, B. Ladewig, S. Liu, M. C. Duke, V. Rudolph and J. C. Diniz da Costa, Sep. Purif. Technol., 2009, 66, 299 CrossRef CAS.
- M. E. Davis, C. Saldarriaga, C. Montes, J. Garces and C. Crowder, Nature, 1988, 331, 698–699 CrossRef CAS; K. Sarkar, S. C. Laha and A. Bhaumik, J. Mater. Chem., 2006, 16, 2439–2444 RSC; J. H. Yu and R. R. Xu, Chem. Soc. Rev., 2006, 35, 593–604 RSC.
- Z. Lixiong, M. D. Jia and M. Enze, Stud. Surf. Sci. Catal., 1997, 105, 2211 CrossRef.
- Y. Y. Tian, L. L. Fan, Z. Y. Wang, S. L. Qiu and G. S. Zhu, J. Mater. Chem., 2009, 19, 7698–7703 RSC.
- J. C. Poshusta, V. A. Tuan, E. A. Pape, R. D. Noble and J. L. Falconer, AIChE J., 2000, 46, 779 CAS.
- S. D. Bhat, N. N. Mallikarjuna and T. M. Aminabhavi, J. Membr. Sci., 2006, 282, 473 CrossRef CAS.
- B. Vaughan, J. Peter, E. Marand and M. Bleha, J. Membr. Sci., 2008, 316, 153 CrossRef CAS.
- C. Covarrubias and R. Quijada, J. Membr. Sci., 2010, 358, 33 CrossRef CAS.
- S. Samanta, S. Laha, N. K. Mal and A. Bhaumik, J. Mol. Catal. A: Chem., 2004, 222, 235–241 CrossRef CAS.
- L. E. Iton, I. Choi, J. A. Desjardins and V. A. Maroni, Zeolites, 1989, 9, 535 CrossRef CAS.
- J. J. Bravo-Suarez, K. K. Bando, T. Fujitani and S. T. Oyama, J. Catal., 2008, 257, 32 CrossRef CAS.
- S. S. Lin and H. S. Weng, Appl. Catal., A, 1993, 105, 289 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2O5
P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCo2+
xCo2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75TEA
0.75TEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20H2O where x = 0.01–0.03. In a typical synthesis, phosphoric acid was mixed with water and cooled to 0 °C and pseudoboehmite was added. Then the desired amount of Co(NO3)2·6H2O was added under vigorous stirring and finally triethylamine was added and the stirring was continued for additional one hour. Prior to coating of the α-alumina tube with CoAPO-5 crystals, both the ends of the alumina tube were wrapped with a Teflon tape in order to prevent deposition of CoAPO-5 in the inner core surface of the alumina tube. Then the alumina tube was sonicated for 15 min in the presence of gel in order to penetrate the gel inside the pores of the alumina tube and then it was mounted vertically using a Teflon base and placed vertically in an autoclave. Then the synthesis gel was filled to desired level and crystallization of CoAPO-5 was carried out at 200 °C for 24 h under static conditions. In order to get the continuous intergrown and completely packed defect-free membrane, the synthesis was repeated two times over the same alumina tube but with different synthesis gel. After the synthesis, the membrane was washed thoroughly with deionized water and dried at 100 °C for 6 h. The templates occluded in the pores/channels of the molecular sieve were removed by calcinations and the heating was done very slowly with a ramping rate of 0.7 °C min−1 until it reaches 500 °C and cooled to room temperature in a period of 3 h. By adapting similar synthesis procedure, the alumina disc with the CoAPO-5 membrane was also fabricated.
20H2O where x = 0.01–0.03. In a typical synthesis, phosphoric acid was mixed with water and cooled to 0 °C and pseudoboehmite was added. Then the desired amount of Co(NO3)2·6H2O was added under vigorous stirring and finally triethylamine was added and the stirring was continued for additional one hour. Prior to coating of the α-alumina tube with CoAPO-5 crystals, both the ends of the alumina tube were wrapped with a Teflon tape in order to prevent deposition of CoAPO-5 in the inner core surface of the alumina tube. Then the alumina tube was sonicated for 15 min in the presence of gel in order to penetrate the gel inside the pores of the alumina tube and then it was mounted vertically using a Teflon base and placed vertically in an autoclave. Then the synthesis gel was filled to desired level and crystallization of CoAPO-5 was carried out at 200 °C for 24 h under static conditions. In order to get the continuous intergrown and completely packed defect-free membrane, the synthesis was repeated two times over the same alumina tube but with different synthesis gel. After the synthesis, the membrane was washed thoroughly with deionized water and dried at 100 °C for 6 h. The templates occluded in the pores/channels of the molecular sieve were removed by calcinations and the heating was done very slowly with a ramping rate of 0.7 °C min−1 until it reaches 500 °C and cooled to room temperature in a period of 3 h. By adapting similar synthesis procedure, the alumina disc with the CoAPO-5 membrane was also fabricated.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 65
SiO2 = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 wt% with a density of 1.6 g cm−3 and porosity of about 47%; the average pore size on the surface of the α-alumina tube ranges from 0.2 to 1.1 μm. CoAPO-5 is a 12-membered ring, wide-pore aluminophosphate with a pore size of 0.73 × 0.73 nm. Table 1 exhibits the cobalt contents in CoAPO-5 composite membranes with various ratios of Co
33 wt% with a density of 1.6 g cm−3 and porosity of about 47%; the average pore size on the surface of the α-alumina tube ranges from 0.2 to 1.1 μm. CoAPO-5 is a 12-membered ring, wide-pore aluminophosphate with a pore size of 0.73 × 0.73 nm. Table 1 exhibits the cobalt contents in CoAPO-5 composite membranes with various ratios of Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P in the mother liquor. As can be seen, the cobalt content of the membranes increases with the amount of cobalt added in the mother liquor but the increase is rather irregular. The CoAPO-5 crystals were coated through sedimentation deposition onto the alumina tube under autogeneous pressure; the SEM picture (Fig. 3B) shows that nearly most of the external surface has been covered with CoAPO-5 crystals after first coating. All the SEM pictures were obtained from calcined alumina/CoAPO-5 membranes. Bundled hexagonal CoAPO-5 crystals with sizes between 2 and 3 μm were observed from the SEM image. Furthermore, in order to obtain a continuous intergrown membrane of CoAPO-5, it was necessary to repeat the coating process over the same α-alumina tube and therefore, the synthesis was carried out two times successively with different synthesis gel. Fig. 3C shows the SEM picture of the alumina tube coated twice; though the surface coverage is improved by second coating still there are a few uncovered patches. In order to maintain the phase purity of CoAPO-5 and avoid microstructure cracking at high film thickness and/or higher loadings, the coating was stopped after two repeated synthesis. The final thickness of the membrane was found to be approximately 20 μm as estimated from the broken structure of the SEM image (Fig. 3D).
P in the mother liquor. As can be seen, the cobalt content of the membranes increases with the amount of cobalt added in the mother liquor but the increase is rather irregular. The CoAPO-5 crystals were coated through sedimentation deposition onto the alumina tube under autogeneous pressure; the SEM picture (Fig. 3B) shows that nearly most of the external surface has been covered with CoAPO-5 crystals after first coating. All the SEM pictures were obtained from calcined alumina/CoAPO-5 membranes. Bundled hexagonal CoAPO-5 crystals with sizes between 2 and 3 μm were observed from the SEM image. Furthermore, in order to obtain a continuous intergrown membrane of CoAPO-5, it was necessary to repeat the coating process over the same α-alumina tube and therefore, the synthesis was carried out two times successively with different synthesis gel. Fig. 3C shows the SEM picture of the alumina tube coated twice; though the surface coverage is improved by second coating still there are a few uncovered patches. In order to maintain the phase purity of CoAPO-5 and avoid microstructure cracking at high film thickness and/or higher loadings, the coating was stopped after two repeated synthesis. The final thickness of the membrane was found to be approximately 20 μm as estimated from the broken structure of the SEM image (Fig. 3D).


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, wt%
P, wt%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49.3
49.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.0
50.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.4
48.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.0
50.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47.7
47.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.0
50.0
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He = 5
He = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mL and the feed rate is 10 mL min−1. Ratio of O2
5 mL and the feed rate is 10 mL min−1. Ratio of O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He = 5
He = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mL and the feed rate is 10 mL min−1.
b Conversion is based on the propane consumed.
5 mL and the feed rate is 10 mL min−1.
b Conversion is based on the propane consumed.
