Enantioselective hydrogenation of prochiral substrates in catalytic membrane reactors†
Pierluigi
Barbaro
*a,
Claudio
Bianchini
a,
Francesca
Liguori
a,
Claudio
Pirovano
b and
Haruo
Sawa
c
aIstituto di Chimica dei Composti Organo Metallici – Consiglio Nazionale delle Ricerche (ICCOM-CNR), via Madonna del Piano 10, 50019 Sesto Fiorentino, Firenze, Italy. E-mail: pierluigi.barbaro@iccom.cnr.it; Fax: +39 055 5225203; Tel: +39 055 5225287
bIstituto di Scienze e Tecnologie Molecolari (ISTM-CNR), via C. Golgi 19, 20133 Milano, Italy. Fax: +39 02 50314405; Tel: +39 02 50314428
cNippon Kodoshi Corporation, 648 Hirookakami, Haruno, Kochi, 781-0395, Japan
First published on 17th February 2011
Abstract
Batch reactors containing catalytic membranes based on molecular asymmetric hydrogenation catalysts immobilized onto hybrid PVA/inorganic polymers were engineered and tested in the enantioselective hydrogenation of prochiral substrates. All reactions were efficiently carried out in mild reaction conditions with ee’s comparable to those of the corresponding homogeneous catalysts as well as minimal metal leaching upon reuse.
The development of sustainable, highly selective processes for the large scale production of fine chemicals is a current major industrial issue.1 Among the solutions proposed to achieve such a goal, the immobilization of chemical catalysts onto solid insoluble support materials provides significant benefits in terms of ease of recovery and reuse of the expensive catalysts, clean catalyst separation from the reaction products as well as integration in existing reactor equipments.2 When preformed enantioselective catalysts are immobilized, this strategy enables the preparation of heterogeneous catalysts featuring predictable asymmetric induction, often comparable with that of the homogeneous counterparts.3 In fact, the chemical industry has a strong preference for heterogeneous catalysts, but limited availability of heterogenized catalysts, together with activity loss and leaching problems, have confined their application to a few industrial processes so far.4 There is therefore a clear need to foster the research in the interdisciplinary domain integrating homogeneous and heterogeneous catalysis and to develop suitable catalytic equipments.
“Catalytic Membrane Reactors” (CMR) embedding a catalyst in a thin layer are receiving increasing attention as efficient and environmentally friendly production devices.5 In particular, compared to metallic or ceramic materials, polymeric-based membranes provide several advantages: (i) the sorption and permeability of reagents and products can be driven so as to improve the catalyst performance, (ii) the catalyst efficiency can be increased by co-incorporation of additives, (iii) the manufacture technology of polymeric membranes is much developed, thus allowing for a wide choice of sizes, morphologies, mechanical, chemical and thermal stability, affinity for reagents and catalysts to be available.6
Nonetheless, very few examples of polymeric catalytic membranes used in enantioselective processes have been reported. The (R,R)-MeDuPHOS–Rh, (S)-BINAP–Ru and (S,S)-SALEN–Mn complexes were supported onto PDMS films and used in the asymmetric hydrogenation and epoxidation reactions of olefins.7‡ These membranes showed good hydrogenation activity upon incorporation of silica or sulfonic acid derivatives, but insufficient stability in terms of metal leaching. Similar results were obtained by occlusion of ((R,R)-MeDuPHOS)–Rh catalysts into PVA membranes.8
During our studies on solid electrolytes for electrochemical applications,9 we recently discovered the possibility to immobilize molecular complexes onto inorganic-modified polymeric membranes that do not act as separation medium between two phases.10,11
Herein we report on the preparation and use of enantioselective catalytic hydrogenation membranes based on asymmetric hydrogenation molecular catalysts immobilized onto hybrid PVA/inorganic polymers and their assembly into CMRs. Preformed hybrid membranes with different PVA, SiO2, WO3, Na3PO4, polystyrenesulfonic acid and PET content,9,10 and rhodium(I) complexes of the general formula [(PP*)Rh(NBD)]PF6 were scrutinized for this purpose (PP* = chiral diphosphino ligand, Scheme 1) (see ESI†, Table S1).
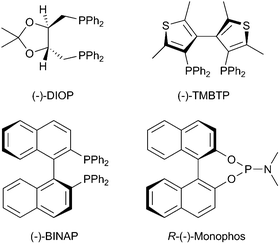 | ||
| Scheme 1 Sketch of the chiral ligands used. | ||
The immobilization of the rhodium compounds was easily and effectively accomplished by stirring a methanol solution of the Rh complex in the presence of the preformed hybrid membrane, followed by washings. Typical Rh loadings in the range 1.6–2.8% (w/w) were obtained in the dry material (ICP-AES and EDS), corresponding to 63–75% of the starting rhodium used. An electron microscope image of a typical catalytic membrane section is reported in Fig. 1. Although the presence of either WO3 or SO3−groups in the membrane is required to achieve an effective anchorage, no direct relation of catalyst loading with membrane composition was apparent. This suggests that the sorption of the rhodium specie is based on a complex combination of interactions whose exact nature is difficult to assess. One can hypothesize that both WO3 (through donor–acceptor interactions as in the case of the Augustine’s method using heteropolyacids)12 and the sulfonate group (through electrostatic bonds as in the case of ion-exchange resins)13 are responsible for the immobilization of the cationic complex.
![ESEM image of a section of the catalytic membrane NK1/[((−)-BINAP)Rh(NBD)]PF6 (secondary electrons, 2650 magnifications).](/image/article/2011/CY/c0cy00030b/c0cy00030b-f1.gif) | ||
| Fig. 1 ESEM image of a section of the catalytic membrane NK1/[((−)-BINAP)Rh(NBD)]PF6 (secondary electrons, 2650 magnifications). | ||
The catalytic membranes obtained were used in the enantioselective hydrogenation of MAA (Scheme 2) in a one-pot, two-step procedure, either in a “fixed-frame” or in a rotating membrane device (Fig. 2). In the latter case, a methanol solution of the substrate was introduced into a Teflon®-covered autoclave with the catalytic membrane prepared in situ. This was stirred for the desired time under 5 bar H2 at room temperature. For comparative purposes, different molecular catalysts were immobilized onto the same membrane type and the hydrogenation reactions were carried out under identical conditions (Table 1 and Table S2, ESI†). The results showed that:
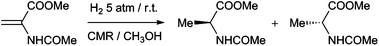 | ||
| Scheme 2 Hydrogenation reaction of MAA. | ||
 | ||
| Fig. 2 Rotating catalytic membrane assembly. | ||
| Membrane type | BINAP | DIOP | Monophos | TMBTP |
|---|---|---|---|---|
a Experimental conditions: CH3OH, rt, 5 bar H2, 120 min, MAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 200 catalyst = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, rotating membrane 150 rpm.
b 1 bar, 10 min.
c 60 min. 1, rotating membrane 150 rpm.
b 1 bar, 10 min.
c 60 min.
|
||||
| Homogeneous | 87.0 (21.7)b | 99.0 (66.0)b | 95.2 (98.0)c | 87 (99.0)b |
| NK-1 | 22.3 (15.0) | 34.8 (17.3) | 20.5 (90.5) | 26.4 (98.5) |
| CSNKW-1 | 93.0 (11.0) | 51.3 (17.6) | 24.4 (90.5) | 91.8 (98.3) |
(1) all catalytic membranes are effective under very undemanding conditions,
(2) the activity and enantioselectivity of the heterogenized catalysts may be compared with those of the corresponding homogeneous counterparts,
(3) the activities are strongly dependent on the membrane type, whereas the ee’s are not significantly affected,14
(4) rhodium leaching in solution is negligible (<1 ppm, GF-AAS) for most catalytic membranes but not acceptable (>1 ppm) for others (e.g.DIOP and TMBTP on CSNKW-1),
(5) none of the hybrid membranes is catalytically active in the absence of anchored rhodium complex,
(6) all reactions are truly heterogeneous, as proved by the absence of catalytic activity of the solutions recovered after catalysis,
(7) except for a minor membrane folding, all catalytic membranes remain intact after catalysis.
A deeper investigation on the reuse of the catalytic membranes was carried out using the immobilized [((−)-Monophos)2Rh(NBD)]PF6 complex as probe catalyst. A sequence of three cycles of 2 hours, followed by one 17 h cycle and one 2 h cycle was adopted as a standard experiment. Selected results are reported in graphical format in Fig. 3. All catalytic membranes showed a slight activity decrease upon recycle, although they were still efficient after 25 h use. Complete conversions were achieved after the appropriate reaction time. The ee’s were fairly constant upon reuse, being highest (>90%) for the NK-1 and CSNKW-1 membranes. In most cases, the rhodium leaching was acceptable and generally proportional to the residence time of the catalytic membrane into the methanol solution (i.e. higher for the overnight cycle). We selected the decay of catalyst activity (expressed as variation of the TOF after 25 h, ESI†) as a reliable parameter to evaluate the overall catalytic membrane performance upon recycle. This parameter did not show any clear relationship with the membrane composition, yet there was a correlation with the amount of rhodium leached in solution. The identification of the factors affecting the Rh leaching is not obvious, however. One evidence is that high rhodium losses are accompanied by a detectable tungsten leaching in solution. Accordingly, it is possible that the leaching of WO3—and thus of the rhodium interacting with it—may be responsible for the deactivation of the catalytic membranes through the release of non-catalytic rhodium species in solution. This fact, coupled with the lower activity decay of the PET-containing membranes, suggests that the membrane mechanical resistance may play a role on their reusability.15
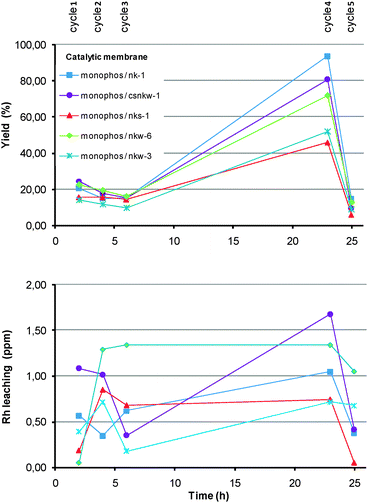 | ||
| Fig. 3 Recycle of catalytic membranes. | ||
In light of these results, we designed a “fixed-frame” CMR in which the flow of the stirred methanol solution is tangent to the membrane and we used this device to scale-up the process by a factor of about ten (ESI†). Under these conditions, the (Monophos)Rh/NK-1 membrane gave a 50% higher TOF in each cycle, as compared to the rotating membrane reactor, with analogous enantioselectivity and Rh leaching.
The possibility to hydrogenate different substrates in diverse solvent systems also testify for the versatility of the technology. Table 2 shows representative results for the reduction of prochiral C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (DDF) in the compounds sketched in Scheme 3. All reactions proceeded with conversions and ee’s comparable with those obtained in the homogeneous phase and with metal leaching below 1 ppm. It is worth mentioning that hydrogenations could be carried out in water (in which solubility of the catalyst hampers the homogenous phase reaction) although with somewhat lower yield and ee’s compared to methanol, but with lower Rh leaching (<0.05 ppm). Lower conversions were observed in other solvents (e.g.ethanol) due to the lower membrane permeation.9
O bonds (DDF) in the compounds sketched in Scheme 3. All reactions proceeded with conversions and ee’s comparable with those obtained in the homogeneous phase and with metal leaching below 1 ppm. It is worth mentioning that hydrogenations could be carried out in water (in which solubility of the catalyst hampers the homogenous phase reaction) although with somewhat lower yield and ee’s compared to methanol, but with lower Rh leaching (<0.05 ppm). Lower conversions were observed in other solvents (e.g.ethanol) due to the lower membrane permeation.9
| Substrate | Solvent | Membrane | Ligand | Yield, ee | Yield, ee |
|---|---|---|---|---|---|
| CMR | Homogeneous | ||||
a Experimental conditions: rt, 5 bar H2, 2 h, substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 200 catalyst = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
b 17 h.
c 24 h.
d 24 h, 40 bar H2. 1.
b 17 h.
c 24 h.
d 24 h, 40 bar H2.
|
|||||
| MAA | CH3OH | NK-1 | Monophos | 93.9, 94.0b | 99.9, 98.0b |
| MAA | H2O | NK-1 | Monophos | 55.2, 70.0b | — |
| DMI | CH3OH | CSNKW-1 | TMBTP | 24.6, 12.4c | 19.6, 14.0 |
| EMC | CH3OH | NK-1 | DIOP | 12.8, 17.0d | 13.0, 17.0d |
| ANL | H2O | NK-1 | Monophos | 15.0, 24.1 | — |
| DDF |
CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NK-1 | Monophos | 33.9, 3.0d | — |
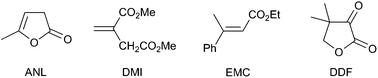 | ||
| Scheme 3 Sketch of the substrates tested. | ||
In conclusion, we communicated here a novel class of enantioselective catalytic membranes prepared by an easy and low-cost procedure devoid of any chemical reaction. Preliminary data show that CMRs based on these membranes result in good reusability, versatility, high ee’s and lower rhodium leaching compared to known polymeric membrane systems. To the best of our knowledge, this is the first Chiral Catalytic Membrane Reactor engineered showing effective enantioselection and remarkable durability, even in polar solvents (i.e. in methanol). Studies aimed at elucidating the interactions between the complexes and the membranes are ongoing in our laboratories and will be reported in due course.
Thanks are due to Prof. S. Recchia and Dr V. Dal Santo (ISTM-CNR) for GF-AAS and ICP-AES analysis.
Notes and references
- Asymmetric Catalysis on Industrial Scale, ed. H. U. Blaser and E. Schmidt, Wiley-VCH, Weinheim, 2004 Search PubMed; N. B. Johnson, I. C. Lennon, P. H. Moran and J. A. Ramsden, Acc. Chem. Res., 2007, 40, 1291 Search PubMed; L. A. Saudan, Acc. Chem. Res., 2007, 40, 1309 CrossRef CAS.
- Catalyst Separation, Recovery and Recycling; Chemistry and Process Design, ed. D. J. Cole-Hamilton and R. P. Tooze, Springer, Dordrecht, 2006 Search PubMed; Recoverable and Recyclable Catalysts, ed. M. Benaglia, Wiley-VCH, Weinheim, 2009 Search PubMed; Recoverable Catalysts and Reagents, ed. J. A. Gladysz, Chem. Rev., special issue, 2002, vol. 102, issue 10; Chiral Catalyst Immobilisation and Recycling, ed. D. E. De Vos, I. F. J. Vankelecom and P. A. Jacobs, Wiley-VCH, Weinheim, 2000 Search PubMed; Fine Chemicals Through Heterogeneous Catalysis, ed. R. A. Sheldon and H. van Bekkum, Wiley-VCH, Weinheim, 2001 Search PubMed; N. End and K. U. Schöning, in Topics in Current Chemistry, Springer, Heidelberg, 2004, vol. 242 Search PubMed; M. D. Jones, R. Raja, J. M. Thomas and B. F. G. Johnson, Top. Catal., 2003, 25, 71 Search PubMed; C. Li, H. Zhang, D. Jiang and Q. Yang, Chem. Commun., 2007, 547 Search PubMed; C. E. Song, Annu. Rep. Prog. Chem., Sect. C, 2005, 101, 143 Search PubMed; J. M. Thomas and R. Raja, Acc. Chem. Res., 2008, 41, 708 Search PubMed; R. T. Baker, S. Kobayashi and W. Leitner, Adv. Synth. Catal., 2006, 348, 1337 Search PubMed.
- Heterogenized Homogeneous Catalysts for Fine Chemicals Production, Catalysis by Metal Complexes Series, vol. 33, ed. P. Barbaro and F. Liguori, Springer, London, 2010 Search PubMed; J. M. Fraile, J. I. Garcia and J. A. Mayoral, Chem. Rev., 2009, 109, 360 Search PubMed; P. Barbaro, Chem.–Eur. J., 2006, 12, 5666 CrossRef CAS for a review on asymmetric homogeneous catalysts, see: Catalytic Asymmetric Synthesis, ed. I. Ojima, Wiley-VCH, Weinheim, 3rd edn, 2010 CrossRef CAS.
- N. Yoneda, T. Minami, K. Hamato, Y. Shiroto and Y. Hosono, J. Jpn. Petrol. Inst., 2003, 46, 229 Search PubMed; P. Pugin and H. U. Blaser, in Heterogenized Homogeneous Catalysts for Fine Chemicals Production, Catalysis by Metal Complexes Series, vol. 33, ed. P. Barbaro and F. Liguori, Springer, London, 2010, ch. 7 Search PubMed.
- E. Drioli and E. Fontanova, in Heterogenized Homogeneous Catalysts for Fine Chemicals Production, Catalysis by Metal Complexes Series, vol. 33, ed. P. Barbaro and F. Liguori, Springer, London, 2010, ch. 6 Search PubMed; S. Miachon and J. A. Dalmon, Top. Catal., 2004, 29, 59 Search PubMed; R. Dittmeyer, K. Svajda and M. Reif, Top. Catal., 2004, 29, 3 CrossRef CAS; J. G. Sanchez Marcano and T. T. Tsotsis, in Catalytic Membranes and Catalytic Membrane Reactors, Wiley-VCH, Weinheim, 2010 CrossRef CAS; Polymer Membranes/Biomembranes, Advances in Polymer Science Series, vol. 224, ed. W. P. Meier and W. Knoll, Springer, London, 2010 CrossRef CAS.
- I. F. J. Vankelecom and P. A. Jacobs, Catal. Today, 2000, 56, 147 CrossRef CAS; I. F. J. Vankelecom, Chem. Rev., 2002, 102, 3779 CrossRef CAS; B. M. L. Dioos, I. F. J. Vankelecom and P. A. Jacobs, Adv. Synth. Catal., 2006, 348, 1413 CrossRef CAS; S. S. Ozdemir, M. G. Buonomenna and E. Drioli, Appl. Catal., A: General, 2006, 307, 167 CrossRef CAS.
- A. Wolfson, S. Janssens, I. Vankelecom, S. Geresh, M. Gottlieb and M. Herskowitz, Chem. Commun., 2002, 388 RSC; I. F. J. Vankelecom, D. Tas, R. F. Parton, V. Van de Vyver and P. A. Jacobs, Angew. Chem., Int. Ed. Engl., 1996, 35, 1346 CrossRef CAS; I. Vankelecom, A. Wolfson, S. Geresh, M. Landau, M. Gottlieb and M. Hershkovitz, Chem. Commun., 1999, 2407 RSC; K. B. M. Janssen, I. Laquiere, W. Dehaen, R. E. Parton, I. E. J. Vankelecom and P. A. Jacobs, Tetrahedron: Asymmetry, 1997, 8, 3481 CrossRef CAS; D. Tas, C. Thoelen, I. F. J. Vankelecom and P. A. Jacobs, Chem. Commun., 1997, 2323 RSC.
- A. Wolfson, S. Geresh, M. Gottlieb and M. Herskowitz, Tetrahedron: Asymmetry, 2002, 13, 465 CrossRef CAS.
- H. Sawa, US Pat. 7396616, 2008; H. Sawa, US Pat. 7101638, 2006; H. Sawa, JP Pat. 3889605, 2007; H. Sawa, JP Pat. 4041422, 2008.
- P. Barbaro, C. Bianchini, F. Liguori, H. Sawa and F. Vizza, PCT/JP, 056288, 2010.
- M. Mulder, Basic Principles of Membrane Technology, Kluwer, Dordrecht, 1991 Search PubMed.
- R. L. Augustine, S. K. Tanielyan, S. Anderson and H. Yang, Chem. Commun., 1999, 1257 RSC; R. L. Augustine and S. K. Tanielyan, US Pat. 6025295, 2000; R. L. Augustine, S. K. Tanielyan, N. Mahata, Y. Gao, A. Zsigmond and H. Yang, Appl. Catal., A, 2003, 256, 69 Search PubMed.
- P. Barbaro and F. Liguori, Chem. Rev., 2009, 109, 515 CrossRef CAS.
- A significant drop in ee’s was observed only for DIOP-based catalysts. Although we have no definitive explanation for this behaviour, a surface effect on the enantioselectivities can be invoked, see: J. M. Fraile, J. I. García, C. I. Herrerías, J. A. Mayoral and E. Pires, Chem. Soc. Rev., 2009, 38, 695 Search PubMed; J. I. García, B. López-Sánchez, J. A. Mayoral, E. Pires and I. Villalba, J. Catal., 2008, 258, 378 RSC.
- Polyethylene glycol-containing membranes showed worse reusability likely due to their greater swellability in methanol, see ref. 9.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of experimental data, procedures and membranes composition. See DOI: 10.1039/c0cy00030b |
| ‡ DuPHOS = 1,2-bis-(2R,5R)-dimethyl(phosphacyclopentyl)-benzene; BINAP = 2,2′-bis(diphenylphospino)-1,1′-binapthyle; SALEN = N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine; PDMS = polydimethylsiloxane; PVA = polyvinyl alcohol; NBD = bicyclo[2.2.1]hepta-2,5-diene; PET = polyethylene terephthalate TMBTP = 4,4′-bis(diphenylphosphino)-2,2′,5,5′-tetramethyl-3,3′-bithiophene; DIOP = 2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino) butane; Monophos = (3,5-dioxa-4-phospha-cyclohepta[2,1-a:3,4-a′]di-naphthalen-4-yl)dimethylamine; MAA = methyl 2-acetamidoacrylate; DMI = dimethyl itaconate; ANL = α-angelica lactone; EMC = ethyltrans-β-methylcinnamate; DDF = dihydro-4,4′-dimethyl-2,3-furandione. |
| This journal is © The Royal Society of Chemistry 2011 |
