Synthesis, characterization and ethylene oligomerization behaviour of 8-(1-aryliminoethylidene)quinaldinylnickel dihalides†
Received
1st October 2010
, Accepted 30th October 2010
First published on 8th February 2011
Abstract
A series of 8-(1-aryliminoethylidene)quinaldines and the nickel halides thereof were synthesized and characterized, and the molecular structures of two representative nickel complexes were confirmed by single-crystal X-ray diffraction studies. Upon treatment with diethylaluminium chloride (Et2AlCl), the nickel pro-catalysts exhibited high activity for ethylene oligomerization (1.24–1.83 × 106 g mol−1(Ni) h−1) with good thermal stability at 60 °C under 10 atm of ethylene. The influence of the reaction parameters on the catalytic behaviour was investigated for these nickel-based systems, including variation of Al/Ni molar ratio and reaction temperature. Furthermore, the effect of the ancillary ligand Ph3P was also probed.
1. Introduction
The Shell higher olefin process (SHOP), which employs a nickel-based catalytic system, is the major route in providing linear α-olefins as industrial chemical substrates.1 In recent years, attention has focused on the use of nickel complexes as pro-catalysts towards ethylene reactivity, primarily due to the discovery of α-diiminonickel complexes as highly active pro-catalysts in ethylene polymerization as reported by Brookhart et al.2 This, together with further successes with other late transition metal systems, has stimulated extensive research, much of which has been the focus of recent reviews.4 In the case of nickel-based pro-catalysts, much of the research has related to the variation of different ligand sets at the metal centre, with bidentate chelate ligands of the type P⁁O,5P⁁P,6N⁁O,7P⁁N,8 and N⁁N,9 and some tridentate ligands of N⁁N⁁O,10N⁁P⁁N,11P⁁N⁁N,12 and N⁁N⁁N,13etc., proving to be popular. However, a critical problem remains within late-transition metal pro-catalysts for ethylene activation, and that is the correlation between the activity and the reaction temperature. It is usually the case that the catalytic activity decreases on elevating the reaction temperature. Such a phenomenon was discussed in the cases of the iron and cobalt-based pro-catalysts,14 but for nickel complexes, only those bearing quinaldine derivatives as bidentate ligands have been evaluated versus temperature.8b,9d As a consequence, here a series of 8-(1-aryliminoethylidene)quinaldines has been synthesized, reacted with nickel dihalides, and the resultant pro-catalysts investigated. Upon activation with Et2AlCl, all the nickel pro-catalysts reported herein showed high activity towards ethylene oligomerization, and the affects of reaction parameters on their catalytic performance has been investigated in detail.
2. Results and discussion
2.1 Synthesis and characterization.
The stoichiometric reaction of 8-acetylquinaldine with various anilines afforded the 8-(1-aryliminoethylidene)quinaldines in moderate to good isolated yields (Scheme 1). All the ligands were characterized by FT-IR, 1H and 13C NMR measurements as well as by elemental analysis. Further reaction of the 8-(1-aryliminoethylidene)quinaldines with an equivalent of a nickel halide [(DME)NiBr2 or NiCl2·6H2O] in ethanol afforded the title nickel complexes (Scheme 1: C1–C5, bromides; C6–C10, chlorides) in high yields. All nickel complexes were characterized by FT-IR spectroscopic and elemental analyses, and were found to be highly stable either in the solid-state or in solution.
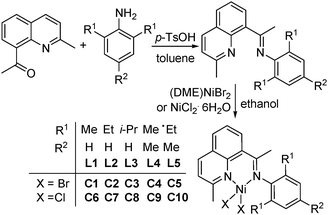 |
| | Scheme 1 Synthetic procedure. | |
In the FT-IR spectra, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequencies of the nickel complexes shifted to lower values (1600–1620 cm−1) with weaker intensity compared with those of the corresponding free ligand. Such changes are in line with the coordination between imino-nitrogen and nickel. The molecular structures of the nickel complexes with C3 and C5 were confirmed by single-crystal X-ray diffraction.
N stretching frequencies of the nickel complexes shifted to lower values (1600–1620 cm−1) with weaker intensity compared with those of the corresponding free ligand. Such changes are in line with the coordination between imino-nitrogen and nickel. The molecular structures of the nickel complexes with C3 and C5 were confirmed by single-crystal X-ray diffraction.
2.2 Crystal structures
Single crystals of the nickel complexes C3 and C5 suitable for X-ray diffraction studies were obtained by slow diffusion of diethyl ether into methanol solution. The molecular structures are shown in Fig. 1 and 2, respectively, with selected bond lengths and angles given in the caption.
 |
| | Fig. 1 Molecular structure of C3. Thermal ellipsoids are shown at the 30% probability level. Hydrogen atoms have been omitted for clarity. Selected bond lengths (Å) and angles (°): Br(1)–Ni(1), 2.3196(11); Br(2)–Ni(1), 2.3850(12); Ni(1)–N(2), 1.958(5); Ni(1)–N(1), 1.994(5); N(2)–C(11), 1.290(8); N(2)–C(13), 1.457(8); N(1)–C(1), 1.331(9); N(1)–C(10), 1.378(8); N(2)–Ni(1)–N(1), 91.2(2); N(2)–Ni(1)–Br(1), 109.25(15); N(1)–Ni(1)–Br(1), 128.58(15); N(2)–Ni(1)–Br(2), 108.35(15); N(1)–Ni(1)–Br(2), 96.19(15); Br(1)–Ni(1)–Br(2), 119.06(4). The dihedral angle of quinolinyl and aryl planes is 64.5°. | |
 |
| | Fig. 2 Molecular structure of C5. Thermal ellipsoids are shown at the 30% probability level. Hydrogen atoms have been omitted for clarity. Selected bond lengths (Å) and angles (°): Br(1)–Ni(1), 2.3486(12); Br(2)–Ni(1), 2.3867(13); Ni(1)–N(2), 1.966(5); Ni(1)–N(1), 2.026(5); N(1)–C(1), 1.336(9); N(1)–C(9), 1.383(8); N(2)–C(11), 1.288(8); N(2)–C(13), 1.454(8); N(2)–Ni(1)–N(1), 89.6(2); N(2)–Ni(1)–Br(1), 118.76(17); N(1)–Ni(1)–Br(1), 132.60(17); N(2)–Ni(1)–Br(2), 104.67(16); N(1)–Ni(1)–Br(2), 98.82(16); Br(1)–Ni(1)–Br(2), 108.17(5). | |
In comparison with the molecular structure of complex C3, the complex C5 also possessed a distorted tetrahedral geometry at nickel (Fig. 2) with associated bond distances Ni–N(1) (2.026(5) Å), Ni–N(2) (1.966(5) Å) Br(1)–Ni(1), (2.3486(12) Å) and Br(2)–Ni(1) (2.3867(13) Å). The slight differences in the bond distances and angles around the nickel centre were caused by the presence of the different substituents on the ligands, which also affects the catalytic behaviour of the nickel complexes.
2.3
Ethylene oligomerization
Various reaction parameters, including the type of alkylaluminium employed as co-catalyst, the molar ratio of co-catalyst to nickel complex (Al/Ni) and reaction temperature, all affect the catalytic activities of the nickel complexes. With regard to the halides involved, the nickel pro-catalysts can be divided into two groups, namely bromide compounds [(L)NiBr2 (C1–C5)] and chloride compounds [(L)NiCl2 (C6–C10)].
2.3.1
Ethylene oligomerization by nickel bromide complexes.
The influence of the various alkylaluminium reagents was evaluated for C3 by employing the co-catalysts methylaluminoxane (MAO), modified methylaluminoxane (MMAO) or diethylaluminium chloride (Et2AlCl)—see Table 1. Ethylene oligomerization was observed in all cases, with the catalytic system using Et2AlCl exhibiting the better activity. In light of these findings, Et2AlCl was chosen as the co-catalyst for further investigation.
| Entry |
Co-catalyst |
Al/Ni |
Activityb |
Oligomer distributionc |
|
α-C4 |
C4/∑C |
C6/∑C |
|
Conditions: 5 μmol Ni, 10 atm C2H4, 30 min, 20 °C, 100 mL toluene.
Activity, 105g mol−1(Ni) h−1.
Determined by GC, ∑C denotes the total a mount of oligomers (%).
|
| 1 |
MAO
|
1000 |
0.13 |
95.2 |
95.5 |
4.5 |
| 2 |
MMAO
|
1000 |
1.33 |
70.5 |
92.4 |
7.6 |
| 3 |
Et2AlCl
|
200 |
5.89 |
95.8 |
97.8 |
3.2 |
In the C3/Et2AlCl system, the molar ratio of Et2AlCl to the nickel pro-catalyst (Al/Ni) greatly affected the catalytic activity (entries 1–4, Table 2), and the optimum Al/Ni molar ratio at 20 °C was found to be 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Upon changing the Al/Ni molar ratio, the selectivity for α-olefins together with the distribution of oligomers varied only slightly. Fixing the catalytic system of C3 with 200 equiv. of Et2AlCl, the influence of reaction temperature was investigated (entries 2 and 5–10, Table 2), and a high activity of 1.83 × 106 g mol−1(Ni) h−1 was observed at 60 °C. Compared with other nickel pro-catalysts which more commonly perform better at lower reaction temperature,15 the current system demonstrates an optimum reaction temperature of 60 °C, which is consistent with our previous observations.13f Interestingly, the content of C6 oligomers was highest at 60 °C, implying more chain propagation and enhanced thermal stability of the active species. Upon the addition of 25 equivalents of PPh3 to the catalytic system, the catalytic activity of C3 was greatly increased, with activities observed as high as 1.72 × 107 g mol−1(Ni) h−1 (entry 11, Table 2), which is almost an order of magnitude higher than that without PPh3 (entry 8, Table 2). In addition, the selectivity for α-C4 was dramatically decreased. It is believed that the auxiliary PPh3 protects the active species during the catalytic reaction, and induces isomerization along with β-hydrogen elimination. Such phenomena are consistent with previous results.9k,16,17
1. Upon changing the Al/Ni molar ratio, the selectivity for α-olefins together with the distribution of oligomers varied only slightly. Fixing the catalytic system of C3 with 200 equiv. of Et2AlCl, the influence of reaction temperature was investigated (entries 2 and 5–10, Table 2), and a high activity of 1.83 × 106 g mol−1(Ni) h−1 was observed at 60 °C. Compared with other nickel pro-catalysts which more commonly perform better at lower reaction temperature,15 the current system demonstrates an optimum reaction temperature of 60 °C, which is consistent with our previous observations.13f Interestingly, the content of C6 oligomers was highest at 60 °C, implying more chain propagation and enhanced thermal stability of the active species. Upon the addition of 25 equivalents of PPh3 to the catalytic system, the catalytic activity of C3 was greatly increased, with activities observed as high as 1.72 × 107 g mol−1(Ni) h−1 (entry 11, Table 2), which is almost an order of magnitude higher than that without PPh3 (entry 8, Table 2). In addition, the selectivity for α-C4 was dramatically decreased. It is believed that the auxiliary PPh3 protects the active species during the catalytic reaction, and induces isomerization along with β-hydrogen elimination. Such phenomena are consistent with previous results.9k,16,17
Table 2 Oligomerization of ethylene with C3/Et2AlCla
| Entry |
T/°C |
Co-catalyst |
Al/Ni |
Activityb |
Oligomer distributionc (%) |
|
α-C4 |
C4/∑C |
C6/∑C |
|
Conditions: 5 μmol Ni, 10 atm C2H4, 30 min, 100 mL toluene.
Activity, 105 g mol−1(Ni) h−1.
Determined by GC, ∑C denotes the total a mount of oligomers (%).
25 equiv. of Ph3P, 20min.
|
| 1 |
20 |
Et2AlCl
|
100 |
1.61 |
95.8 |
96.9 |
3.1 |
| 2 |
20 |
Et2AlCl
|
200 |
5.89 |
95.8 |
97.8 |
3.2 |
| 3 |
20 |
Et2AlCl
|
300 |
1.30 |
92.5 |
96.7 |
3.3 |
| 4 |
20 |
Et2AlCl
|
400 |
1.27 |
76.1 |
91.3 |
8.7 |
| 5 |
30 |
Et2AlCl
|
200 |
5.90 |
93.1 |
94.2 |
5.8 |
| 6 |
40 |
Et2AlCl
|
200 |
5.92 |
95.1 |
95.1 |
4.9 |
| 7 |
50 |
Et2AlCl
|
200 |
7.26 |
78.4 |
95.4 |
4.6 |
| 8 |
60 |
Et2AlCl
|
200 |
18.3 |
63.4 |
86.5 |
13.5 |
| 9 |
70 |
Et2AlCl
|
200 |
8.62 |
72.3 |
89.5 |
10.5 |
| 10 |
85 |
Et2AlCl
|
200 |
1.61 |
85.5 |
92.1 |
7.9 |
| 11d |
60 |
Et2AlCl
|
200 |
113 |
17.2 |
94.4 |
5.6 |
To compare the influence of the ligand environment on the catalytic behaviour of the nickel pro-catalysts, nickel bromide pro-catalysts C1–C5 were investigated under optimum reaction condition of molar ratio Et2AlCl/Ni at 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 60 °C and 10 atm ethylene (see Table 3). High activities were observed for all nickel pro-catalysts, which varied in the order C3 > C1 > C2 > C4 > C5. The catalytic activities were likely affected by both the steric and electronic influences of the ligands. An exception is the complex C3 bearing the bulky 2,6-diisopropyl substituted ligand, which protects the active species and affords highest activity. Despite the ethyl group being bulkier than the methyl group, the rotation of the σ-bond in the ethyl group reduces the steric hindrance for the ethyl compared with the methyl group. The activity order C1 > C2, C4 > C5, C1 > C4 and C2 > C5 seems to be governed by the electronic influences of the ligands. The presence of the additional methyl group in the ligands enhances the solubility, which can be helpful for activating the complexes,9k,13f,18 but can also cause lower activity due to a lower net charge at the nickel center.19
1, 60 °C and 10 atm ethylene (see Table 3). High activities were observed for all nickel pro-catalysts, which varied in the order C3 > C1 > C2 > C4 > C5. The catalytic activities were likely affected by both the steric and electronic influences of the ligands. An exception is the complex C3 bearing the bulky 2,6-diisopropyl substituted ligand, which protects the active species and affords highest activity. Despite the ethyl group being bulkier than the methyl group, the rotation of the σ-bond in the ethyl group reduces the steric hindrance for the ethyl compared with the methyl group. The activity order C1 > C2, C4 > C5, C1 > C4 and C2 > C5 seems to be governed by the electronic influences of the ligands. The presence of the additional methyl group in the ligands enhances the solubility, which can be helpful for activating the complexes,9k,13f,18 but can also cause lower activity due to a lower net charge at the nickel center.19
| Entry |
Pro-catalyst |
Activityb |
Oligomer distributionc (%) |
|
α-C4 |
C4/∑C |
C6/∑C |
|
Conditions: 5 μmol Ni, 200 equiv. Et2AlCl, 60 °C, 10 atm C2H4, 30 min, 100 mL of toluene.
Activity, 105 g mol−1(Ni) h−1.
Determined by GC. ∑C denotes the total amount of oligomers.
|
| 1 |
C1
|
15.9 |
66.4 |
88.3 |
11.7 |
| 2 |
C2
|
13.6 |
66.4 |
93.4 |
6.6 |
| 3 |
C3
|
18.3 |
63.4 |
86.5 |
13.5 |
| 4 |
C4
|
10.5 |
68.1 |
92.7 |
7.3 |
| 5 |
C5
|
8.61 |
70.4 |
92.5 |
7.5 |
2.3.2
Ethylene oligomerization by nickel chloride complexes.
In a similar procedure, complex C8 was used in exploring suitable co-catalysts (entries 1–3 in Table 4) for optimum oligomerization. Both the catalytic systems with MMAO and Et2AlCl exhibited highest activities, however the system with Et2AlCl used less aluminium and exhibited higher ratios of C6 fractions and selectivity for α-C4. Given this, the co-catalyst Et2AlCl was further explored. In the range of Al/Ni molar ratio from 100 to 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, highest activity was observed at 200
1, highest activity was observed at 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entries 3–6 in Table 4). On varying the reaction temperature from 20 to 70 °C (entries 3 and 7–10 in Table 4), the highest activity value was obtained at 60 °C, consistent with the bromide analogues above. Similarly, on adding 25 equivalents of PPh3 to the catalytic system, the activity greatly increased (up to 8.43 × 106 g mol−1(Ni) h−1).
1 (entries 3–6 in Table 4). On varying the reaction temperature from 20 to 70 °C (entries 3 and 7–10 in Table 4), the highest activity value was obtained at 60 °C, consistent with the bromide analogues above. Similarly, on adding 25 equivalents of PPh3 to the catalytic system, the activity greatly increased (up to 8.43 × 106 g mol−1(Ni) h−1).
| Entry |
T/°C |
Co-catalyst |
Al/Ni |
Activityb |
Oligomer distributionc (%) |
|
α-C4 |
C4/∑C |
C6/∑C |
|
Conditions: 5 μmol Ni, 10 atm C2H4, 30 min, 100 mL toluene.
Activity, 105 g mol−1(Ni) h−1.
Determined by GC, ∑C denotes the total amount of oligomers.
25 equiv. of Ph3P, 20 min.
|
| 1 |
20 |
MAO
|
1000 |
2.01 |
94.4 |
98.2 |
2.8 |
| 2 |
20 |
MMAO
|
1000 |
0.79 |
60.7 |
86.7 |
13.3 |
| 3 |
20 |
Et2AlCl
|
200 |
1.81 |
96.1 |
94.9 |
5.1 |
| 4 |
20 |
Et2AlCl
|
100 |
0.51 |
98.2 |
91.3 |
8.7 |
| 5 |
20 |
Et2AlCl
|
300 |
1.50 |
93.4 |
95.3 |
4.7 |
| 6 |
20 |
Et2AlCl
|
500 |
1.03 |
87.9 |
96.0 |
4.0 |
| 7 |
30 |
Et2AlCl
|
200 |
2.12 |
93.2 |
94.8 |
5.2 |
| 8 |
50 |
Et2AlCl
|
200 |
2.91 |
93.0 |
94.6 |
5.4 |
| 9 |
60 |
Et2AlCl
|
200 |
11.0 |
73.5 |
90.7 |
9.3 |
| 10 |
70 |
Et2AlCl
|
200 |
2.19 |
84.8 |
93.3 |
6.7 |
| 11d |
60 |
Et2AlCl
|
200 |
84.3 |
17.3 |
93.9 |
6.1 |
In a similar manner to the bromide analogues, ethylene oligomerization by the C6–C10/Et2AlCl systems was investigated under optimum condition (Al/Ni molar ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 60 °C—see Table 5). Relatively lower activities were exhibited relative to those of the bromide analogues (Table 3), presumably because of the better solubility associated with the latter. A similar trend for catalytic activities was displayed in regard to the nature of the ligands present, such that the order C8 > C6 ≈ C9 > C7 > C10 was observed. It was also noted that the methyl group at the arylimino para-position of ligands exerted a stronger influence on the catalytic activities for the chloride pro-catalysts (compared with their bromide analogues).
1 at 60 °C—see Table 5). Relatively lower activities were exhibited relative to those of the bromide analogues (Table 3), presumably because of the better solubility associated with the latter. A similar trend for catalytic activities was displayed in regard to the nature of the ligands present, such that the order C8 > C6 ≈ C9 > C7 > C10 was observed. It was also noted that the methyl group at the arylimino para-position of ligands exerted a stronger influence on the catalytic activities for the chloride pro-catalysts (compared with their bromide analogues).
| Entry |
Pro-catalyst |
Activityb |
Oligomer distributionc (%) |
|
α-C4 |
C4/∑C |
C6/∑C |
|
Conditions: 5 μmol Ni, 200 equiv. Et2AlCl, 60 °C, 10 atm C2H4, 30 min, 100 mL of toluene.
Activity, 105 g mol−1(Ni) h−1.
Determined by GC. ∑C denotes the total amount of oligomers.
|
| 1 |
C6
|
6.89 |
78.1 |
94.1 |
5.9 |
| 2 |
C7
|
1.88 |
90.8 |
93.4 |
6.6 |
| 3 |
C8
|
11.0 |
73.5 |
90.7 |
9.3 |
| 4 |
C9
|
6.74 |
76.1 |
99.7 |
0.3 |
| 5 |
C10
|
1.24 |
74.9 |
90.0 |
10.0 |
3. Conclusion
A series of nickel(II) halide complexes bearing 8-(1-aryliminoethylidene)quinaldines was synthesized and fully characterized by FT-IR and elemental analyses. Upon activation with Et2AlCl, all the nickel(II) complexes showed good activity in ethylene oligomerization with enhanced thermal stability at 60 °C and high selectivity for α-C4. The better thermo-stable property provides a potential advantage for such nickel pro-catalysts for industrial considerations. Solubility and the electronic influence of the ligand substituents played an important role and pro-catalysts bearing ligands with more electron-donating features had lower activity. The addition of PPh3 dramatically increased the catalytic activity of the system for ethylene oligomerization.
4. Experimental
4.1 General considerations
All operations of air- and moisture-sensitive compounds were manipulated under an atmosphere of nitrogen using standard Schlenk techniques. Toluene was refluxed over sodium benzophenone and distilled under argon prior to use. Methylaluminoxane (MAO, 1.46 M solution in toluene) and modified ethylaluminoxane (MMAO, 1.93 M in heptane) were purchased from Akzo Nobel Corporation. Diethylaluminium chloride (Et2AlCl, 1.7 M in toluene) was purchased from Acros Chemicals. Other reagents were purchased from Aldrich or Acros Chemicals. The 1H and 13C NMR spectra were recorded on Bruker DMX 400 MHz instruments at ambient temperature using TMS as an internal standard. FT-IR spectra were recorded on a Perkin-Elmer System 2000 FT-IR spectrometer. Elemental analyses was carried out using a Flash EA 1112 microanalyzer. GC analyses were performed with a Varian CP-3800 gas chromatograph equipped with a flame ionization detector and a 30 m (0.2 mm i.d., 0.25 μm film thickness) CP-Sil 5 CB column. The yield of oligomers was calculated by referencing with the mass of the solvent on the basis of the prerequisite that the mass of each fraction was approximately proportional to its integrated areas in the GC trace. Selectivity for the linear α-olefin was defined as (amount of linear α-olefin of all fractions)/(total amount of oligomer products) in percent.
4.2 Syntheses
(E)-2,6-dimethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine
L1.
A mixture of 8-acetylquinaldine (1.542 g, 0.008 mol) and 2,6-dimethylbenzenamine (0.7922 g, 0.008 mol, 1 equiv.) with excess p-toluenesulfonic acid monohydrate (0.200 g, 0.006 mol) was stirred at 140 °C for 24 h in 100 mL toluene. After the mixture was cooled to room temperature, the solvent was evaporated under reduced pressure. Following purification by column chromatography (alumina column 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, petroleum ether (60–90)-ethyl acetate), L1 (0.92 g, 41% yield) was obtained as a white powder (Found: C 83.29, H 7.01, N 9.70. C20H20N2 requires C 83.30, H 6.99, N 9.71%); Mp: 60–61 °C; δH (400 MHz; CDCl3; Me4Si) 8.06 (d, 1 H, J = 8.4 Hz, quinoline7), 7.81 (d, 1 H, J = 8.4 Hz, quinoline4), 7.81 (d, 1 H, J = 8.2 Hz, quinoline5), 7.54 (t, 1 H, J = 7.6 Hz, quinoline6), 7.30 (d, 1 H, J = 8.4 Hz, quinoline3), 7.09 (d, 2 H, J = 7.5 Hz, m-Ar), 6.95 (t, 1 H, J = 6.9 Hz, p-Ar), 2.72 (s, 3 H, –CH3), 2.27 (s, 6 H, 2 × –CH3), 2.18 (s, 3 H, –CH3); δC (100 MHz; CDCl3; Me4Si) 171.2 (N
1, petroleum ether (60–90)-ethyl acetate), L1 (0.92 g, 41% yield) was obtained as a white powder (Found: C 83.29, H 7.01, N 9.70. C20H20N2 requires C 83.30, H 6.99, N 9.71%); Mp: 60–61 °C; δH (400 MHz; CDCl3; Me4Si) 8.06 (d, 1 H, J = 8.4 Hz, quinoline7), 7.81 (d, 1 H, J = 8.4 Hz, quinoline4), 7.81 (d, 1 H, J = 8.2 Hz, quinoline5), 7.54 (t, 1 H, J = 7.6 Hz, quinoline6), 7.30 (d, 1 H, J = 8.4 Hz, quinoline3), 7.09 (d, 2 H, J = 7.5 Hz, m-Ar), 6.95 (t, 1 H, J = 6.9 Hz, p-Ar), 2.72 (s, 3 H, –CH3), 2.27 (s, 6 H, 2 × –CH3), 2.18 (s, 3 H, –CH3); δC (100 MHz; CDCl3; Me4Si) 171.2 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 158.9, 153.3, 144.3, 135.6, 131.3, 130.5, 130.2, 130.1, 126.0, 127.4, 127.1, 126.0, 126.6, 122.1, 25.2, 16.0. FT-IR (KBr disc, cm−1) 3042, 1645, 1611, 1594, 1567, 1498, 1465, 1438, 1357, 1280, 1253, 1183, 1094, 834, 755.
C), 158.9, 153.3, 144.3, 135.6, 131.3, 130.5, 130.2, 130.1, 126.0, 127.4, 127.1, 126.0, 126.6, 122.1, 25.2, 16.0. FT-IR (KBr disc, cm−1) 3042, 1645, 1611, 1594, 1567, 1498, 1465, 1438, 1357, 1280, 1253, 1183, 1094, 834, 755.
(E)-2,6-diethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine
L2.
Using a similar procedure as for the synthesis of L1, L2 was obtained as a white powder in 46% yield (Found: C 83.49, H 7.65, N 8.84. C22H24N2 requires C 83.50, H 7.64, N 8.85%); Mp: 66–67 °C;δH (400 MHz; CDCl3; Me4Si) 8.07 (d, 1 H, J = 8.4 Hz, quinoline7), 7.82 (d, 2 H, J = 7.4 Hz, quinoline4,5), 7.53 (t, 1 H, J = 7.6 Hz, quinoline6), 7.31 (d, 1 H, J = 8.4 Hz, quinoline3), 7.29 (d, 2 H, J = 7.5 Hz, m-Ar), 7.13 (t, 1 H, J = 6.9 Hz, p-Ar), 2.71 (s, 3 H, –CH3), 2.60–2.54 (m, 4 H, –CH2–), 2.30 (s, 3 H, –CH3), 1.27 (s, 6 H, 2 × –CH3); δC (100 MHz; CDCl3; Me4Si) 171.7 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 158.7, 147.9, 146.0, 141.6, 136.1, 132.4, 128.5, 127.7, 126.7, 126.2, 125.7, 123.4, 122.2, 25.72, 24.82, 23.55, 14.41. FT-IR (KBr disc, cm−1) 3049, 2970, 1656, 1610, 1598, 1571, 1498, 1450, 1439, 1367, 1350, 1254, 1183, 1059, 970, 890, 832, 590.
C), 158.7, 147.9, 146.0, 141.6, 136.1, 132.4, 128.5, 127.7, 126.7, 126.2, 125.7, 123.4, 122.2, 25.72, 24.82, 23.55, 14.41. FT-IR (KBr disc, cm−1) 3049, 2970, 1656, 1610, 1598, 1571, 1498, 1450, 1439, 1367, 1350, 1254, 1183, 1059, 970, 890, 832, 590.
(E)-2,6-diisopropyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine
L3.
Using a similar procedure as for the synthesis of L1, L3 was obtained as a white powder in 56% yield (Found: C 83.49, H 8.21, N 8.10. C24H28N2 requires C 83.68, H 8.19, N 8.13%); Mp: 93–94 °C; δH (400 MHz; CDCl3; Me4Si) 8.07 (d, 1 H, J = 8.4 Hz, quinoline7), 7.81 (d, 1 H, J = 7.4 Hz, quinoline4), 7.79 (d, 1 H, J = 8.4 Hz, quinoline5), 7.51 (t, 1 H, J = 7.6 Hz, quinoline6), 7.31 (d, 1 H, J = 8.4 Hz, quinoline3), 7.21 (d, 2 H, J = 7.5 Hz, m-Ar), 7.13 (t, 1 H, J = 6.9 Hz, p-Ar), 3.31–3.12 (m, 2 H, –CH), 2.71 (s, 3 H, –CH3), 2.30 (s, 3 H, –CH3), 1.33 (s, 6 H, 2 × –CH3),1.21 (d, 6 H, J = 6.8 Hz, –CH3); δC (100 MHz; CDCl3; Me4Si) 171.8 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 158.5, 146.1, 145.7, 141.5, 136.9, 135.8, 128.2, 127.2, 126.4, 125.5, 123.5, 122.9, 121.9, 27.9, 25.4, 23.5, 23.4, 23.3, 22.4. FT-IR (KBr disc, cm−1) 3057, 2960, 1656, 1605, 1571 1497, 1460, 1434, 1357, 1326, 1279, 1183, 1034, 933, 793, 657.
C), 158.5, 146.1, 145.7, 141.5, 136.9, 135.8, 128.2, 127.2, 126.4, 125.5, 123.5, 122.9, 121.9, 27.9, 25.4, 23.5, 23.4, 23.3, 22.4. FT-IR (KBr disc, cm−1) 3057, 2960, 1656, 1605, 1571 1497, 1460, 1434, 1357, 1326, 1279, 1183, 1034, 933, 793, 657.
(E)-2,4,6-trimethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine
L4.
Using a similar procedure as for the synthesis of L1, L4 was obtained as a white powder in 28% yield (Found: C 83.38, H 7.37, N 9.21. C21H22N2 requires C 83.40, H 7.33, N 9.26%); Mp: 70–71 °C;δH (400 MHz; CDCl3; Me4Si) 8.06 (d, 1 H, J = 8.4 Hz, quinoline7), 7.87 (d, 1 H, J = 8.4 Hz, quinoline4), 7.81 (d, 1 H, J = 8.4 Hz, quinoline5), 7.54 (t, 1 H, J = 7.6 Hz, quinoline6), 7.30 (d, 1 H, J = 8.4 Hz, quinoline3), 6.91 (d, 2 H, J = 7.5 Hz, m-Ar), 2.70 (s, 3 H, –CH3), 2.30 (s, 3 H, –CH3), 2.26 (s, 3 H, –CH3), 2.22 (s, 3 H, –CH3), 2.18 (s, 3 H, –CH3); δC (100 MHz; CDCl3; Me4Si) 171.4 (N = C), 158.9, 150.3, 144.3, 136.7, 135.6, 131.3, 130.4, 130.1, 130.2, 129.2, 126.6, 126.0, 122.1, 25.2, 24.9, 16.3, 16.0. FT-IR (KBr disc, cm−1): 3057, 2960, 1656, 1605, 1571, 1497, 1460, 1434, 1357, 1326, 1279, 1183, 1034, 933, 793, 657.
(E)-2,6-diethyl-4-methyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine
L5.
Using a similar procedure as for the synthesis of L1, L5 was obtained as a yellow powder in 49% yield (Found: C 83.49, H 7.97, N 8.45. C23H26N2 requires C 83.59, H 7.93, N 8.48%); Mp: 75–76 °C; δH (400 MHz; CDCl3; Me4Si) 8.06 (d, 1 H, J = 8.4 Hz, quinoline7), 7.83 (d, 2H, J = 7.4 Hz, quinoline4,5), 7.54 (t, 1 H, J = 7.6 Hz, quinoline6), 7.31 (d, 1 H, J = 8.4 Hz, quinoline3), 7.25 (s, 2 H, J = 7.5 Hz, m-Ar), 2.71 (s, 3 H, –CH3), 2.60–2.54 (m, 4 H, –CH2), 2.35 (s, 3 H, –CH3), 2.30 (s, 3 H, –CH3), 1.27 (s, 6 H, 2 × –CH3); δC (100 MHz; CDCl3; Me4Si) 171.7 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 158.5, 145.8, 145.2, 141.6, 135.9, 132.3, 132.2, 128.3, 127.5, 126.8, 126,5, 125.5, 122.0, 25.54, 24.64, 23.30, 21.18, 14.36. FT-IR (KBr disc, cm−1) 2962, 2928, 1635, 1600, 1567, 1498, 1431, 1257, 1204, 1186, 1147, 951, 898, 836, 767, 630, 557.
C), 158.5, 145.8, 145.2, 141.6, 135.9, 132.3, 132.2, 128.3, 127.5, 126.8, 126,5, 125.5, 122.0, 25.54, 24.64, 23.30, 21.18, 14.36. FT-IR (KBr disc, cm−1) 2962, 2928, 1635, 1600, 1567, 1498, 1431, 1257, 1204, 1186, 1147, 951, 898, 836, 767, 630, 557.
[(E)-2,6-dimethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dibromonickel C1.
To a solution of the ligand (E)-2,6-dimethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine (0.288, 1.0 mmol) in ethanol (5 mL), (DME)NiBr2 (0.308 g, 1.0 mmol) was added. The reaction mixture was stirred at room temperature for 12 h to afford a brown precipitate from the reaction mixture (C1, 0.491 g, 82% yield) (Found: C 47.45, H 3.97, N 5.51. C20H20Br2N2Ni requires C 47.39, H 3.98, N 5.53%). FT-IR (KBr disc, cm−1) 1620, 1596, 1567, 1506, 1466, 1370, 1282, 1207, 862, 843, 794.
[(E)-2,6-diethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dibromonickel C2.
Using the same procedure as for the synthesis of C1, C2 was obtained in 86% yield (Found: C 49.42, H 4.56, N 5.21. C22H24Br2N2Ni requires C 49.40, H 4.52, N 5.24%). FT-IR (KBr disc, cm−1): 3409, 1620, 1593, 1563, 1509, 1439, 1309, 1277, 1206, 1178, 855, 774, 665.
[(E)-2,6-diisopropyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dibromonickel C3.
Using the same procedure as for the synthesis of C1, C3 was obtained in 93% yield (Found: C 50.99, H 5.10, N 4.93. C24H28Br2N2Ni requires C 51.20, H 5.01, N 4.98%). FT-IR (KBr disc, cm−1): 3048, 1619, 1596, 1585, 1508, 1445, 1381, 1361, 1277, 1204, 1149, 1056, 935, 846, 806, 771.
[(E)-2,4,6-trimethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dibromonickel C4.
Using the same procedure as for the synthesis of C1, C4 was obtained in 88% yield (Found: C 48.53, H 4.27, N 5.32. C21H22Br2N2Ni requires C 48.42, H 4.26, N 5.38%). FT-IR (KBr disc, cm−1) 2970, 1618, 1593, 1563, 1509, 1431, 1371, 1276, 1206, 1150, 850, 767.
[(E)-2,6-diethyl-4-methyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dibromonickel C5.
Using the same procedure as for the synthesis of C1, C5 was obtained in 89% yield (Found: C 60.34, H 4.79, N 5.07. C23H26Br2N2Ni requires C 60.32, H 4.77, N 5.10%). FT-IR (KBr disc, cm−1) 2962, 1612, 1594, 1565, 1505, 1458, 1371, 1278, 1147, 1031, 875, 798, 483.
[(E)-2,6-dimethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dichloronickel C6.
To a solution of the ligand (E)-2,6-dimethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine (0.288 g, 1.0 mmol) in ethanol (5 mL), NiCl2·6H2O (0.236 g, 1 mmol) was added. The reaction mixture was stirred at room temperature for 12 h to afford a light yellow precipitate from the reaction mixture. C6 was obtained in 76% yield (Found: C 57.52, H 4.86, N 6.64. C20H20Cl2N2Ni requires C 57.47, H 4.82, N 6.70%). FT-IR (KBr disc, cm−1) 3391, 1606, 1536, 1365, 1260, 1191, 1143, 848, 503.
[(E)-2,6-diethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dichloronickel C7.
Using the same procedure as for the synthesis of C6, C7 was obtained in 75% yield (Found: C 59.14, H 5.45, N 6.21. C22H24Cl2N2Ni requires C 59.24, H 5.42, N 6.28%). FT-IR (KBr disc, cm−1) 3406, 1607, 1534, 1408, 1259, 1190, 1147, 853.
[(E)-2,6-diisopropyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dichloronickel C8.
Using the same procedure as for the synthesis of C6, C8 was obtained in 87% yield (Found: C 60.89, H 5.94, N 5.89. C24H28Cl2N2Ni requires C 60.80, H 5.95, N 5.91%). FT-IR (KBr disc, cm−1) 3424, 1609, 1557, 1503, 1468, 1429, 1365, 1339, 1224, 1193, 1147, 952, 844, 772, 755, 717, 480.
[(E)-2,4,6-trimethyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dichloronickel C9.
Using the same procedure as for the synthesis of C6, C9 was obtained in 79% yield (Found: C 68.35, H 5.17, N 6.42. C21H22Cl2N2Ni requires C 58.38, H 5.13, N 6.48%). FT-IR (KBr disc, cm−1) 3411, 2360, 2341, 1606, 1535, 1486, 1409, 1259, 1210, 1150, 1032, 851, 768, 668.
[(E)-2,6-diethyl-4-methyl-N-(1-(2-methylquinoline-8-yl)ethylidene)benzenamine]dichloronickel C10.
Using the same procedure as for the synthesis of C6, C10 was obtained in 80% yield (Found: C 60.00, H 5.71, N 6.05. C23H26Cl2N2Ni requires C 60.04, H 5.70, N 6.09%). FT-IR (KBr disc, cm−1) 3397, 3075, 2968, 2359, 1604, 1532, 1457, 1425, 1258, 1222, 1191, 1143, 1025, 852, 763, 602.
4.3 General procedure for ethylene oligomerization
Ethylene oligomerization at 10 atm ethylene pressure was performed in a stainless steel autoclave (0.25 L capacity) equipped with a mechanical stirrer, a temperature controller and gas ballast through a solenoid clave for continuous feeding of ethylene at constant pressure. The catalyst precursor was dissolved in 50 mL toluene in a Schlenk tube stirred with a magnetic stirrer and injected into the reactor under an ethylene flux. With Et2AlCl, 50 mL toluene was added. When the reaction temperature had been reached, ethylene with the desired pressure was introduced to initiate the reaction. After the reaction mixture had been stirred for the desired period of time, the reaction was stopped and about 1 mL of the reaction solution was collected, terminated by the addition of 10% aqueous hydrogen chloride. The organic layer was analyzed by gas chromatography (GC) for determining the composition and mass distribution of the oligomers obtained.
4.4 Crystal structure determinations
Diffraction data of C3 and C5 were collected on a Rigaku R-AXIS Rapid IP diffractometer with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å). Cell parameters were obtained by global refinement of the positions of all collected reflections. Intensities were corrected for Lorentz and polarization effects and empirical absorption. Data were collected using phi-scans and the structures were solved by direct methods (SIR-97) using the SHELX-97 software20 and the refinement was by full-matrix least squares on F2. All non-hydrogen atoms were refined anisotropically. Crystal data and processing parameters for pro-catalysts C3 and C5 are summarized in Table 6.
| |
C3
|
C5
|
| Crystal colour |
Brown |
Green |
| Empirical formula |
C24H28Br2N2Ni
|
C23H26Br2N2Ni
|
|
FW
|
563.01 |
548.99 |
|
T/K |
293(2) |
293(2) |
|
λ/Å |
0.71073 |
0.71073 |
| Crystal system |
Monoclinic |
Monoclinic |
| Space group |
C2/c |
P21/n |
|
a/Å |
29.331(6) |
15.834(3) |
|
b/Å |
10.775(2) |
8.6441(17) |
|
c/Å |
15.226(3) |
17.665(4) |
|
α/° |
90 |
90 |
|
β/° |
105.30(3) |
114.28(3) |
|
γ/° |
90 |
90 |
|
V/Å3 |
4641.8(16) |
2203.9(8) |
|
Z
|
8 |
4 |
|
D
c/mg m−3 |
1.611 |
1.655 |
|
μ/mm−1 |
4.292 |
4.517 |
|
F(000) |
2272 |
1104 |
| Crystal size/mm |
0.20 × 0.20 × 0.20 |
0.12 × 0.11 × 0.04 |
|
θ range/° |
1.44–27.48 |
1.46–27.52 |
| Limiting indices |
−30 ≤ h ≤ 37 |
−20 ≤ h ≤ 20 |
| −13 ≤ k ≤ 13 |
−11 ≤ k ≤ 10 |
| −19 ≤ l ≤ 19 |
−22 ≤ l ≤ 22 |
| No. of reflections collected |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631 631 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
| No. unique reflections [Rint] |
5284(0.0635) |
5043(0.0730) |
| Completeness to θ (%) |
99.2% |
99.3% |
| Absorption correction |
None |
None |
| data/restraints/parameters |
5284/0/262 |
5043/0/253 |
| Goodness of fit on F2 |
1.062 |
1.368 |
| Final R indices [I > 2σ(I)] |
R
1 = 0.0755 |
R
1 = 0.0855 |
|
wR2 = 0.1768 |
wR2 = 0.1510 |
|
R indices (all data) |
R
1 = 0.0899 |
R
1 = 0.0954 |
|
wR2 = 0.1857 |
wR2 = 0.1581 |
| Largest diffraction peak and hole/e Å−3 |
0.485 and −0.659 |
0.526 and −0.623 |
Acknowledgements
This work is supported by MOST 863 program No. 2009AA033601. The EPSRC is thanked for the award of a travel grant (to CR).
References
-
(a) W. Keim, F. H. Kowaldt, R. Goddard and C. Kruger, Angew. Chem., Int. Ed. Engl., 1978, 17, 466 CrossRef;
(b) W. Keim, A. Behr, B. Limbäcker and C. Krüger, Angew. Chem., Int. Ed. Engl., 1983, 22, 503 CrossRef.
-
(a) L. K. Johnson, C. M. Killian and M. Brookhart, J. Am. Chem. Soc., 1995, 117, 6414 CrossRef CAS;
(b) C. M. Killan, L. K. Johnson and M. Brookhart, Organometallics, 1997, 16, 2005 CrossRef CAS.
-
(a)
A. M. A. Bennett, WO9827124 Al, 1998-06-25, 1998;
(b) B. L. Small, M. Brookhart and A. M. A. Bennett, J. Am. Chem. Soc., 1998, 120, 4049 CrossRef CAS;
(c) G. J. P. Britovsek, V. C. Gibson, B. S. Kimberley, P. J. Maddox, S. J. McTavish, G. A. Solan, A. J. P. White and D. J. Williams, Chem. Commun., 1998, 849 RSC.
-
(a) S. D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169 CrossRef CAS;
(b) V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283 CrossRef CAS;
(c) F. Speiser, P. Braunstein and L. Saussine, Acc. Chem. Res., 2005, 38, 784 CrossRef CAS;
(d) V. C. Gibson, C. Redshaw and G. A. Solan, Chem. Rev., 2007, 107, 1745 CrossRef CAS;
(e) C. Bianchini, G. Giambastiani, L. Luconi and A. Meli, Coord. Chem. Rev., 2010, 254, 431 CrossRef CAS;
(f) Y. Song, S. Zhang, Y. Deng, S. Jie, L. Li, X. Lu and W.-H. Sun, Kinet. Catal., 2007, 48, 664 CrossRef CAS;
(g) M. Zhang, T. Xiao and W.-H. Sun, Acta Polym. Sin., 2009, 9, 600 Search PubMed.
-
(a) J. Pietsch, P. Braunstein and Y. Chauvin, New J. Chem., 1998, 22, 467 RSC;
(b) W. Liu, J. M. Malinoski and M. Brookhart, Organometallics, 2002, 21, 2836 CrossRef CAS.
-
(a) N. A. Cooley, S. M. Green, D. F. Wass, K. Heslop, A. G. Orpen and P. G. Pringle, Organometallics, 2001, 20, 4769 CrossRef CAS;
(b) Z. Guan and W. J. Marshall, Organometallics, 2002, 21, 3580 CrossRef CAS.
-
(a) C. Wang, S. Friedrich, T. R. Younkin, R. T. Li, R. H. Grubbs, D. A. Bansleben and M. W. Day, Organometallics, 1998, 17, 3149 CrossRef CAS;
(b) T. R. Younkin, E. F. Connor, J. I. Henderson, S. K. Friedrich, R. H. Grubbs and D. A. Bansleben, Science, 2000, 287, 460 CrossRef CAS;
(c) T. Hu, L.-M. Tang, X.-F. Li, Y.-S. Li and N.-H. Hu, Organometallics, 2005, 24, 2628 CrossRef CAS;
(d) L. Wang, W.-H. Sun, L. Han, Z. Li, Y. Hu, C. He and C. Yan, J. Organomet. Chem., 2002, 650, 59 CrossRef CAS;
(e) A. Kermagoret and P. Braunstein, Dalton Trans., 2008, 1564 RSC.
-
(a) W. Keim, S. Killat, C. F. Nobile, G. P. Suranna, U. Englert, R. Wang, S. Mecking and D. L. SchrÖder, J. Organomet. Chem., 2002, 662, 150 CrossRef CAS;
(b) W.-H. Sun, Z. Li, H. Hu, B. Wu, H. Yang, N. Zhu, X. Leng and H. Wang, New J. Chem., 2002, 26, 1474 RSC;
(c) F. Speiser, P. Braunstein and L. Saussine, Organometallics, 2004, 23, 2625 CrossRef CAS;
(d) F. Speiser, P. Braunstein and L. Saussine, Organometallics, 2004, 23, 2633 CrossRef CAS;
(e) F. Speiser, P. Braunstein, L. Saussine and R. Welter, Inorg. Chem., 2004, 43, 1649 CrossRef CAS;
(f) Z. Weng, S. Teo and T. S. A. Hor, Organometallics, 2006, 25, 4878 CrossRef CAS.
-
(a) C. M. Killian, D. J. Tempel, L. K. Johnson and M. Brookhart, J. Am. Chem. Soc., 1996, 118, 11664 CrossRef;
(b) S. A. Svejda and M. Brookhart, Organometallics, 1999, 18, 65 CrossRef CAS;
(c) B. Y. Lee, X. Bu and G. C. Bazan, Organometallics, 2001, 20, 5425 CrossRef CAS;
(d) Z. L. Li, W. H. Sun, Z. Ma, Y. L. Hu and C. X. Shao, Chin. Chem. Lett., 2001, 12, 691 CAS;
(e) J. M. Benito, E. de Jesús, F. J. de la Mata, J. C. Flores, R. Gómez and P. Gómez-Sal, Organometallics, 2006, 25, 3876 CrossRef CAS;
(f) X. Tang, W.-H. Sun, T. Gao, J. Hou, J. Chen and W. Chen, J. Organomet. Chem., 2005, 690, 1570 CrossRef CAS;
(g) S. Jie, D. Zhang, T. Zhang, W.-H. Sun, J. Chen, Q. Ren, D. Liu, G. Zheng and W. Chen, J. Organomet. Chem., 2005, 690, 1739 CrossRef CAS;
(h) T. V. Laine, M. Klinga and M. Leskelä, Eur. J. Inorg. Chem., 1999, 959 CrossRef CAS;
(i) C. Shao, W.-H. Sun, Z. Li, Y. Hu and L. Han, Catal. Commun., 2002, 3, 405 CrossRef CAS;
(j) J. Li, T. Gao, W. Zhang and W.-H. Sun, Inorg. Chem. Commun., 2003, 6, 1372 CrossRef CAS;
(k) X. Tang, Y. Cui, W.-H. Sun, Z. Miao and S. Yan, Polym. Int., 2004, 53, 2155 CrossRef CAS;
(l) P. Hao, S. Zhang, W.-H. Sun, Q. Shi, S. Adewuyi, X. Lu and P. Li, Organometallics, 2007, 26, 2439 CrossRef CAS.
- Q.-Z. Yang, A. Kermagoret, M. Agostinho, O. Siri and P. Braunstein, Organometallics, 2006, 25, 5518 CrossRef CAS.
- F. Speiser, P. Braunstein and L. Saussine, Dalton Trans., 2004, 1539 RSC.
-
(a) J. Hou, W.-H. Sun, S. Zhang, H. Ma, Y. Deng and X. Lu, Organometallics, 2006, 25, 236 CrossRef CAS;
(b) C. Zhang, W.-H. Sun and Z.-X. Wang, Eur. J. Inorg. Chem., 2006, 4895 CrossRef CAS.
-
(a) L. Wang, W.-H. Sun, L. Han, H. Yang, Y. Hu and X. Jin, J. Organomet. Chem., 2002, 658, 62 CrossRef CAS;
(b) F. A. Kunrath, R. F. Souza, O. L. Casagrande, Jr., N. R. Brooks and V. G. Young, Jr., Organometallics, 2003, 22, 4739 CrossRef CAS;
(c) N. Ajellal, M. C. A. Kuhn, A. D. G. Boff, M. Hörner, C. M. Thomas, J.-F. Carpentier and O. L. Casagrande, Jr., Organometallics, 2006, 25, 1213 CrossRef CAS;
(d) W.-H. Sun, S. Zhang, S. Jie, W. Zhang, Y. Li, H. Ma, J. Chen, K. Wedeking and R. Fröhlich, J. Organomet. Chem., 2006, 691, 4196 CrossRef CAS;
(e) W.-H. Sun, K. Wang, K. Wedeking, D. Zhang, S. Zhang, J. Cai and Y. Li, Organometallics, 2007, 26, 4781 CrossRef CAS;
(f) R. Gao, M. Zhang, T. Liang, F. Wang and W.-H. Sun, Organometallics, 2008, 27, 5641 CrossRef CAS.
- S. Jie, W.-H. Sun and T. Xiao, Chin. J. Polym. Sci., 2010, 28, 299 CAS.
-
(a) S. Jie, S. Zhang and W.-H. Sun, Eur. J. Inorg. Chem., 2007, 5584 CrossRef CAS;
(b) M. Zhang, S. Zhang, P. Hao, S. Jie, W.-H. Sun, P. Li and X. Lu, Eur. J. Inorg. Chem., 2007, 3816 CrossRef CAS;
(c) S. Zhang, W.-H. Sun, X. Kuang, I. Vystorop and J. Yi, J. Organomet. Chem., 2007, 692, 5307 CrossRef CAS.
-
(a) W.-H. Sun, W. Zhang, T. Gao, X. Tang, L. Chen, Y. Li and X. Jin, J. Organomet. Chem., 2004, 689, 917 CrossRef CAS;
(b) X. Tang, D. Zhang, S. Jie, W.-H. Sun and J. Chen, J. Organomet. Chem., 2005, 690, 3918 CrossRef CAS;
(c) S. Al-Benna, M. J. Sarsfield, M. Thornton-Pett, D. L. Ormsby, P. J. Maddox, P. Brès and M. Bochmann, J. Chem. Soc., Dalton Trans., 2000, 4247 RSC;
(d) W.-H. Sun, S. Zhang, S. Jie, W. Zhang, Y. Li, H. Ma, J. Chen, K. Wedeking and R. Fröhlich, J. Organomet. Chem., 2006, 691, 4196 CrossRef CAS;
(e) J. C. Jenkins and M. Brookhart, Organometallics, 2003, 22, 250 CrossRef CAS.
- W. Keim, Angew. Chem., Int. Ed. Engl., 1990, 29, 235 CrossRef.
-
(a) Y. Chen, P. Hao, W. Zuo, K. Gao and W.-H. Sun, J. Organomet. Chem., 2008, 693, 1829 CrossRef CAS;
(b) W.-H. Sun, P. Hao, G. Li, S. Zhang, W. Wang, J. Yi, M. Asma and N. Tang, J. Organomet. Chem., 2007, 692, 4506 CrossRef CAS.
-
(a) T. Zhang, W.-H. Sun, T. Li and X. Yang, J. Mol. Catal. A: Chem., 2004, 218, 119 CrossRef CAS;
(b) J. Liu, Y. Zheng, Y. Li, L. Pan, Y. Li and N. Hu, J. Organomet. Chem., 2005, 690, 1233 CrossRef CAS.
-
G. M. Sheldrick, SHELXTL-97, Program for the Refinement of Göttingen, Germany, 1997 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequencies of the nickel complexes shifted to lower values (1600–1620 cm−1) with weaker intensity compared with those of the corresponding free ligand. Such changes are in line with the coordination between imino-nitrogen and nickel. The molecular structures of the nickel complexes with C3 and C5 were confirmed by single-crystal X-ray diffraction.
N stretching frequencies of the nickel complexes shifted to lower values (1600–1620 cm−1) with weaker intensity compared with those of the corresponding free ligand. Such changes are in line with the coordination between imino-nitrogen and nickel. The molecular structures of the nickel complexes with C3 and C5 were confirmed by single-crystal X-ray diffraction.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Upon changing the Al/Ni molar ratio, the selectivity for α-olefins together with the distribution of oligomers varied only slightly. Fixing the catalytic system of C3 with 200 equiv. of Et2AlCl, the influence of reaction temperature was investigated (entries 2 and 5–10, Table 2), and a high activity of 1.83 × 106 g mol−1(Ni) h−1 was observed at 60 °C. Compared with other nickel pro-catalysts which more commonly perform better at lower reaction temperature,15 the current system demonstrates an optimum reaction temperature of 60 °C, which is consistent with our previous observations.13f Interestingly, the content of C6 oligomers was highest at 60 °C, implying more chain propagation and enhanced thermal stability of the active species. Upon the addition of 25 equivalents of PPh3 to the catalytic system, the catalytic activity of C3 was greatly increased, with activities observed as high as 1.72 × 107 g mol−1(Ni) h−1 (entry 11, Table 2), which is almost an order of magnitude higher than that without PPh3 (entry 8, Table 2). In addition, the selectivity for α-C4 was dramatically decreased. It is believed that the auxiliary PPh3 protects the active species during the catalytic reaction, and induces isomerization along with β-hydrogen elimination. Such phenomena are consistent with previous results.9k,16,17
1. Upon changing the Al/Ni molar ratio, the selectivity for α-olefins together with the distribution of oligomers varied only slightly. Fixing the catalytic system of C3 with 200 equiv. of Et2AlCl, the influence of reaction temperature was investigated (entries 2 and 5–10, Table 2), and a high activity of 1.83 × 106 g mol−1(Ni) h−1 was observed at 60 °C. Compared with other nickel pro-catalysts which more commonly perform better at lower reaction temperature,15 the current system demonstrates an optimum reaction temperature of 60 °C, which is consistent with our previous observations.13f Interestingly, the content of C6 oligomers was highest at 60 °C, implying more chain propagation and enhanced thermal stability of the active species. Upon the addition of 25 equivalents of PPh3 to the catalytic system, the catalytic activity of C3 was greatly increased, with activities observed as high as 1.72 × 107 g mol−1(Ni) h−1 (entry 11, Table 2), which is almost an order of magnitude higher than that without PPh3 (entry 8, Table 2). In addition, the selectivity for α-C4 was dramatically decreased. It is believed that the auxiliary PPh3 protects the active species during the catalytic reaction, and induces isomerization along with β-hydrogen elimination. Such phenomena are consistent with previous results.9k,16,17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 60 °C and 10 atm ethylene (see Table 3). High activities were observed for all nickel pro-catalysts, which varied in the order C3 > C1 > C2 > C4 > C5. The catalytic activities were likely affected by both the steric and electronic influences of the ligands. An exception is the complex C3 bearing the bulky 2,6-diisopropyl substituted ligand, which protects the active species and affords highest activity. Despite the ethyl group being bulkier than the methyl group, the rotation of the σ-bond in the ethyl group reduces the steric hindrance for the ethyl compared with the methyl group. The activity order C1 > C2, C4 > C5, C1 > C4 and C2 > C5 seems to be governed by the electronic influences of the ligands. The presence of the additional methyl group in the ligands enhances the solubility, which can be helpful for activating the complexes,9k,13f,18 but can also cause lower activity due to a lower net charge at the nickel center.19
1, 60 °C and 10 atm ethylene (see Table 3). High activities were observed for all nickel pro-catalysts, which varied in the order C3 > C1 > C2 > C4 > C5. The catalytic activities were likely affected by both the steric and electronic influences of the ligands. An exception is the complex C3 bearing the bulky 2,6-diisopropyl substituted ligand, which protects the active species and affords highest activity. Despite the ethyl group being bulkier than the methyl group, the rotation of the σ-bond in the ethyl group reduces the steric hindrance for the ethyl compared with the methyl group. The activity order C1 > C2, C4 > C5, C1 > C4 and C2 > C5 seems to be governed by the electronic influences of the ligands. The presence of the additional methyl group in the ligands enhances the solubility, which can be helpful for activating the complexes,9k,13f,18 but can also cause lower activity due to a lower net charge at the nickel center.19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, highest activity was observed at 200
1, highest activity was observed at 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entries 3–6 in Table 4). On varying the reaction temperature from 20 to 70 °C (entries 3 and 7–10 in Table 4), the highest activity value was obtained at 60 °C, consistent with the bromide analogues above. Similarly, on adding 25 equivalents of PPh3 to the catalytic system, the activity greatly increased (up to 8.43 × 106 g mol−1(Ni) h−1).
1 (entries 3–6 in Table 4). On varying the reaction temperature from 20 to 70 °C (entries 3 and 7–10 in Table 4), the highest activity value was obtained at 60 °C, consistent with the bromide analogues above. Similarly, on adding 25 equivalents of PPh3 to the catalytic system, the activity greatly increased (up to 8.43 × 106 g mol−1(Ni) h−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 60 °C—see Table 5). Relatively lower activities were exhibited relative to those of the bromide analogues (Table 3), presumably because of the better solubility associated with the latter. A similar trend for catalytic activities was displayed in regard to the nature of the ligands present, such that the order C8 > C6 ≈ C9 > C7 > C10 was observed. It was also noted that the methyl group at the arylimino para-position of ligands exerted a stronger influence on the catalytic activities for the chloride pro-catalysts (compared with their bromide analogues).
1 at 60 °C—see Table 5). Relatively lower activities were exhibited relative to those of the bromide analogues (Table 3), presumably because of the better solubility associated with the latter. A similar trend for catalytic activities was displayed in regard to the nature of the ligands present, such that the order C8 > C6 ≈ C9 > C7 > C10 was observed. It was also noted that the methyl group at the arylimino para-position of ligands exerted a stronger influence on the catalytic activities for the chloride pro-catalysts (compared with their bromide analogues).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, petroleum ether (60–90)-ethyl acetate), L1 (0.92 g, 41% yield) was obtained as a white powder (Found: C 83.29, H 7.01, N 9.70. C20H20N2 requires C 83.30, H 6.99, N 9.71%); Mp: 60–61 °C; δH (400 MHz; CDCl3; Me4Si) 8.06 (d, 1 H, J = 8.4 Hz, quinoline7), 7.81 (d, 1 H, J = 8.4 Hz, quinoline4), 7.81 (d, 1 H, J = 8.2 Hz, quinoline5), 7.54 (t, 1 H, J = 7.6 Hz, quinoline6), 7.30 (d, 1 H, J = 8.4 Hz, quinoline3), 7.09 (d, 2 H, J = 7.5 Hz, m-Ar), 6.95 (t, 1 H, J = 6.9 Hz, p-Ar), 2.72 (s, 3 H, –CH3), 2.27 (s, 6 H, 2 × –CH3), 2.18 (s, 3 H, –CH3); δC (100 MHz; CDCl3; Me4Si) 171.2 (N
1, petroleum ether (60–90)-ethyl acetate), L1 (0.92 g, 41% yield) was obtained as a white powder (Found: C 83.29, H 7.01, N 9.70. C20H20N2 requires C 83.30, H 6.99, N 9.71%); Mp: 60–61 °C; δH (400 MHz; CDCl3; Me4Si) 8.06 (d, 1 H, J = 8.4 Hz, quinoline7), 7.81 (d, 1 H, J = 8.4 Hz, quinoline4), 7.81 (d, 1 H, J = 8.2 Hz, quinoline5), 7.54 (t, 1 H, J = 7.6 Hz, quinoline6), 7.30 (d, 1 H, J = 8.4 Hz, quinoline3), 7.09 (d, 2 H, J = 7.5 Hz, m-Ar), 6.95 (t, 1 H, J = 6.9 Hz, p-Ar), 2.72 (s, 3 H, –CH3), 2.27 (s, 6 H, 2 × –CH3), 2.18 (s, 3 H, –CH3); δC (100 MHz; CDCl3; Me4Si) 171.2 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 158.9, 153.3, 144.3, 135.6, 131.3, 130.5, 130.2, 130.1, 126.0, 127.4, 127.1, 126.0, 126.6, 122.1, 25.2, 16.0. FT-IR (KBr disc, cm−1) 3042, 1645, 1611, 1594, 1567, 1498, 1465, 1438, 1357, 1280, 1253, 1183, 1094, 834, 755.
C), 158.9, 153.3, 144.3, 135.6, 131.3, 130.5, 130.2, 130.1, 126.0, 127.4, 127.1, 126.0, 126.6, 122.1, 25.2, 16.0. FT-IR (KBr disc, cm−1) 3042, 1645, 1611, 1594, 1567, 1498, 1465, 1438, 1357, 1280, 1253, 1183, 1094, 834, 755.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 158.7, 147.9, 146.0, 141.6, 136.1, 132.4, 128.5, 127.7, 126.7, 126.2, 125.7, 123.4, 122.2, 25.72, 24.82, 23.55, 14.41. FT-IR (KBr disc, cm−1) 3049, 2970, 1656, 1610, 1598, 1571, 1498, 1450, 1439, 1367, 1350, 1254, 1183, 1059, 970, 890, 832, 590.
C), 158.7, 147.9, 146.0, 141.6, 136.1, 132.4, 128.5, 127.7, 126.7, 126.2, 125.7, 123.4, 122.2, 25.72, 24.82, 23.55, 14.41. FT-IR (KBr disc, cm−1) 3049, 2970, 1656, 1610, 1598, 1571, 1498, 1450, 1439, 1367, 1350, 1254, 1183, 1059, 970, 890, 832, 590.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 158.5, 146.1, 145.7, 141.5, 136.9, 135.8, 128.2, 127.2, 126.4, 125.5, 123.5, 122.9, 121.9, 27.9, 25.4, 23.5, 23.4, 23.3, 22.4. FT-IR (KBr disc, cm−1) 3057, 2960, 1656, 1605, 1571 1497, 1460, 1434, 1357, 1326, 1279, 1183, 1034, 933, 793, 657.
C), 158.5, 146.1, 145.7, 141.5, 136.9, 135.8, 128.2, 127.2, 126.4, 125.5, 123.5, 122.9, 121.9, 27.9, 25.4, 23.5, 23.4, 23.3, 22.4. FT-IR (KBr disc, cm−1) 3057, 2960, 1656, 1605, 1571 1497, 1460, 1434, 1357, 1326, 1279, 1183, 1034, 933, 793, 657.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 158.5, 145.8, 145.2, 141.6, 135.9, 132.3, 132.2, 128.3, 127.5, 126.8, 126,5, 125.5, 122.0, 25.54, 24.64, 23.30, 21.18, 14.36. FT-IR (KBr disc, cm−1) 2962, 2928, 1635, 1600, 1567, 1498, 1431, 1257, 1204, 1186, 1147, 951, 898, 836, 767, 630, 557.
C), 158.5, 145.8, 145.2, 141.6, 135.9, 132.3, 132.2, 128.3, 127.5, 126.8, 126,5, 125.5, 122.0, 25.54, 24.64, 23.30, 21.18, 14.36. FT-IR (KBr disc, cm−1) 2962, 2928, 1635, 1600, 1567, 1498, 1431, 1257, 1204, 1186, 1147, 951, 898, 836, 767, 630, 557.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631
631![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210
210