Selective oxidation of cyclohexane over gold nanoparticles supported on mesoporous silica prepared in the presence of thioether functionality†
Pingping
Wu
ab,
Zhigang
Xiong
a,
Kian Ping
Loh
bc and
X. S.
Zhao
*ab
aDepartment of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive, 117576, Singapore. E-mail: chezxs@nus.edu.sg; Fax: +65 6779-1936; Tel: +65 6516-4727
bNanoscience and Nanotechnology Initiative, National University of Singapore, 117576, Singapore
cDepartment of Chemistry, National University of Singapore, 3 Science Drive, 117543, Singapore. E-mail: chmlohkp@nus.edu.sg; Fax: +65 6779-1691; Tel: +65 6516-4402
First published on 16th February 2011
Abstract
Gold (Au) nanoparticles (NPs) supported on mesoporous silica were prepared using a one-pot synthesis method in the presence of thioether functionality -(CH2)3-S-S-S-S-(CH2)3. Bis(triethoxysilyl) propane tetrasulfide was used as the source of thioether functionality. The complexation of thioether groups with tetrachloroauric anion (AuCl4−) led to a good dispersion of Au NPs on mesoporous silica. Removal of the template and the thioether functional groups by calcination resulted in the reduction of the Au species, leaving behind Au NPs well dispersed on mesoporous silica. Selective oxidation of cyclohexane with molecular oxygen was used to evaluate the catalytic properties of the Au NP catalysts prepared using the present method and compared with that of a Au catalyst prepared using the hydrogen reduction method.
Introduction
Cyclohexanol and cyclohexanone (K/A oil) are important intermediates for the bulk production of polyamides and plastics. The main route to producing K/A oil is the selective oxidation of cyclohexane catalyzed by a homogeneous catalyst, such as transition metal salts. Such homogeneous catalysts are undesirable in these environmentally conscious and energy-intensive days. Solid catalysts, such as titanium silicalite-1 (TS-1),1,2 metal-substituted aluminophosphate molecular sieves,3–6 metal-incorporated mesoporous and microporous aluminosilicate molecular sieves,7–10 and nanostructured catalysts (Fe2O3, Co3O4 and mixed Fe–Co oxide nanoparticles)11–13 have been explored as heterogeneous catalysts for cyclohexane oxidation. These catalysts in general exhibit a relatively low activity and/or poor selectivity.Very recently, gold (Au) nanoparticles (NPs) supported on mesoporous silica have been found to display a high catalytic activity in oxidation reactions.14–17 As Au NPs are unstable and liable to aggregation, ligands such as chloro-group,18 amine,19 phenyl20 and thioether groups14 have been found to be effective for stabilizing Au NPs. Bis(triethoxysilyl) propane tetrasulfide (TESPTS) with thioether functionality -(CH2)3-S-S-S-S-(CH2)3- has been shown to be the most effective stabilizing ligand.14
In this work, Au NPs supported on mesoporous silica were prepared using a one-pot synthesis method in the presence of thioether functionality. The interaction of the Au species with TESPTS was studied and the evolution of the Au species was monitored using a real-time ultraviolet–visible (UV–vis) technique. The Au NP catalysts exhibited an outstanding catalytic performance. It was observed that the presence of TESPTS in the one-pot synthesis method played an important role in the dispersion of Au NPs on the silica support.
Results and discussion
Catalyst preparation and characterization
Fig. 1 shows the X-ray diffraction (XRD) patterns of the Au catalysts prepared with different amounts of TESPTS. Three reflection peaks indexed as (1 0 0), (1 1 0) and (2 0 0) diffractions (Fig. 1a and b) can be seen on the SBA-15 silica (template) and catalyst Au/0.625TESPTS–SiO2-cal, indicating a highly ordered 2D hexagonal structure of both materials. With increasing x, the (1 1 0) and (2 0 0) diffraction peaks became less intense (Fig. 1c) and eventually disappeared (Fig. 1d and e). Only one diffraction peak can be observed from catalysts Au/2.5TESPTS–SiO2-cal and Au/5TESPTS–SiO2-cal. In addition, the intensity of the peak was lowered, revealing a decrease in the regularity of the 2D hexagonal ordered structure. This was probably due to the presence of TESPTS, which affected the structure of the surfactant micelles, leading to the formation of a poor hexagonal structure. The right shift of the main (1 0 0) diffraction peak with increasing x indicated increased framework shrinkage after removal of the template. | ||
| Fig. 1 Low-angle XRD patterns of (a) SBA-15, (b) Au/0.625TESPTS–SiO2-cal, (c) Au/1.25TESPTS–SiO2-cal, (d) Au/2.5TESPTS–SiO2-cal, and (e) Au/5TESPTS–SiO2-cal. | ||
The pore diameters (DBJH) shown in Table 1, estimated from the adsorption branches using the BJH method, showed that the pore diameters decreased with increasing x, as well as the wall thickness. This trend can be rationalized in terms of different interactions between the surfactant molecules and the silicate and/or organosilicate precursors in the hydrothermal system.21,22 A strong hydrophobic interaction between the thioether group (-CH2-CH2-CH2-S-S-S-S-CH2-CH2-CH2-) and the hydrophobic PPO blocks of P123 induced the penetration of silicate species into the micelle core, leading to the reduction of channel diameters. Thus, the amount of TESPT introduced in the synthesis mixture played a crucial role in determining the mesostructure of the resultant catalysts.
| Catalysts | Au loadinga/wt% | d 100/nm | a 0 /nm | S BET/m2 g−1 | Vtotal/cm3 g−1 | D BJH/nm | Wall thickness/nm |
|---|---|---|---|---|---|---|---|
a Measured using the ICP-MS technique.
b
a
0 is the lattice parameter calculated from the d100 spacing according to the equation of  . SBET, surface area calculated by the BET method. Vtotal, total pore volume calculated at P/Po = 0.998. DBJH, pore diameter calculated from the adsorption branch using BJH method. Wall thickness obtained by subtracting pore size from lattice parameter. . SBET, surface area calculated by the BET method. Vtotal, total pore volume calculated at P/Po = 0.998. DBJH, pore diameter calculated from the adsorption branch using BJH method. Wall thickness obtained by subtracting pore size from lattice parameter.
|
|||||||
| SBA-15 | — | 8.7 | 10.0 | 782 | 1.0 | 7.9 | 2.1 |
| Au/0.625TESPTS–SiO2-cal | 0.75 | 9.5 | 11.0 | 960 | 1.09 | 9.2 | 1.8 |
| Au/1.25TESPTS–SiO2-cal | 0.94 | 8.8 | 10.2 | 1050 | 1.17 | 8.4 | 1.8 |
| Au/2.5TESPTS–SiO2-cal | 1.07 | 8.3 | 9.6 | 945 | 0.89 | 7.7 | 1.9 |
| Au/5TESPTS–SiO2-cal | 1.10 | 8.1 | 9.4 | 919 | 0.78 | 5.6 | 3.8 |
| Au/2.5TESPTS–SiO2-H2 | 0.76 | 9.1 | 10.5 | 665 | 0.75 | 7.4 | 3.1 |
The wide-angle XRD patterns of the Au catalysts prepared with different TESPTS/TEOS ratios are shown in Fig. 2 and the Au particle sizes calculated from the Scherrer equation are presented in Table 2. Four peaks at 38.18°, 44.43°, 64.55° and 77.65° are the characteristic peaks of the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) reflections of cubic Au nanoparticles (JCPDS card no.: 4-784). The Au nanoparticle size calculated from the XRD data showed an optimized TEMPTS/TEOS ratio of about 1/40 f(x = 2.5), for which well dispersed Au NPs with an average Au particle size of about 5.5 nm were obtained. When the TESPTS/TEOS ratio was less than 1/40, the size of Au particles increased with the decrease of TESPTS, as shown in the cases of catalysts Au/1.25TESPTS–SiO2-cal and Au/0.625TESPTS–SiO2-cal, which exhibited average Au particle sizes of 6.6 nm and 24.4 nm, respectively.
 | ||
| Fig. 2 Wide-angle XRD patterns of (a) Au/0.625TESPTS–SiO2-cal, (b) Au/1.25TESPTS–SiO2-cal, (c) Au/2.5TESPTS–SiO2-cal and (d) Au/5TESPTS–SiO2-cal. | ||
| Catalysts | Au NPs sizea/nm | Time/h | C6H12 conversion/mol% | Selectivity/mol% | TOFc/h−1 | ||
|---|---|---|---|---|---|---|---|
| C6H12OH | C6H12O | By-productsb | |||||
| C6H12, cyclohexane. C6H12OH, cyclohexanol. C6H12O, cyclohexanone.a Au particle sizes were estimated from the XRD patterns using the Scherrer equation, t = 0.9λ/Bcos(θ), where t is the crystallite size, λ is the wavelength, B is the full width at the half maximum, and θ is the diffraction angle.b By-products are mainly ring-opened acids such as n-butyric, n-valeric, succinic, glutaric and adipic acid.c Moles of K/A oil produced per mole of Au per hour. | |||||||
| SBA-15 | — | — | — | — | — | — | — |
| Au/0.625TESPTS–SiO2-cal | 24.4 | 0.5 | 10.3 | 40.3 | 56.7 | 3.0 | 9900 |
| 1 | 19.4 | 30.7 | 59.5 | 9.8 | |||
| 2 | 27.8 | 17.7 | 50.4 | 31.8 | |||
| Au/1.25TESPTS–SiO2-cal | 6.6 | 0.5 | 13.4 | 37.3 | 60.5 | 2.2 | 10394 |
| 1 | 24.2 | 34.4 | 60.0 | 5.6 | |||
| 2 | 31.4 | 20.0 | 57.3 | 22.7 | |||
| Au/2.5TESPTS–SiO2-cal | 5.5 | 0.5 | 15.9 | 35.7 | 62.6 | 1.7 | 11653 |
| 1 | 31.7 | 24.0 | 69.5 | 6.5 | |||
| 2 | 33.4 | 18.5 | 56.6 | 24.8 | |||
| Au/5TESPTS–SiO2-cal | 6.4 | 0.5 | 13.6 | 35.8 | 61.7 | 2.5 | 9886 |
| 1 | 27.3 | 31.0 | 62.7 | 6.3 | |||
| 2 | 34.5 | 18.8 | 61.6 | 19.6 | |||
| Au/2.5TESPTS–SiO2-H2 | — | 0.5 | — | — | — | 0 | 0 |
| 1 | 3.3 | 60.6 | 38.0 | 1.4 | |||
| 2 | 11.2 | 55.4 | 41.0 | 3.6 | |||
The Au contents in different catalysts were measured by using the inductive-coupled plasma-mass spectrometer (ICP-MS) technique and the results are shown in Table 1. It is seen that when the amount of TESPTS was equal to or larger than 1.25%, almost all (≧94%) Au precursors were introduced into the mesoporous silica to form Au NPs, indicating a very high Au loading efficiency. However, if less than 1.25% of TESPTS was added, only ∼75% of Au precursors was immobilized in the silica.
The TEM images and Au NPs size distribution histograms of catalysts Au/xTESPTS–SiO2-cal are shown in Fig. 3. When the amount of TESPTS was equal to 0.625%, unevenly distributed Au NPs (with sizes in the range of 4–15 nm) were obtained on the mesoporous silica support (Fig. 3a), consistent with XRD results. With the increase of the TESPTS ratio, uniformly dispersed Au NPs in the range of 3–8 nm were observed on catalysts Au/1.25TESPTS–SiO2-cal (Fig. 3b) and Au/2.5TESPTS–SiO2-cal (Fig. 3c) with a TESPTS ratio of 1.25% and 2.5%, respectively. However, Au NPs began to aggregate and were unevenly distributed (Au NPs with sizes in the range of 4–12 nm) with a further increase of the TESPTS ratio (Fig. 3d) due to the deterioration of the mesoporous structure.
 | ||
| Fig. 3 TEM images and Au NPs size distribution histograms of catalysts (a) Au/0.625TESPTS–SiO2-cal, (b) Au/1.25TESPTS–SiO2-cal, (c) Au/2.5TESPTS–SiO2-cal and (d) Au/5TESPTS–SiO2-cal. | ||
The incorporation of thioether groups into the silica framework of catalyst Au/2.5TESPTS–SiO2-H2 was verified by the 29Si and 13C CP MAS NMR spectra as shown in Fig. 4. From the 29Si MAS NMR spectra, the signals in the range of −90 to −150 ppm can be assigned to the silicon resonances of Si(OSi)4 (Q4, δ = −110 ppm), (OH)Si(OSi)3 (Q3, δ = −102 ppm) and (OH)2Si(OSi)2 (Q2, δ = −91.7 ppm), and the signals in the range of −50 to −70 ppm can be assigned to the silicon resonances of Tx [(SiO)x(OH)3−xSiC] sites.23 The resonances at about −65 ppm and −56 ppm attributed to the resonances of T3 and T2 for the organosiloxanes were observed on sample Au/2.5TESPTS–SiO2-as and catalyst Au/2.5TESPTS–SiO2-H2. The predominant T3 resonance over T2 resonance indicates the existence of cross-linked organosiloxanes in sample Au/2.5TESPTS–SiO2-as and catalyst Au/2.5TESPTS–SiO2-H2. Moreover, the enhancement of T3 resonance on catalyst Au/2.5TESPTS–SiO2-H2 was due to further dehydration of organosiloxane [(SiO)2(OH)SiC] (T2) sites to [(SiO)3SiC] (T3) sites during the H2 reduction at 250 °C. However, on catalyst Au/2.5TESPTS–SiO2-cal, no T3 or T2 peak was observed because of the complete removal of functional groups. The presence of the peaks in the range of 10–40 ppm on the 13C CP MAS NMR spectra further confirmed the presence of organosiloxane in sample Au/2.5TESPTS–SiO2-as and catalyst Au/2.5TESPTS–SiO2-H2. Three different carbon chemical environments with chemical shifts of δ = 10 ppm, 21 ppm, and 40 ppm in the 13C CP MAS NMR spectrum can be assigned to C1, C2 and C3 of the thioether group (Si-1CH2-2CH2-3CH2-S-S-S-S-3CH2-2CH2-1CH2-Si), respectively.23 A featureless 13C CP MAS NMR spectrum observed on catalyst Au/2.5TESPTS–SiO2-cal verified the complete removal of functional groups.
 | ||
| Fig. 4 29Si and 13C MAS NMR spectra of catalysts (a) Au/2.5TESPTS–SiO2-as, (b) Au/2.5TESPTS–SiO2-H2 and (c) Au/2.5TESPTS–SiO2-cal. | ||
The TEM image in Fig. 5 clearly shows the well dispersed Au NPs on catalyst Au/2.5TESPTS–SiO2-H2. The diameters of the Au particles were estimated to be in the range of 2–6 nm. But the Au NP sizes were in the range of 3–7 nm for catalyst Au/2.5TESPTS–SiO2-cal (see Fig. 3c). The larger Au NPs of catalyst Au/2.5TESPTS–SiO2-cal may be due to the aggregation of Au particles during the calcination (500 °C for 6 h). The Au loadings on catalysts Au/2.5TESPTS–SiO2-H2 and Au/2.5TESPTS–SiO2-cal were 0.76 wt% and 1.07 wt% (shown in Table 1), respectively, with an expected Au loading of 1.0 wt%. The lower Au loading efficiency on catalyst Au/2.5TESPTS–SiO2-H2 (76%) may be due to the leaching of Au species during the template removal.
Fig. 6 shows the UV-vis spectra of pure SBA-15 (Fig. 6a), catalysts Au/2.5TESPTS–SiO2-H2 (Fig. 6b) and Au/2.5TESPTS–SiO2-cal (Fig. 6c). A featureless spectrum was observed for the pure SBA-15 sample, while a strong Au surface plasma resonance (SPR) peak at about 513 nm can be seen on catalyst Au/2.5TESPTS–SiO2-cal, which is a typical SPR absorbance of nano-sized Au particles (510 nm).24 However, a significant shift of the SPR peak occurred for catalyst Au/2.5TESPTS–SiO2-H2, which exhibited an absorption peak at ca. 423 nm. This blue shift is probably due to the interactions between thioether groups and Au NPs.25 The different colours of the catalysts (Fig. 6, inset) justified the different complexation forms of Au NPs on catalysts Au/2.5TESPTS–SiO2-H2 and Au/2.5TESPTS–SiO2-cal, confirming the existence of strong interactions between Au NPs and thioether groups in the H2-reduced catalyst.
 | ||
| Fig. 6 UV-visible spectra of (a) SBA-15, (b) Au/2.5TESPTS–SiO2-H2 and (c) Au/2.5TESPTS–SiO2-cal. | ||
The one-pot synthesis chemistry
Fig. 7 shows the real-time UV-vis spectra of the synthesis mixtures in the absence and presence of TESPTS. The pure HAuCl4 solution (Fig. 7, curve 0) with a yellow colour exhibited two absorption bands centred at ∼220 nm and ∼310 nm, which originated from the gold(III) chloride and partially hydrolyzed gold chloride (Au(OH)xCl4−x−) species, respectively.26 After adding the AuCl4− solution into the synthesis mixture, the UV-vis spectra of the solution are shown in Fig. 7a and b. Without introducing the functional precursor TESPTS, the absorption peak shifted slightly to a longer wavelength (∼325 nm) which is due to the hindering effect of the strong acidic condition (pH < 1) on the hydrolysis of AuCl4− to (Au(OH)xCl4−x−). The concentration of AuCl4− in the solution exhibited a slight decrease after 1 h reaction, which may be due to the adsorption of AuCl4− on silicate species. On the other hand, with the presence of TESPTS, the two absorption spectra shifted to shorter wavelengths and developed new adsorption bands at about 210 nm and 280 nm. The intensity of these two peaks gradually enhanced, indicating that a certain form of complex gradually formed between AuCl4− and functionality TESPTS.27 | ||
| Fig. 7 Evolution of UV-visible spectra by introducing HAuCl4 into the synthesis gel with and without TESPTS addition. | ||
The evolution of this complex under the hydrothermal treatment conditions was studied by comparing the UV-vis spectra of the synthesis mixture with sample Au/2.5TESPTS–SiO2-as (Fig. 8a and b). Similar spectra were observed in the range of 200–300 nm due to the absorption band of the thioether–AuCl4− complex; while an enhanced UV-vis spectrum was seen on catalyst Au/2.5TESPTS–SiO2-as in the range of 400–500 nm, indicating that Au NPs with a size of less than 2 nm (Au clusters) may be formed during the hydrothermal treatment.28
 | ||
| Fig. 8 UV-visible spectra of (a) synthesis gel after 60 min reaction and (b) Au/2.5TESPTS–SiO2-as. | ||
The XPS results shown in Fig. 9a and d supported the above conclusion. As seen from Fig. 9a, sample Au/2.5TESPTS–SiO2-as exhibited the Au 4f7/2 and 4f5/2 doublet with binding energies of 84.5 eV and 88.2 eV, respectively, similar to those of thiolate-passivated Au clusters (Au:SR clusters) at 84.4 eV,29 indicating the presence of thioether-stabilized Au clusters in the as-synthesized sample. The S 2p spectrum for sample Au/2.5TESPTS–SiO2-as showed doublet peaks with binding energies of 163.9 eV and 165.1 eV (Fig. 9d), confirming the existence of interaction between S and Au.30 Herein, it can be concluded that the Au clusters began to form during the hydrothermal process and Au species were partially present as thioether-stabilized Au clusters in sample Au/2.5TESPTS–SiO2-as. After H2 reduction or high temperature calcination, the UV-vis spectra showed the formation of thioether-stabilized Au NPs on catalyst Au/2.5TESPTS–SiO2-H2 (Fig. 6b) and the formation of Au NPs on catalyst Au/2.5TESPTS–SiO2-cal (Fig. 6c). The XPS results shown in Fig. 9 verified this conclusion. Au 4f7/2 with binding energies of 84.0 eV and 84.1 eV were observed on catalysts Au/2.5TESPTS–SiO2-H2 (Fig. 9b) and Au/2.5TESPTS–SiO2-cal (Fig. 9c), respectively, due to metallic Au0 (84.0 eV),31 indicating that the Au species were completely reduced after H2 reduction or high temperature calcination. The S 2p spectrum shown in Fig. 9e confirmed the presence of interaction between S and Au NPs on catalyst Au/2.5TESPTS–SiO2-H2. The obvious decrease of spectrum intensity may be due to the partial decomposition of thioether groups during the H2 reduction. The S 2p peaks are absent on catalyst Au/2.5TESPTS–SiO2-cal (Fig. 9f). The evolution of Au species is also verified by the colour of the sample. The as-synthesized sample showed a light yellow colour, indicating the presence of AuCl4− while the ruby colour of catalyst Au/2.5TESPTS–SiO2-cal indicated the presence of Au NPs (insets in Fig. 6 and 8).
 | ||
| Fig. 9 Au (4f) (a, b, c) and S (2p) (d, e, f) XPS spectra of catalysts Au/2.5TESPTS–SiO2-as, Au/2.5TESPTS–SiO2-H2 and Au/2.5TESPTS–SiO2-cal. | ||
Based on the above results, a possible formation mechanism of Au NP catalysts was proposed as illustrated in Scheme 1. Upon mixing of the silica precursors, TEOS and TESPTS, with the gold precursor (HAuCl4) and the surfactant solution, a mesoporous silica phase was formed. At the same time, complexation of AuCl4− with thioether groups occurred to form AuCl4−–thioether complexes (Step I). With the co-condensation of the silica precursors to form the silica framework under the hydrothermal treatment (100 °C), part of AuCl4−–thioether complexes decomposed to form Au clusters stabilized by thioether groups (Step II) as proved by the UV-vis and XPS data of sample Au/2.5TESPTS–SiO2-as. The template was removed by ethanol extraction or high temperature calcination. The extracted sample was reduced by H2 (Step III). The residual AuCl4−–thioether complexes were completely reduced and thioether-stabilized Au clusters assembled to form thioether-stabilized Au NPs as proved by the UV-vis spectrum of catalyst Au/2.5TESPTS–SiO2-H2. On the other hand, after high-temperature calcination (Step III′), the template and functional groups were completely removed and AuCl4−–thioether complexes and thioether-stabilized Au clusters decomposed and further deposited to form Au NPs. The removal of the functional groups (e.g., -CH2-CH2-CH2-S-S-S-S-CH2-CH2-CH2-) led to the formation of structure defects in the silica framework, which were the favourable sites for the formation of Au NPs, and the silica framework prohibited the growth of Au NPs during the high temperature treatment.14
 | ||
| Scheme 1 Evolution of Au species during the one-pot synthesis process: (I) mixing of all reaction precursors in one pot; (II) hydrothermal treatment at 100 °C for 24 h; (III) ethanol extraction followed by H2 reduction, and (III′) high-temperature calcination. | ||
Catalytic performance
The catalytic performance of the catalysts prepared with different TESPTS/TEOS ratios or reduced by different methods was investigated on selective oxidation of cyclohexane with molecular oxygen. The catalytic reaction profile with time was studied on catalyst Au/xTESPTS–SiO2-cal and the H2-reduced catalyst. The conversion, selectivity, turnover frequency (TOF) and product distribution are summarized in Table 2. It can be seen that the calcined catalysts showed a good catalytic activity (the cyclohexane conversion ranged from 19.4% to 31.7%) and K/A oil selectivity (>90%) in the first hour reaction. After that, the selectivity of K/A oil declined with a slight increase in the cyclohexane conversion (27.8–34.5%). The results indicated that control over the reaction kinetics is important in terms of avoiding further oxidation of cyclohexanol to cyclohexanone and further oxidation of K/A oil to by-products. During the reaction, the formation of by-products is inevitable with the increase of reaction time. The TOF was measured in moles of product K/A oil produced per mole of Au per hour. The remarkably highest TOF number (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653 h−1) for the first half an hour reaction was observed on catalyst Au/2.5TESPTS–SiO2-cal, indicating the highest initial reaction rate of cyclohexane oxidation on this catalyst, followed by catalysts Au/1.25TESPTS–SiO2-cal (10
653 h−1) for the first half an hour reaction was observed on catalyst Au/2.5TESPTS–SiO2-cal, indicating the highest initial reaction rate of cyclohexane oxidation on this catalyst, followed by catalysts Au/1.25TESPTS–SiO2-cal (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 394 h−1), Au/5TESPTS–SiO2-cal (9886 h−1) and Au/0.625TESPTS–SiO2-cal (9900 h−1). This trend was closely related to the Au NP sizes of the catalysts. Catalyst Au/2.5TESPTS–SiO2-cal exhibited the smallest and most uniform Au particles (with an average Au NPs size of about 5.5 nm), followed by catalysts Au/1.25TESPTS–SiO2-cal (with an average Au NPs size of 6.6 nm), Au/5TESPTS–SiO2-cal (with an average Au NPs size of 6.4 nm) and Au/0.625TESPTS–SiO2-cal (with an average Au NPs size of 24.4 nm). The higher reaction activity on catalyst with smaller Au NPs size can be explained below. More surface-free Au atoms were presented as the active sites on the catalysts with smaller Au NPs. Judging from the characterization and reaction results, the optimized TESPTS/TEOS ratio was obtained as 1/40 (at the value of x = 2.5) with which well-dispersed Au NPs were obtained and a high Au loading efficiency was achieved, thus leading to a high catalytic activity.
394 h−1), Au/5TESPTS–SiO2-cal (9886 h−1) and Au/0.625TESPTS–SiO2-cal (9900 h−1). This trend was closely related to the Au NP sizes of the catalysts. Catalyst Au/2.5TESPTS–SiO2-cal exhibited the smallest and most uniform Au particles (with an average Au NPs size of about 5.5 nm), followed by catalysts Au/1.25TESPTS–SiO2-cal (with an average Au NPs size of 6.6 nm), Au/5TESPTS–SiO2-cal (with an average Au NPs size of 6.4 nm) and Au/0.625TESPTS–SiO2-cal (with an average Au NPs size of 24.4 nm). The higher reaction activity on catalyst with smaller Au NPs size can be explained below. More surface-free Au atoms were presented as the active sites on the catalysts with smaller Au NPs. Judging from the characterization and reaction results, the optimized TESPTS/TEOS ratio was obtained as 1/40 (at the value of x = 2.5) with which well-dispersed Au NPs were obtained and a high Au loading efficiency was achieved, thus leading to a high catalytic activity.
Table 2 also shows that catalyst Au/2.5TESPTS–SiO2-cal exhibited a much higher cyclohexane conversion than catalyst Au/2.5TESPTS–SiO2-H2. The lower activity of the latter, on the other hand, minimized the deep oxidation of K/A oil to by-products, thus leading to a high K/A oil selectivity. The catalytic data collected in this study showed that catalyst Au/2.5TESPTS–SiO2-H2 with Au particle sizes in the range of 2–6 nm exhibited a lower catalytic activity than the calcined catalyst with Au particle sizes in the range of 3–7 nm. The lower cyclohexane conversion on catalyst Au/2.5TESPTS–SiO2-H2 (0.76 wt%) is probably due to the lower Au loading than that on catalyst Au/2.5TESPTS–SiO2-cal (1.07 wt%). But the extremely lower TOF of catalyst Au/2.5TESPTS–SiO2-H2 (0 h−1) than that of catalyst Au/2.5TESPTS–SiO2-cal during the first half an hour reaction indicated a lower initial reaction rate of the former. This is most probably due to the partial coordination of active Au sites with capping agent TESPTS, which hampered the accessibility of the active Au sites to the reactants.32
Based on above reaction results, catalyst Au/2.5TESPTS–SiO2-cal synthesized with a TESPTS/TEOS ratio of 1/40 and reduced by calcination exhibited the best catalytic activity for cyclohexane oxidation. Furthermore, the optimized reaction time for this catalyst was 1 h with respect to achieving a high cyclohexane conversion and K/A oil selectivity. The results obtained in present work are more significant than the previous reported results shown in Table S1 (see ESI†).15,18,33–35 Compared to these materials, the present catalyst Au/2.5TESPTS–SiO2-cal emerged as the most promising catalyst for cyclohexane oxidation with respect to both high cyclohexane conversion (∼32%) and high TOF (11633 h−1). The high reaction rate and cyclohexane conversion on catalyst Au/2.5TESPTS–SiO2-cal is because of more surface-free Au atoms acting as active sites after complete removal of functional groups. Correlating the above reaction results with the catalyst properties, it was realized that the available surface-free Au atoms determined the catalytic activity of Au nanoparticles supported on functionalized mesoporous silica, while the presence of functional groups on the resultant catalyst may poison the catalytic activity.
Catalyst stability and recyclability
Recycling tests with repeated use of catalysts Au/2.5TESPTS–SiO2-cal and Au/2.5TESPTS–SiO2-H2 in six consecutive reactions were carried out. The catalyst was removed from the reaction system by filtration after 1 h reaction and washed thoroughly with ethanol, followed by drying at 80 °C overnight and then subjected to the next cycle. The recycling results shown in Fig. 10 indicated that a slight decrease in conversion occurred after the 2nd run on catalyst Au/2.5TESPTS–SiO2-cal and no obvious activity loss was observed in the following 4 cycles, demonstrating a good stability of catalyst Au/2.5TESPTS–SiO2-cal. The slight decrease of catalytic activity during the first two cycles is due to the aggregation of small Au NPs on the surface of the silica support. | ||
| Fig. 10 Recyclability of catalyst Au/2.5TESPTS–SiO2-cal. (a) Fresh catalyst, (b) catalyst after two reaction cycles and (c) catalyst after six reaction cycles. | ||
Fig. 11 shows the recyclability of catalyst Au/2.5TESPTS–SiO2-H2. It is interesting to note that the catalytic activity kept increasing in the first several runs and decreased after the 4th run. This is due to the fact that part of the Au surface was coordinated by the functional ligand in the fresh catalyst. The functional groups would be decomposed under the high temperature reaction. Thus, after one or two runs the active sites were completely released from coordination, leading to the higher activity in the 3rd and 4th cycles. However, these free Au NPs may easily aggregate to larger Au particles; explaining that after the 4th reaction cycle, the catalytic activity decreased again. The colours of the fresh and used catalysts shown in Fig. 10 and 11 justified the above results. For catalyst Au/2.5TESPTS–SiO2-cal, the appearance of catalyst became slightly dark red after two reaction cycles and no obvious change of colour occurred after the 2nd reaction cycle until the 6th cycle, indicating that no further aggregation occurred after the initial aggregation of surface Au NPs. On the other hand, the colour changed significantly in each cycle of catalyst Au/2.5TESPTS–SiO2-H2, indicating the decomposition of functional groups, the release of Au NPs and the further aggregation of Au NPs.
 | ||
| Fig. 11 Recyclability of catalyst Au/2.5TESPTS–SiO2-H2. (a) Fresh catalyst, (b) catalyst after one reaction cycle, (c) catalyst after two reaction cycles and (d) catalyst after six reaction cycles. | ||
Fig. 12 shows the S 2p XPS spectra of catalyst Au/2.5TESPTS–SiO2-H2 before and after use. It is seen that the thioether groups with a binding energy of 163.8 eV were oxidized to form SO3− with a binding energy of 168.8 eV after the 1st reaction cycle, and then decomposed after the 3rd reaction cycle. This exactly explains that the catalyst preserved the highest catalytic activity in the 4th reaction cycle and after that aggregation of Au nanoparticles occurred. As is seen from the TEM images in Fig. 13, no obvious aggregation of Au NPs was observed on catalyst Au/2.5TESPTS–SiO2-cal, while distinct aggregation of Au NPs was found on catalyst Au/2.5TESPTS–SiO2-H2. The excellent stability and recyclability of catalyst Au/2.5TESPTS–SiO2-cal is due to the confinement of Au NPs in the silica framework, while the poor stability of catalyst Au/2.5TESPTS–SiO2-H2 is attributed to the attachment of Au NPs with functional groups, which were unstable at high temperatures. These reaction results confirmed the validity of the formation mechanism proposed in Scheme 1 that for catalyst Au/2.5TESPTS–SiO2-H2 the Au NPs were attached and stabilized by thioether groups, while on catalyst Au/2.5TESPTS–SiO2-cal, the Au NPs were protected by the silica framework.
 | ||
| Fig. 12 S 2p XPS spectra of fresh and used catalyst Au/2.5TESPTS–SiO2-H2. (a) Fresh catalyst, (b) after one reaction cycle, (c) after two reaction cycles and (d) after three reaction cycles. | ||
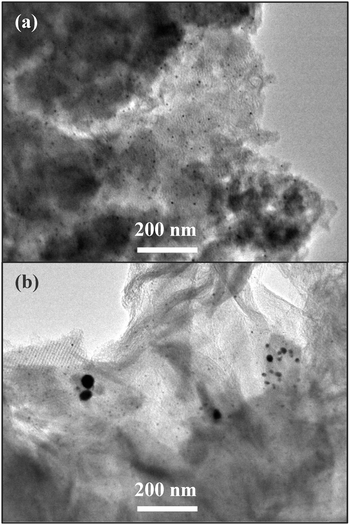 | ||
| Fig. 13 TEM images of catalysts (a) Au/2.5TESPTS–SiO2-cal and (b) Au/2.5TESPTS–SiO2-H2 after 6 cycles of reaction. | ||
Experimental
Chemicals
HAuCl4·xH2O (Aldrich), tetraethyl orthosilicate (TEOS, 98%, Acros Organics), triblock co-polymer PEO20PPO70PEO20 (P123, Aldrich),bis(triethoxysilyl) propane tetrasulfide (TESPTS, 97%, Aldrich), hydrochloric acid (37%, Merck), cyclohexane (99.99%, Fisher) and absolute ethanol (99.98%, Merck) were used as received without further purification.Catalyst preparation
In a typical synthesis, 4 g of P123 was dissolved in 30 mL of deionized water at room temperature followed by adding 120 mL of a 0.74 M HCl solution into this solution. A mixture of TEOS and TESPTS was slowly added. Then, 6.4 mL of 0.02 M HAuCl4·xH2O solution was added. After stirring at 40 °C for 24 h, the mixture was transferred to a Teflon-lined stainless steel autoclave to undergo a static hydrothermal treatment at 100 °C for 24 h. The solid products were filtered off, washed with deionized water till no Cl− was detected using a AgNO3 solution, and dried at 80 °C in a vacuum oven overnight. To investigate the effect of TESPTS on the loading of Au, the molar percentage of TESPTS/TEOS = x% was varied by changing x from 0.625 to 5. The samples thus obtained, herein denoted as Au/xTESPTS–SiO2-as, were calcined in air at 500 °C for 6 h to remove the template. During the calcination process, Au species were reduced to Au nanoparticles. The final solid products are designated as Au/xTESPTS–SiO2-cal, which were used directly as catalysts without further reduction.For comparison purpose, sample Au/2.5TESPTS–SiO2-as was treated with ethanol to remove the template, followed by hydrogen reduction at 250 °C for 2 h. This catalyst is designated as Au/2.5TESPTS–SiO2-H2.
Catalyst characterization
X-Ray powder diffraction (XRD) patterns were recorded on a XRD-6000 (Shimadzu, Japan) system with a Cu-Kα radiation of wavelength λ = 0.15418 nm. N2 adsorption–desorption isotherms were measured at –196 °C on an automatic volumetric sorption analyzer (Micromeritics, ASAP2020). Prior to adsorption, the samples were degassed at 200 °C for 4 h under vacuum. The solid ultraviolet–visible (UV-vis) spectra were measured on a UV-vis-NIR (UV-visible Near Infra-Red) scanning spectrophotometer (Shimadzu, UV-3101 PC) with an ISR-3100 integrating sphere attachment and BaSO4 as an internal reference. Chemical analyses of Au in the catalysts were carried out on an Agilent 7500 series inductive-coupled plasma-mass spectrometer (ICP-MS), after dissolving the solids by attacking with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of HNO3/HF. X-Ray photoelectron spectroscopy (XPS) spectra were recorded on an AXIS HIS 165 spectrometer (Kratos Analytical) with a monochromatized Al-Kα X-ray source. The Au 4f signals were recorded in a 0.05 eV step with a pass energy of 40 eV. Solid-state magic-angle spinning (MAS) nuclear magnetic resonance (NMR) spectra were obtained on a Bruker DRX400 FT-NMR spectrometer. A 4 mm rotor was used. The MAS speed was 8 kHz. The cross-polarization (CP) technique was used for 13C measurements while the single-pulse method was used for 29Si spectrum collections. The microstructures of the catalysts were observed on a transmission electron microscope (JEM 2010 from JEOL) operated at 200 kV.
1 mixture of HNO3/HF. X-Ray photoelectron spectroscopy (XPS) spectra were recorded on an AXIS HIS 165 spectrometer (Kratos Analytical) with a monochromatized Al-Kα X-ray source. The Au 4f signals were recorded in a 0.05 eV step with a pass energy of 40 eV. Solid-state magic-angle spinning (MAS) nuclear magnetic resonance (NMR) spectra were obtained on a Bruker DRX400 FT-NMR spectrometer. A 4 mm rotor was used. The MAS speed was 8 kHz. The cross-polarization (CP) technique was used for 13C measurements while the single-pulse method was used for 29Si spectrum collections. The microstructures of the catalysts were observed on a transmission electron microscope (JEM 2010 from JEOL) operated at 200 kV.
Measurement of catalytic properties
The selective oxidation of cyclohexane was carried out in a 200 mL Parr batch reactor with a polytetrafluoroethylene (PTFE) liner. In a typical oxidation reaction, 20 mL of cyclohexane and 50 mg solid catalyst were added into the reactor and the reaction was conducted under the conditions of 150 °C and a pressure of 1 MPa controlled by O2. After the reaction, the mixture was dissolved by ethanol and an excessive amount of triphenylphosphine (Ph3P) was added to the reaction mixture to completely reduce the cyclohexyl hydroperoxide (CHHP), an intermediate in the cyclohexane oxidation to cyclohexanol. The products were analyzed using a gas chromatogram (HP 7890 series GC) with a mass spectrometer detector (HP 5973 mass selective detector) and a capillary column (HP 5MS).Conclusions
Gold nanoparticles supported on mesoporous silica have been prepared using a simple one-pot synthesis method in the presence of bis(triethoxysilyl) propane tetrasulfide (TESPTS) as a ligand. The amount of TESPTS present in the synthesis system was found to play a crucial role in the dispersion of the final gold nanoparticles because of the complexation effect of thioether groups with AuCl4− species. Calcination of the as-synthesized solids for removing the template and the organic ligand directly yielded ruby-colored gold catalysts. Removal of the template by using alcohol extraction followed by hydrogen reduction yielded a light-brown-colored gold catalyst. The catalyst prepared by direct calcination exhibited a higher catalytic activity in selective oxidation of cyclohexane with molecular oxygen than the catalyst prepared using the hydrogen reduction method because of the smaller gold nanoparticle size of the former catalyst and partial coverage of the gold particle surface by organic functional groups in the latter catalyst. The gold catalyst prepared using the one-pot synthesis method also displayed an excellent stability and recyclability.Acknowledgements
P. W. wishes to thank National University of Singapore Nanoscience and Nanotechnology (NUSNNI) for offering a scholarship.References
- E. V. Spinace, H. O. Pastore and U. Schuchardt, J. Catal., 1995, 157, 631–635 CrossRef CAS.
- M. H. Zahedi-Niaki, M. P. Kapoor and S. Kaliaguine, J. Catal., 1998, 177, 231–239 CrossRef CAS.
- S. S. Lin and H. S. Weng, Appl. Catal., A, 1993, 105, 289–308 CrossRef CAS.
- R. Zhao, Y. Q. Wang, Y. L. Guo, Y. Guo, X. H. Liu, Z. G. Zhang, Y. S. Wang, W. C. Zhan and G. Z. Lu, Green Chem., 2006, 8, 459–466 RSC.
- B. Moden, B. Z. Zhan, J. Dakka, J. G. Santiesteban and E. Iglesia, J. Phys. Chem. C, 2007, 111, 1402–1411 CrossRef CAS.
- J. Li, X. Li, Y. Shi, D. S. Mao and G. Z. Lu, Catal. Lett., 2010, 137, 180–189 CrossRef CAS.
- S. E. Dapurkar, A. Sakthivel and P. Selvam, New J. Chem., 2003, 27, 1184–1190 RSC.
- S. Samanta, N. K. Mal and A. Bhaumik, J. Mol. Catal. A: Chem., 2005, 236, 7–11 CrossRef CAS.
- H. Zhao, J. C. Zhou, H. Luo, C. Y. Zeng, D. H. Li and Y. J. Liu, Catal. Lett., 2006, 108, 49–54 CrossRef CAS.
- J. Li, Y. Shi, L. Xu and G. Z. Lu, Ind. Eng. Chem. Res., 2010, 49, 5392–5399 CrossRef CAS.
- N. Perkas, Y. Koltypin, O. Palchik, A. Gedanken and S. Chandrasekaran, Appl. Catal., A, 2001, 209, 125–130 CrossRef CAS.
- V. Kesavan, D. Dhar, Y. Koltypin, N. Perkas, O. Palchik, A. Gedanken and S. Chandrasekaran, in 11th European Conference on Analytical Chemistry (EUROANALYSIS 11), Lisbon, Portugal, Int Union Pure Applied Chemistry, 2000, pp. 85–91 Search PubMed.
- J. M. Thomas, B. F. G. Johnson, R. Raja, G. Sankar and P. A. Midgley, Acc. Chem. Res., 2003, 36, 20–30 CrossRef CAS.
- L. F. Chen, J. C. Hu and R. Richards, J. Am. Chem. Soc., 2009, 131, 914–915 CrossRef CAS.
- R. Zhao, D. Ji, G. M. Lv, G. Qian, L. Yan, X. L. Wang and J. S. Suo, Chem. Commun., 2004, 904–905 RSC.
- G. M. Lu, D. Ji, G. Qian, Y. X. Qi, X. L. Wang and J. S. Suo, Appl. Catal., A, 2005, 280, 175–180 CrossRef.
- B. P. C. Hereijgers and B. M. Weckhuysen, J. Catal., 2010, 270, 16–25 CrossRef CAS.
- K. K. Zhu, J. C. Hu and R. Richards, Catal. Lett., 2005, 100, 195–199 CrossRef CAS.
- H. G. Zhu, B. Lee, S. Dai and S. H. Overbury, Langmuir, 2003, 19, 3974–3980 CrossRef CAS.
- T. Maddanimath, A. Kumar, J. D'Arcy-Gall, P. G. Ganesan, K. Vijayamohanan and G. Ramanath, Chem. Commun., 2005, 1435–1437 RSC.
- J. C. Hu, L. F. Chen, K. K. Zhu, A. Suchopar and R. Richards, in Gold 2006 Meeting, Limerick, Ireland, Elsevier Science Bv, 2006, pp. 277–283 Search PubMed.
- H. Liu, J. Yang, Q. H. Yang, G. Wang and Y. Li, Adv. Funct. Mater., 2005, 15, 1297–1302 CrossRef CAS.
- J. Liu, Q. H. Yang, L. Zhang, D. M. Jiang, X. Shi, J. Yang, H. Zhong and C. Li, Adv. Funct. Mater., 2007, 17, 569–576 CrossRef CAS.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 4212–4217 CrossRef CAS.
- Y. Negishi and T. Tsukuda, J. Am. Chem. Soc., 2003, 125, 4046–4047 CrossRef CAS.
- F. Moreau, G. C. Bond and A. O. Taylor, J. Catal., 2005, 231, 105–114 CrossRef CAS.
- K. Torigoe, A. Suzuki and K. Esumi, J. Colloid Interface Sci., 2001, 243, 528 CrossRef.
- T. Shimizu, T. Teranishi, S. Hasegawa and M. Miyake, J. Phys. Chem. B, 2003, 107, 2719–2724 CrossRef CAS.
- S. R. Johnson, S. D. Evans, S. W. Mahon and A. Ulman, Langmuir, 1997, 13, 51–57 CrossRef CAS.
- D. G. Castner, K. Hinds and D. W. Grainger, Langmuir, 1996, 12, 5083–5086 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC.
- V. C. L. Butselaar-Orthlieb, A. Quintanilla, C. Kwakernaak, W. G. Sloof, M. T. Kreutzer and F. Kapteijn, J. Catal., 2010, 271, 104–114 CrossRef CAS.
- G. M. Lu, R. Zhao, G. Qian, Y. X. Qi, X. L. Wang and J. S. Suo, Catal. Lett., 2004, 97, 115–118 CrossRef.
- L. X. Xu, C. H. He, M. Q. Zhu and S. Fang, Catal. Lett., 2007, 114, 202–205 CrossRef CAS.
- L. X. Xu, C. H. He, M. Q. Zhu, K. J. Wu and Y. L. Lai, Catal. Lett., 2007, 118, 248–253 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0cy00025f |
| This journal is © The Royal Society of Chemistry 2011 |

