Thermal-regulated PEG1000-based ionic liquid/PM for one-pot three-component synthesis of 2,4,5-trisubstituted imidazoles
Dong
Fang
ab,
Jinming
Yang
b and
Changmei
Jiao
ab
aJiangsu Provincial Key Laboratory of Coast Wetland Bioresources & Environment Protection, Yancheng 224002, Jiangsu, P. R. China. E-mail: fangdong106@yahoo.com.cn
bSchool of Chemistry & Chemical Engineering, Yancheng Normal University, Yancheng 224002, P. R. China
First published on 10th February 2011
Abstract
A novel recyclable temperature-dependant phase-separation catalytic system comprised of PEG1000-based functional dicationic acidic ionic liquid and propylene glycol monomethyl ether was developed. This catalytic system could be used for the synthesis of 2,4,5-trisubstituted imidazoles via one-pot three-component condensation with various aldehydes, benzil and ammonium acetate in reasonable to good yield of 81–95%. The reaction was accomplished homogeneously at 70 °C, while the products was separated from the catalyst system by liquid/liquid phasic-separation at room-temperature, and the catalyst could be reused at least eight times without significantly decreasing the catalytic activity.
1. Introduction
The imidazole nucleus constitutes the core unit of many natural products. Also, imidazole derivatives are a kind of very interesting N-heterocyclic compounds for their versatile pharmacological properties and play important roles in biochemical processes for over a century.1 Multi-aryl substituted imidazoles, especially tri-substituted compounds, could have potential novel therapeutic activities. In addition, many of the 2,4,5-triarylimidazoles are known as herbicides, fungicides, or as inhibitors of IL-1 or P38 MAP kinase.2 Recent advances in green chemistry and organometallic catalysis have extended the application of imidazoles as ionic liquids and N-heterocyclic substantial compounds, hence, great progress has been made to develop more efficient methods for these compounds.3 Therefore, the synthesis of this class of compounds has received considerable attention, originally, these compounds can be produced by the condensation of benzil, ammonium acetate, and aldehydes in the presence of protic acids, Lewis acids, and heteropolyacids4 as catalysts, as well as with the assistance of microwave5 or ultrasound irradiation.6 However, the development of a simple, efficient , cost effective and environment friendly protocol remains a challenging task for the synthetic chemists. Today and tomorrow, pollutants reduction and environmental protection are of great importance for chemistry research and chemical engineering, and ionic liquids (ILs) are widely thought as green solvents/catalysts and have been extensively studied in green chemistry and catalysis, especially for the functionalized ionic liquids (FILs), which have been one of the most attractive topics,3e.g., use of FILs as catalysts and solvents for synthesis of 2,4,5-trisubstituted imidazoles via multi-component reactions (MCRs) has been reported in recent years.7It is believed that homogeneous catalytic reaction systems have attracted much attention for their high activity and selectivity under mild conditions; however, they often suffer from drawbacks such as the laborious separation and recycling of catalyst. On the other hand, substrate and catalyst transfer is less efficient though the separation is relatively simple for the heterogeneous case. The search for more efficient catalytic systems that might combine the advantages of both homogeneous and heterogeneous catalysis is one of the most exciting challenges of modern chemistry. Currently, some novel temperature-dependent ionic liquid biphasic catalytic systems including organic solvent/ionic liquid, supercritical carbon dioxide/ionic liquid have been reported8 and found that these catalytic system possess some advantages, such as high conversions and selectivity, separation and recovery of catalyst with high efficiency.
In continuation of our work in ionic liquid catalysis and considering the importance of combined catalytic system,9 we now report an efficient and convenient homogeneous reaction-heterogeneous separation procedure for the synthesis of 2,4,5-trisubstituted imidazoles catalyzed by PEG1000-based dicationic ionic liquid (PEG1000-DAIL) (Fig. 1).
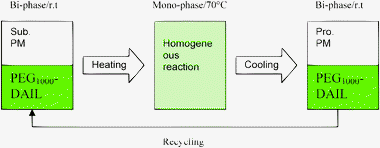 | ||
| Fig. 1 Temperature-dependant PEG1000-DAIL catalytic system. | ||
2. Experimental
2.1 Materials and methods
Melting points were determined by an X6-Data microscope apparatus. IR spectra were recorded on a Bruker Vectter 22 spectrometer and expressed in cm−1 (KBr). 1H NMR were recorded on a Bruker DRX300 (300 MHz) spectrometer. 13C NMR were recorded on a Bruker DRX300 (75.5 MHz) spectrometer. Mass spectra were obtained with automated Fininigan Trace Ultra-Trace DSQ GC-MS spectrometer. All other chemicals (AR grade) were commercially available and used without further purification unless otherwise stated.2.2 Synthesis of PEG1000-based functional dicationic acidic ionic liquid (PEG1000-DAIL)
The PEG1000-DAIL was prepared according to the literature.8e For comparison purpose, some other FILs including acyclic ammonium-, pyridine-, imidazole-based ([TMPSA][HSO4], [MIMPS][HSO4], [PyPS][HSO4]) were prepared according to our reported methods,9 their structure (Scheme 1) was analyzed by 1H-NMR and MS spectral data.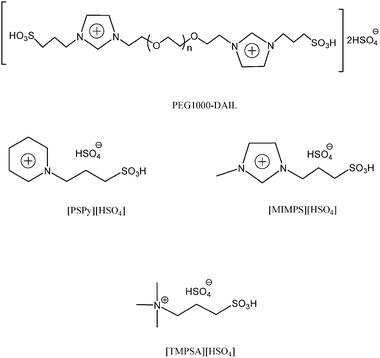 | ||
| Scheme 1 Structures of the acidic functional ionic liquids. | ||
2.3 General procedure for the synthesis of 2,4,5-trisubstituted imidazoles
In a typical experiment (Scheme 2), to a tube reactor pre-loaded with PEG1000-DAIL (2 mL) and propylene glycol monomethyl ether (PM) (2 mL) was charged with benzil (0.21 g, 1 mmol) 1, aldehyde (1 mmol) 2 and ammonium acetate (2.4 mmol) 3, respectively, under stirring. The mixture was then stirred vigorously for a length of time at 70 °C (Fig. 1). After completion of the condensation (monitored by TLC), the reaction mixture was then cooled to room temperature and the upper phase of PM layer was separated by decantation, then concentrated under vacuum, the resulting solid was recrystallized from acetone/water (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or by chromatography through a column of silica-gel using ethyl acetate/petroleum ether (1
1) or by chromatography through a column of silica-gel using ethyl acetate/petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as an eluent to yield the desired substituted imidazoles 4. The products were identified by 1H-NMR, and physical data (m.p.) with those reported in the literatures. The under layer of PEG1000-DAIL was reused without any treatment.
3) as an eluent to yield the desired substituted imidazoles 4. The products were identified by 1H-NMR, and physical data (m.p.) with those reported in the literatures. The under layer of PEG1000-DAIL was reused without any treatment.
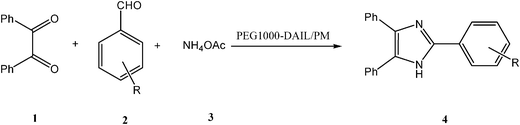 | ||
| Scheme 2 Synthesis of 2,4,5-trisubstituted imidazoles catalyzed by ionic liquid. | ||
3. Results and discussion
In the initial catalytic activity experiments, the PEG1000-DAIL/PM temperature-regulated biphasic catalytic system was applied to prepare 2,4,5-trisubstituted imidazoles by one-pot three-component condensation of aldehyde, benzil and ammonium acetate, some reaction media including other type of ionic liquids were compared (Table 1). From the table it can be found that, nearly trace desirable product could be detected in PM in the absence of any catalyst (entry 1). Only 36% yield of product was obtained in present of conc. H2SO4 (entry 2). In case of acyclic ammonium- (entry 3), pyridine- (entry 4), imidazole-based acidic ionic liquids (entry 5) as well as PEG1000-DAIL (entry 6) were used as dual solvent/catalyst, the yields would be improved to 73 to 82%, respectively. In comparison, the PEG1000-DAIL/EM system gave the best result (entry 7) due to lower viscosity and homogenous catalytic system at reaction temperature. Additionally, PM is expected to be a safe substitute for toxic solvents owing to its negligible toxicity; hence, it is relatively safe when compared with the toluene to form such catalytic system.| Entry | Catalytic system | t/h | T/°C | Yield (%) |
|---|---|---|---|---|
| a 1 mmol benzil, 1 mmol aldehyde, 2.4 mmol ammonium acetate, 2 mL catalyst, 2 mL PM, 70 °C. | ||||
| 1 | PM(catalyst free) | 6 | 70 | Trace |
| 2 | H2SO4 | 3 | 70 | 36 |
| 3 | [TMPSA][HSO4] | 1 | 70 | 81 |
| 4 | [PyPS][HSO4] | 1 | 70 | 82 |
| 5 | [MIMPS][HSO4] | 1 | 70 | 79 |
| 6 | PEG1000-DAIL | 1 | 70 | 73 |
| 7 | PEG1000-DAIL/PM | 1 | 70 | 93 |
Subsequently, the recycling performance of PEG1000-DAIL/PM biphasic catalytic system was investigated using the above same model reaction. When complete, the reaction system was stilled and cooled to room temperature, liquid/liquid bi-phase was then formed and the upper phase of PM containing desired product was removed by decantation , then the fresh substrates and PM were charged into the residual PEG1000-DAIL (without further purification) and the mixture was reacted under the same reaction conditions. The data listed in Fig. 2 showed that the PEG1000-DAIL could be reused at least eight times without obviously decreasing of the catalytic activity. To compare with the traditional homogenous catalysis, the easy separation and recycling performance is absolutely an attractive property.
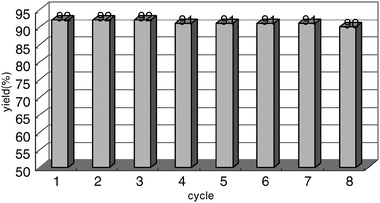 | ||
| Fig. 2 The recycling performance of temperature-dependant PEG1000-DAIL catalytic system | ||
Then, this condensation reaction with various aldehydes, benzil, and ammonium acetate in the presence of PEG1000-DAIL/PM as the catalyst was explored under the optimized reaction conditions and the results are presented in Table 2.
| Entry | R | t/min | Yieldb (%) | mp/°C | Ref. |
|---|---|---|---|---|---|
| a 1 mmol benzil, 1 mmol aldehyde, 2.4 mmol ammonium acetate, 2 mL catalyst, 2 mL PM, 70 °C. b Isolated yields. | |||||
| 1 | 4-CH3OC6H4 | 60 | 95 | 220–222 | 222–2256 |
| 2 | 2-CH3OC6H4 | 60 | 90 | 205–207 | 207–2086 |
| 3 | 4-CH3C6H4 | 60 | 94 | 226–228 | 227–2295 |
| 4 | C6H5 | 60 | 93 | 274–276 | 275–2765 |
| 5 | 4-ClC6H4 | 60 | 81 | 258–260 | 258–2605 |
| 6 | 2-ClC6H4 | 90 | 87 | 198–200 | 197–1996 |
| 7 | 2,4-Cl2C6H3 | 90 | 89 | 171–173 | 172–1736 |
| 8 | 4-NO2C6H4 | 60 | 91 | 237–239 | 239–2426 |
| 9 | 3-NO2C6H4 | 60 | 92 | 312–315 | 313–3155 |
| 10 | 4-HOC6H4 | 90 | 86 | 261–263 | 2666 |
It can easily be seen that this one-pot, three-component condensation completed within 60–90 min and the products were isolated in good yields. For aromatic aldehydes carrying either electron-donation (entries 1–3) or electron-withdrawing substituents (entries 8–9) could afford good yields of 2,4,5-trisubstituted imidazoles.
The result obtained with aldehyde, benzil, and ammonium acetate under the optimized conditions was compared with the results of other catalysts reported in the literatures, the data listed in Table 3 showed that the PEG1000-DAIL/PM was relatively a good catalytic system for this condensation reaction.
Conclusions
In summary, the PEG1000-DAIL/PM temperature-dependent biphasic catalytic system was found to be a novel procedure for the synthesis of 2,4,5-trisubstituted imidazoles, and this catalytic system has the advantages of both homogeneous and heterogeneous reaction, including short reaction time, good yields, operational simplicity, and environmentally benign.Acknowledgements
This work was financially supported by the Educational Commission of Jiangsu Provinces (05KJD150251), Jiangsu Provincial Key Laboratory of Coastal Wetland Bioresources & Environmental Protection (JLCBE 09023) and the Professional Elite Foundation of Yancheng Normal University.References
- F. Bellina, S. Cauteruccio, S. Monti and R. Rossi, Bioorg. Med. Chem. Lett., 2006, 16, 5757 CrossRef CAS.
- (a) J. C. Lee, J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. Blumenthal, J. R. Heys, S. W. Landvatter, J. E. Strickler, M. M. McLaughlin, I. R. Siemens, S. M. Fisher, J. P. Livi, J. R. White, J. L. Adams and P. R. Young, Nature, 1994, 372, 739 CrossRef CAS; (b) A. Pesquet, A. Daich and L. Van Hijfte, J. Org. Chem., 2006, 71, 5303 CrossRef CAS.
- P. Wasserscheid, R. Hal and A. Bosmann, Green Chem., 2002, 4, 400 RSC.
- (a) S. Sarshar, D. Siev and A. M. M. Mjalli, Tetrahedron Lett., 1996, 37, 835 CrossRef CAS; (b) D. E. Frantz, L. Morency, A. Soheili, J. A. Murry, E. J. J. Grabowski and R. D. Tillyer, Org. Lett., 2004, 6, 843 CrossRef CAS; (c) H. Weinmann, M. Harre, K. Koeing, E. Merten and U. Tilstam, Tetrahedron Lett., 2002, 43, 593 CrossRef CAS; (d) S. D. Sharma, P. Hazarika and D. Konwar, Tetrahedron Lett., 2008, 49, 2216 CrossRef; (e) M. H. Majid, B. Khadijeh, A. O. Hossein and T. Shima, J. Mol. Catal. A: Chem., 2007, 263, 279 CrossRef CAS; (f) M. H. Majid, F. Derikvand and F. F. Bamoharram, J. Mol. Catal. A: Chem., 2007, 263, 112 CrossRef.
- H. A. Oskooei, Z. Alimohammadi and M. M. Heravi, Heteroat. Chem., 2006, 17, 699 CrossRef CAS.
- H. Zang, Q. H. Su, Y. M. Mo, B. W. Cheng and S. Jun, Ultrason. Sonochem., 2010, 17, 749 CrossRef CAS.
- (a) A. R. Khosropour, Can. J. Chem., 2008, 86, 264 CrossRef CAS; (b) M. Xia and Y. D. Lu, J. Mol. Catal. A: Chem., 2007, 265, 205 CrossRef CAS; (c) S. A. Siddiqui, U. C. Narkhede, S. S. Palimkar, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Tetrahedron, 2005, 61, 3539 CrossRef CAS.
- (a) P. B. Webb, M. F. Sellin and T. E. Kunene, J. Am. Chem. Soc., 2003, 125, 15577 CrossRef CAS; (b) J. van den Broeke, F. Winter, B. J. Deelman and G. van Koten, Org. Lett., 2002, 4, 3851 CrossRef CAS; (c) L. Wei, J. Y. Jiang, Y. H. Wang and Z. L. Jin, J. Mol. Catal. A: Chem., 2004, 221, 47 CrossRef CAS; (d) Y. Leng, J. Wang, D. R. Zhu, X. Q. Ren, H. Q. Ge and L. Shen, Angew. Chem., Int. Ed., 2008, 48, 168 CrossRef; (e) H. Z. Zhi, C. X. Lu, Q. Zhang and J. Luo, Chem. Commun., 2009, 3, 1 Search PubMed; (f) Y. L. Hu, G. Qiang, Y. He and M. Lu, ChemCatChem, 2010, 2, 392 Search PubMed.
- (a) D. Fang, Q. R. Shi, J. Cheng, K. Gong and Z. L. Liu, Appl. Catal., A, 2008, 345, 158 CrossRef CAS; (b) D. Fang, Z. H. Fei and Z. L. Liu, Catal. Commun., 2009, 10, 1566.
| This journal is © The Royal Society of Chemistry 2011 |
