Hydrogen production from ethanol via inorganic membrane reactors technology: a review
A.
Iulianelli
and
A.
Basile
*
ITM-CNR, via P. Bucci Cubo 17/C, University of Calabria, Rende (CS)—87036, Italy. E-mail: a.basile@itm.cnr.it; Fax: +39 0984 402103; Tel: +39 0984 492013
First published on 7th March 2011
Abstract
Nowadays, hydrogen is the most viable energy carrier useful for further applications and in particular for fuel cells. Furthermore, from an environmentally-friendly point of view, hydrogen production from ethanol, mainly producible from biomass, is considered as a new opportunity for exploiting renewable sources. The present paper reviews the current state of the art about hydrogen production from ethanol reforming processes performed through membrane reactors technology. In particular, inorganic membrane reactors are described because they are useful to perform reactions limited by the equilibrium conversion owing to the hydrogen separation capability of an inorganic membrane. The benefits and the main drawbacks of inorganic membrane reactors are examined and the performances in terms of hydrogen yield, hydrogen recovery, conversion and so on are qualitatively compared to those of conventional systems. In addition, an overview on the most relevant scientific results on ethanol reforming processes carried out in inorganic membrane reactors is also reported and discussed.
1. Introduction
In the last few decades, the need to reduce atmospheric pollution and greenhouse gas emissions has led to the development of new technologies for mitigating these phenomena. Proton exchange membrane fuel cells (PEMFCs) are some of the most interesting candidates to cover this need. PEMFCs are fuelled by highly pure hydrogen, which is industrially produced as a hydrogen-rich stream mainly via steam reforming of natural gas;1 it is then further purified to achieve the desired purity. There has been growing attention towards hydrogen production in the scientific community, as reflected by the increasing number of scientific publications present in the open literature, Fig. 1. | ||
| Fig. 1 Number of scientific publications on H2 production vs. year. Scopus database: www.scopus.com. | ||
According to the International Energy Agency, at the moment the world economy is unfortunately devoted to exploiting mainly derived fossil fuels such as oil, coal, methane, etc.2 Owing to the increase of environmental pollution due to emissions of CO2 and other greenhouse gases caused by the burning of fossil fuels, the development of new technologies coupled with the use of renewable sources is a top priority. As previously mentioned, fuel cells could be one of the most promising technologies for applications both in large-scale stationary systems and small-scale portable power supplying devices.3 In particular, PEMFCs transform the chemical energy produced by the electrochemical reaction between hydrogen and oxygen into electrical energy, besides the direct combustion of hydrogen and oxygen gases to produce thermal energy without pollutants. Furthermore, PEMFCs produce more power for a given volume or weight of fuel cell with respect to other kinds of fuel cells, making them compact and lightweight. They commonly operate at a temperature lower than 100 °C, therefore allowing a rapid start-up. These characteristics make PEMFCs top candidates as an alternative technology. As a main drawback, PEMFCs are supplied by pure hydrogen because the anodic Pt catalysts tolerate only a few ppm of CO (<10). As mentioned above, industrial hydrogen is produced via the steam reforming reaction of fossil fuels such as natural gas, gasoline, etc. It is conventionally carried out in fixed bed reactors (FBRs) and produces a reformed hydrogen rich-stream containing other byproduct gases, mainly such as CO, CH4 and CO2. Therefore, to supply a PEMFC, hydrogen needs to be purified by means of further processes like water gas shift (WGS) reaction, pressure swing adsorption (PSA) and/or Pd membrane separation, etc. In this contest, membrane reactor (MR) technology plays an important role as an alternative solution to the conventional systems, offering the opportunity of combining the reforming reaction for producing hydrogen and its separation in the same device.4
Fig. 2 illustrates the number of publications in this field, pointing out how, at a scientific level, many efforts in terms of scientific studies have been made for proposing this technology convincingly on a larger scale.
 | ||
| Fig. 2 Number of scientific papers on H2 production by MR technology vs. year. Scopus database: www.scopus.com. | ||
As a key task, it is possible to summarize the benefits of utilizing inorganic MRs on the conventional reactors5,6 taking into account that they:
• combine chemical reaction and hydrogen separation in only one system, reducing the capital costs;
• allow the conversion enhancement of equilibrium limited reactions;
• reach higher conversions than FBRs (operated at the same MRs conditions) or, in contrast, the same conversion, but operating at milder conditions than FBRs;
• allow both the hydrogen yield and hydrogen selectivity to be improved;
• are able to produce directly a high purity hydrogen stream (in the case of dense Pd-based MRs).
Among the several kinds of inorganic membranes, special attention should be paid to dense palladium-based membranes owing to their full hydrogen perm-selectivity.7 The hydrogen permeation through dense Pd-based membranes follows a transport mechanism called solution-diffusion.8 However, when at temperatures lower than 300 °C a dense palladium membrane is exposed to a hydrogen flow, the embrittlement phenomenon takes place after a few cycles of α ⇆ β transitions of pure palladium.9–11 These transitions do not involve a change of the lattice structure, but only in a lattice dilatation. Alloying palladium with elements such as silver, copper or other metals, Pd–H phases show an increased reticular step and the ability to anticipate the reticular expansion from hydrogen.12
Since the 1960s, hydrogen production by inorganic MRs technology has been deeply studied using both dense Pd-based and porous membranes. Nevertheless, the superiority of palladium membranes overcomes that of all the other materials owing to their high solubility of hydrogen and the aforementioned full perm-selectivity to hydrogen permeation.
The aim of this review is to discuss the progress at a scientific level of coupling inorganic MRs technology with the exploitation of ethanol as a renewable source. This could constitute an important goal in the area of hydrogen production, offering several advantages over the current technology, based on conventional systems and derived fossil fuel sources.
2. Ethanol as a viable bio-source for producing hydrogen through conventional reactors
As previously introduced, the decrease of fossil fuels reserves coupled with the need for reducing the atmospheric pollution caused by their exploitation for power production determined in the last decades the incentive to consider alternative feedstocks and processes. In the viewpoint of hydrogen production via reforming reactions, compared to the most commonly derived fossil feedstocks used for producing hydrogen via reforming reactions, ethanol represents an emerging and alternative source because it is producible renewably from biomass. Furthermore, ethanol constitutes an important bio-source that, compared to other alternative and renewable liquid fuels such as methanol, glycerol, acetic acid, diethylether, etc., seems to be more suitable because of its very low toxicity.13 As a further benefit, when ethanol is processed via the steam reforming reaction, 6 moles of hydrogen are produced per mole of ethanol. Therefore, owing to what stated above, in the last two decades the scientific community involved in the field of hydrogen production and reforming processes has paid particular attention to study the reforming processes using ethanol as a main feedstock. The majority of the research in this area has been realized by studying ethanol reforming reactions by means of conventional reactors and, only in the last decade, this reaction has been also considered by the membranologists, applying the inorganic membrane reactor technology with the intent of improving the ESR performances obtained using conventional systems.Currently, in the specialized literature there is a misleading approach when describing the use of bio-ethanol as a bio-source. Indeed, many authors indicate bio-ethanol as pure ethanol derived from biomass after the distillation and the extraction procedures, whereas bio-ethanol is an aqueous solution containing between 8 and 12 wt% of ethanol and other by-products.14–16 Conventionally, a hydrogen-rich stream can be obtained by converting pure ethanol mixed with steam or directly bio-ethanol via reforming reactions such as autothermal reforming, steam reforming and oxidative steam reforming. In the open literature, the majority of scientific publications deals with performing the ethanol steam reforming (ESR) reaction in FBRs. Fig. 3 illustrates the percentage distribution of the main catalysts used for the ESR reaction in FBRs. It appears quite evident that there is a greater utilization of non-noble metal catalysts, probably because many researches are devoted to maximizing hydrogen production, taking into account the economic advantage of proposing cheaper catalysts than noble-metal catalysts.
 | ||
| Fig. 3 Percentage distribution of the catalyst types used in scientific studies focusing on ESR reaction in FBRs. Database: http://scienceserver.cilea.it. | ||
Via FBRs, several authors investigated ESR performances in terms of ethanol conversion, hydrogen yield and hydrogen selectivity by using various catalysts17–22 and Fig. 4 shows the percentage distribution of the different metal phases in both noble-metal (Fig. 4a) and non-noble metal catalysts (Fig. 4b) mainly utilized in many studies present in the specialized literature.
 | ||
| Fig. 4 Percentage distribution of (a) noble and (b) non-noble metal catalysts in scientific studies focusing on ESR reaction performed in FBRs. Database: http://scienceserver.cilea.it. | ||
In two interesting studies,17,18 the current state of the art for the ESR reaction in FBRs was reviewed, making a comparative analysis on the performances using different catalysts such as Rh-, Ru-, Pd-, Pt-, Ni-, Co- and Cu-based catalysts. The authors pointed out that FBRs performances can vary greatly depending on the catalyst choice and reaction conditions. Nevertheless, Haga et al.19 affirm that a Co–Al2O3 catalyst seems to be the most selective towards ESR reaction. Furthermore, other authors support the role of cobalt as the most effective catalyst for the ESR reaction owing to its high catalytic activity and because it is more cost effective than noble metal-based catalysts like Rh, Pt and Pd.20–22
However, taking into account, as previously mentioned, the use of a bio-ethanol mixture instead of an ethanol/water solution, it is well known that a water excess during the ESR reaction reduces the formation of carbon monoxide in the reformed stream. Therefore, it could be advantageous to supply directly into an FBR a real bio-ethanol mixture (corresponding to a water/ethanol feed molar ratio between 29.0/1–18.7/1), without making any distillation of further ethanol separation/purification process. This approach could be economically relevant, considering that in the purification of ethanol, extracting 99% water presents a high cost owing to the ethanol/water azeotrope. Ni et al.15 studied the bio-ethanol steam reforming (BESR) reaction performed in FBRs, reviewing the crucial role played by the catalyst choice in terms of hydrogen production, concluding that Rh- and Ni-based catalysts are the most commonly used catalysts for the BESR reaction. Moreover, other authors paid attention to both the autothermal and oxidative BESR in FBRs to highlight viable methods for improving the hydrogen yield and lowering the CO content in the reformed streams.23–25
In the following, the stoichiometric reaction for hydrogen production via gas phase ESR reaction (1) is proposed:
| C2H5OH + 3H2O = 2CO2 + 6H2 ΔH°298K = 157.0 kJ mol−1 | (1) |
| C2H5OH = CH4 + CO + H2 ΔH°298K = 33.16 kJ mol−1 | (2) |
| CO + 3H2 = CH4 + H2O ΔH°298K = −206.2 kJ mol−1 | (3) |
| C2H5OH = CH3CHO + H2 (dehydrogenation) ΔH°298K = 17.36 kJ mol−1 | (4) |
| C2H5OH = C2H4 + H2O (dehydration) ΔH°298K = 28.99 kJ mol−1 | (5) |
| C2H5OH + H2O = 2CO + 4H2 (incomplete reforming) ΔH°298K = 239.31 kJ mol−1 | (6) |
| 2C2H5OH = (C2H5)2O + H2O (dehydrative coupling) ΔH°298K = −88.48 kJ mol−1 | (7) |
| CH4 + 2H2O = CO2 + 4H2 (methane steam reforming) ΔH°298K = 206.2 kJ mol−1 | (8) |
| CO + H2O = CO2 + H2 (water gas shift) ΔH°298K = −41.02 kJ mol−1 | (9) |
| 2CO = CO2 + C (Boudouard reaction) ΔH°298K = −172.46 kJ mol−1 | (10) |
Hence, considering that it would be economically desirable to limit the number of operation units for the hydrogen separation/purification, in the membranologists' area many researchers propose to replace the conventional system (reformer + gas cleaning units) with an MR, able to perform both the reaction and the hydrogen separation/purification processes in the same device.27 In the following paragraph, the MR technology will be outlined, paying special attention to the main characteristics of the inorganic membrane reactors useful for hydrogen production.
3. The outlook of inorganic membranes and membrane reactors
According to IUPAC,28 a membrane is defined as a material possessing lateral dimensions much higher than its thickness, through which a mass transfer may take place as a consequence of a driving force occurring as a gradient of concentration, pressure, temperature, electric potential, etc. Membranes are classified depending on their nature, geometry and separation regime.29Classification by nature distinguishes membranes into both biological and synthetic. Biological membranes are easily manufactured, although they present relevant drawbacks related to the limited operating temperature (below 100 °C), pH range, etc.30 Synthetic membranes can be further classified into organic (polymeric) and inorganic (ceramic, metallic) depending on the operative temperature limit. Indeed, polymeric membranes generally operate up to 200 °C, preferentially under 100–150 °C, making them unusable for the reforming process in MRs. Otherwise, inorganic membranes operate above 250 °C and are stable between 300–800 °C. In some cases, they can operate at elevated temperatures (ceramic membranes) over 1000 °C.31 However, they may be further subdivided into porous and metallic. Porous membranes are classified according to their pore diameter as microporous (dp < 2 nm), mesoporous (2 nm < dp < 50 nm) and macroporous (dp > 50 nm).28
Metallic membranes can be categorized into supported and unsupported. There are different separation mechanisms, which are based on the specific properties of the materials,4 and determine the classification of the membranes, respectively, depending on the different mechanisms occurring, such as porous (1), dense (2) and ion-exchange (3):
(1) Knudsen mechanism (representing one of the possible transport mechanisms for porous membranes), separation depending on molecules/membrane surface interactions (e.g. multi-layer diffusion) and/or difference between the average pore diameter and free path of fluid molecules;
(2) solution/diffusion mechanism, separation depending on the difference in diffusivity and solubility of certain substances in the membrane;
(3) electrochemical effect, separation occurring related to the charge difference within the species to be separated.
However, based on the dimension of the membrane pores, Table 1 summarizes the mechanism taking place in porous and dense membranes.
Inorganic membranes are also categorized depending on the structural material as reported below:
• metal membranes,
• ceramic membranes,
Metal membranes are conventionally studied for hydrogen separation from gas mixtures and in the MR area.4,7,9 Palladium and its alloys are the dominant materials for preparing this kind of membrane owing to their high solubility and permeability to hydrogen. Nevertheless, other dense membranes are selectively permeable only to hydrogen such as those based on tantalum, vanadium, nickel and titanium. Moreover, they are less expensive than palladium and its alloy. Unfortunately, metal membranes have the important drawback of surface poisoning, which is more significant when they are very thin.
Ceramic membranes are generally made from aluminium, titanium or silica oxides.4 They are chemically inert and stable at high temperatures and, therefore, particularly useful for food, biotechnology and pharmaceutical applications as well as for gas separation and in MR applications. This kind of membrane is constituted of porous solids containing constricted apertures approaching the molecular dimensions of diffusing gas molecules, which are separated through molecular sieving. At the moment, carbon membranes are identified as promising candidates for gas separation as well as for application in MRs.32 Nevertheless, today, in the open literature, no studies are present on the ESR reaction for hydrogen production via carbon membrane reactors, but they could constitute a next research application taking into account the fact that they were used for hydrogen production via the steam reforming reaction of methanol.32
Zeolites are microporous crystalline alumina-silicates with a uniform pore size and their applications are mainly devoted to uses as catalysts or adsorbents in the form of micron or submicron-sized crystallites embedded in millimetre-sized granules.33 Also in this case, the application of zeolite membranes as membrane reactors in the field of ESR for hydrogen production is today quite scarce. However, the limited number of applications of both carbon and zeolite MRs in the field of ESR reaction to produce hydrogen is probably due to the possibility of losing reactants such as ethanol and steam not yet reacted. This is because of their permeation through the pores of these kinds of membranes, which causes a detrimental effect on the performances of the MRs.
3.1 Membrane reactors
Industrially, several heterogeneous gas–solid catalytic processes (conventionally performed by means of fixed, fluidised or trickle bed reactors) are developed by combining operations at high temperatures and in a chemically harsh environment. Therefore, inorganic membranes are more adequate than polymeric membranes as useful materials for MRs. Generally, the separation capability of an inorganic membrane housed in an MR is advantageously utilized for improving the performances of a catalytic system. Currently, it is possible to distinguish two main generic approaches concerning an MR as a selective product separation (Extractor) and selective reactant addition (Distributor).34 The first MR approach makes possible the in situ removal of one of the reaction products. As an example, when a steam reforming reaction is performed in a conventional reactor, hydrogen yield and CO2 product selectivities are limited by thermodynamics. Using an MR (with an Extractor approach), the selective removal of hydrogen from the reaction side allows the thermodynamic equilibrium restrictions to be overcome. This potentiality is the well-known “shift effect”, through which both higher hydrogen yields and ethanol conversions than those of a conventional reactor can be achieved. Moreover, this effect allows operation at milder reaction conditions in terms of temperature and pressure than the FBRs.35The second MR approach involves the use of the membrane to control the contact within reactants. Both full perm-selective and non-full perm-selective membranes can be useful to distribute the supply of one of the reactants in the reaction zone. For example, in partial oxidation reactions performed in FBRs, on the one hand oxygen rich-feed results in low product selectivity and high reactant conversions. On the other hand, low oxygen content means high product selectivity but lower conversions. Using a membrane for distributive oxygen supply in the reaction zone, both high conversions and product selectivities can be reached.36,37 Furthermore, since the reactants and oxygen are not premixed, mixtures are avoided and the possibility of flame back firing into the feed is considerably lowered. Nevertheless, until today no inorganic membrane reactors were used with a Distributor approach for ESR reaction to produce a hydrogen-rich stream. In particular, the inorganic membranes indicated in this review are devoted to favour the selective permeation of hydrogen as a product of ESR reaction (Extractor approach), allowing as much as possible a high purity of hydrogen in the permeate stream to be achieved.
3.2 The role of palladium membranes in MR technology
Owing to the aforementioned “shift effect” that permits the thermodynamic equilibrium restrictions of an FBR to be overcome, the main characteristic and advantage when utilizing MRs for reforming reactions are related to the improvement of the reaction conversion, hydrogen yield, etc. Depending on the difference in the inorganic membranes housed in the MRs, in the specialized literature it is reported that the MR performances in terms, for example, of conversion are a function of the parameter “H”, defined as the permeation to reaction rate ratio.38 When H = 0, an FBR is represented because obviously no permeation takes place. Otherwise, at H > 0, different types of MRs are considered. In the case of low H values (i.e. low permeation to reaction rate ratio takes place) microporous, dense and mesoporous MRs show the same behaviour.38 At higher H values, it is possible to appreciate the differences among the different kinds of MRs.MRs with a finite separation factor (representing the ratio between the permeability of a certain gas to that of a gas assumed as a blank, generally an inert such as He or N2) achieve an optimum in terms of the permeability/reaction rate. Above the optimum, the reactant loss caused by permeation induces a detrimental effect on the conversion. In contrast, higher separation factors correspond to higher conversions.
MRs showing infinite separation factors, for example for hydrogen, do not give any drawbacks in terms of conversion because loss of reactants does not occur.
However, generally MRs performances could be, at least, the same as a FBR working at the same MRs operating conditions.
In the field of inorganic membranes, dense metallic membranes have attracted great interest among many researchers.39 In particular, dense palladium membranes are most considered owing to their full hydrogen perm-selectivity. However, between 0–700 °C other metals such as niobium, vanadium and tantalum offer higher hydrogen permeability than palladium, although they have a stronger surface resistance to hydrogen transport than palladium. Therefore, dense palladium membranes are more considered, although their commercialization is limited by some drawbacks such as the low hydrogen permeability and high costs.
The hydrogen molecular transport in palladium membranes takes place through a solution/diffusion mechanism and, as indicated by Koros and Fleming,40 it involves six different activated steps: dissociation of molecular hydrogen at the gas/metal interface, adsorption of the atomic hydrogen on the membrane surface, dissolution of atomic hydrogen into the palladium matrix, diffusion of atomic hydrogen through the membrane, re-combination of atomic hydrogen to form hydrogen molecules at the gas/metal interface and desorption of hydrogen molecules. Basile41 identified that each of these steps may control hydrogen permeation through the dense film depending on temperature, pressure, gas mixture composition and membrane thickness. As a result, the hydrogen flux permeating through the membrane can be expressed as reported below:42
| JH2 = PeH2(pnH2,retentate − pnH2,permeate)/δ | (11) |
| JH2,Sieverts−Fick = PeH2(p0.5H2,retentate − p0.5H2,permeate)/δ | (12) |
| JH2 = PeH2(pH2,retentate − pH2,permeate)/δ | (13) |
| PeH2 = Pe0H2exp(−Ea/RT) | (14) |
Therefore, when the Sieverts–Fick law is valid, the hydrogen flux is expressed as indicated by Richardson's eqn (15):
| JH2 = Pe0H2[exp(−Ea/RT)](p0.5H2,retentate − p0.5H2,permeate)/δ | (15) |
 | ||
| Fig. 5 Conventional system (a) and dense Pd-based MR (b) for pure H2 production from reforming reaction. | ||
3.3 Palladium-based MR drawbacks
MRs housing dense Pd-based membranes may present crucial problems that are summarized in the following. Associated to pure palladium membranes, there is the already cited “hydrogen embrittlement” phenomenon, taking place below 300 °C and 2.0 MPa. It can be solved by alloying palladium with other metals, commonly with silver, which displays its electron donating behaviour, being largely similar to that of the hydrogen atom in palladium, making possible competition for the filling of electron holes within the silver and hydrogen.6Another critical drawback for the Pd-based MRs is that the palladium surface can be contaminated by the presence of hydrogen sulfide, SO2, Hg vapour, thiophene, arsenic, unsaturated hydrocarbons, or chlorine carbon from organic materials, etc. More in detail, the poisoning through exposure to hydrogen sulfide negatively affects Pd-coated membranes, which can be destroyed rapidly and the poisoning effects would be irreversible.47,48 However, by performing ESR in Pd-based MRs, H2S, arsenic, SO2, thiophene, etc. are not formed during the reaction. Typically, in certain conditions ethylene, ethane and acetaldehyde are formed as byproducts besides H2, CO, CO2 and CH4. These byproducts are carbon coke precursors. Coke affects negatively both the hydrogen permeation (covering the membrane surface and lowering the hydrogen permeating flux) and the catalyst (lowering its activity), reducing the overall performances of the ESR MR. Generally, using catalysts with a non-acidic support and working in a no carbon region (i.e. high water to ethanol feed ratio), coke formation and its effects on the Pd-based membranes and ESR process are avoided.26
The presence of CO in an MR could cause the decrease of the hydrogen permeation performances of the membrane, because the adsorbed CO displaces the adsorbed hydrogen, blocking the hydrogen adsorption sites. This reduction is more significant at low temperature (below 150 °C) or at high CO feed concentration.49
The poisoning effect of water vapour on hydrogen permeability can be more consistent than the CO effect. It affects the water vapour dissociation/recombinative desorption, which contaminates the palladium surface with adsorbed oxygen.50
Finally, when an MR allocates thin palladium membranes in contact with coke at high temperature, the hydrogen permeation characteristics are lowered. This poisoning is caused by the carbon atoms that penetrate into the palladium lattice, provoking membrane failure owing to the expansion of the palladium lattice.51
Thin palladium or palladium alloy laminated membranes avoid the formation of oxides on the metallic surfaces, lowering the hydrogen adsorption activation energy and, as a consequence, emphasizing the hydrogen permeation characteristics. Generally, palladium alloys have some advantages over pure palladium membranes. Besides avoiding the embrittlement phenomenon, certain palladium alloys improve the membrane chemical resistance. As an example, Pd–Cu and Pd–Au alloyed membranes are more resistant to H2S poisoning.54
3.4 Brief history on palladium-based MRs applied to hydrogen production
In 1866, Graham was the first to apply palladium membranes for separating hydrogen from gases mixtures.55 In 1915, Snelling patented a system constituted of palladium or platinum tubes for the hydrogen removal from a reactor, packed with a granular catalyst for dehydrogenation reactions.52 In 1964, Gryaznov studied the application of a tubular and full perm-selective palladium-based membrane reactor for hydrogen consumption, where the palladium acts also as a catalyst.56 In the same year, the first commercial application of a dense Pd–Ag membrane was registered by Johnson Matthey for purifying a hydrogen rich-stream.57 Johnson Matthey also developed a Pd-based MR for producing hydrogen from a methanol/water mixture. This system was used on a small scale by the British Antarctic Survey in 1975.58,59 Successively, Tokyo Gas Company Ltd. realized the first pilot-scale Pd-based MR for direct ultra-pure hydrogen production. Nowadays, palladium-based MR technology is mainly studied and developed at lab scale and is an alternative solution to conventional systems for pure hydrogen production for supplying PEMFCs. In detail, Table 2 summarizes some of the chemical reactions performed by using Pd-based MRs technology with the purpose of producing pure hydrogen.| Reaction type | Membrane | Material |
|---|---|---|
| Decomposition of ammonia | Dense | Pd |
| Dehydrogenation of cyclohexane to benzene | Dense | Pd/Ag |
| Dehydrogenation of ethylbenzene to styrene | Porous | Pd |
| Dehydrogenation of ethane to ethylene | Dense | Pd/Ag |
| Dehydrogenation of isopropyl alcohol to acetone | Dense | Pd |
| Dehydrogenation of water-gas shift reaction |
Dense
Porous |
Pd, Pd/Ag Pd |
| Dehydrogenation of n-heptane to toluene + benzene | Dense | Pd/Rh |
| Dehydrogenation of butane to butadiene | Dense | Pd |
| Dehydrogenation of 1,2-cyclohexanediol | Dense | Pd/Cu |
| Dry reforming of methane | Dense | Pd-alloy |
| Methane conversion into hydrogen rich-gas | Porous | Pd-alloy |
| Octane reforming | Dense | Pd and Pd-alloy |
| Partial oxidation of methane | Porous | Pd-alloy |
| Steam reforming of ethanol | Dense | Pd and Pd-alloy |
| Steam reforming of methane | Dense | Pd-alloy |
| Steam reforming of glycerol | Dense | Pd-alloy |
| Steam reforming of acetic acid | Dense | Pd-alloy |
| Steam reforming of methanol | Dense | Pd/Pd-alloy |
| Water gas shift | Dense–porous | P/Pd-alloy–Silica/etc. |
At the moment, different processes are based on hydrogen production via reforming reaction of biofuels, such as methanol, glycerol, ethanol, biogas, etc. and there are a consistent number of publications found in the open literature concerning the application of Pd-based MR technology.
4. ESR, oxidative ESR and ethanol dehydrogenation reactions in MRs
As extensively introduced in the beginning of this review, in the last few years MR technology has been applied with the intent to produce hydrogen based on the exploitation of ethanol as a renewable source. Fig. 6 illustrates the number of scientific studies per year dealing with hydrogen production via the ESR reaction combined to MR technology with respect to the total number of publications in this field, comprising both FBR and MR applications.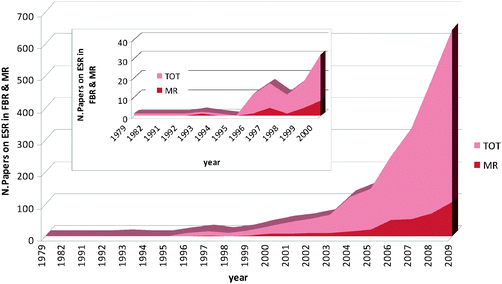 | ||
| Fig. 6 Number of scientific publications vs. year on ESR reaction in both FBR and MR. | ||
Furthermore, Fig. 7 highlights the percentage distribution of the most used catalysts for ethanol reforming reactions in MRs. Ni- and Ru-based catalysts are preferentially used, probably because they are less expensive than other catalysts.
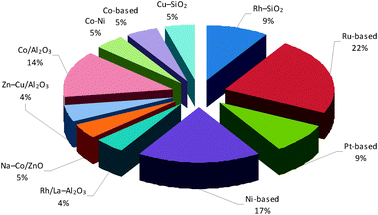 | ||
| Fig. 7 Percentage distribution of the most used catalysts in ethanol reforming reactions performed in MRs. | ||
However, the main aim of the scientists involved in this field is oriented to emphasizing the role of the membrane by analyzing the performances of the reaction system in terms of ethanol conversion, hydrogen yield (defined as the ratio within hydrogen produced during the reaction and that theoretically producible from the stoichiometry of the reaction) and hydrogen recovery (hydrogen collected in the permeate side vs. the total hydrogen produced during the reaction). Table 3 summarizes the most significant performances of several scientific studies present in the specialized literature.
| Authors | H2O/EtOH | O2/EtOH | T/°C | p/bar | Catalyst | H2 recovery [%] | H2 yield [%] | Membrane type |
|---|---|---|---|---|---|---|---|---|
| a High purity H2 yield = H2 permeate/6·EtOHfeed. b Calculated. c At H2O/EtOH = 9/1. | ||||||||
| Tosti et al.60 | 13/1 | — | 450 | 2.0 | Ru–Al2O3 | — | 80 | Dense self-supported Pd–Ag |
| Tosti et al.61 | 13/1 | — | 450 | 2.0 | Pt–Al2O3 | — | 60 | Dense self-supported Pd–Ag |
| Iulianelli and Basile62 | 18.7/1 | — | 400 | 1.5 | Co–Al2O3 | 30 | 18a | Dense self-supported Pd–Ag |
| Iulianelli et al.63 | 18.7/1 | — | 400 | 3.0 | Co–Al2O3 | 90 | 53a | Dense self-supported Pd–Ag |
| Basile et al.64 | 3/1 | — | 400 | 1.3 | Ru–Al2O3 | 22 | 56c | Dense self-supported Pd–Ag |
| Basile et al.65 | 11/1 | — | 500 | 3.6 | Ru–Al2O3 | 25 | — | Dense self-supported Pd–Ag |
| Papadias et al.66 | 12/1 | — | 700 | 6.9 | Rh–LaAl2O3 | — | 65 | Supported Pd–Ag |
| Gallucci et al.67 | 4.5/1 | — | 600 | 1.3 | Ru–Al2O3 | — | 35b | Pd–Ag supported onto TiO2-Al2O3 |
| Lin and Chang68 | — | — | 450 | 10.0 | Cu–Zn/Al2O3 | 80 | — | Pd–Ag supported onto PSS |
| Lin et al.69 | 1/1 | 0.2/1 | 450 | 9.0 | — | — | 40 | Pd–Ag supported onto PSS |
| Iulianelli et al.70 | 11/1 | 0.6/1 | 400 | 2.5 | Ru–Al2O3 | 30 | 18 | Dense self-supported Pd–Ag |
| Amandusson et al.71 | — | — | 350 | 1.2 | Cu–Zn/Al2O3 | — | 40b | Laminated Pd–Ag |
| Lim et al.72 | 3/1 | — | 350 | — | Co–ZnO | — | 23 | Silica–alumina composite |
| Yu et al.73 | 5/1 | — | 600 | — | Rh–SiO2 | 85 | 50 | SiO2 supported onto PSS |
These experimental data are qualitatively summarized with the scope of illustrating the various performances achievable using different types of MR. In detail, the table shows the operating conditions of the MRs exercised as well as the type of inorganic membranes housed inside. As a general consideration, it appears quite evident that Pd-based membranes are the most used both in terms of laminated and supported membranes. With the aim of improving both conversion and hydrogen yield, Tosti et al.60,61 and Iulianelli et al.62,63 analyzed the ESR reaction paying particular attention to the influence of high steam to ethanol feed molar ratio and working at relatively low reaction temperature (400–450 °C) using a self-supported Pd–Ag membrane reactor. The membranes used in these studies are produced both by cold-rolling and welding technique.42,44 They are around 50 μm thick and joined to two stainless steel ends useful for the membrane housing inside the MR. In all cases, the Pd–Ag membranes are full perm-selective to hydrogen permeation. At 13/1 of steam to ethanol feed ratio and at 2.0 bar, Tosti et al. achieved ∼80% of hydrogen yield using a Ru–Al2O3 catalyst instead of around 60% by means of a Pt–Al2O3 catalyst, demonstrating that the latter catalyst is more active for achieving better hydrogen yields. In the meanwhile, Tosti et al. confirmed that higher steam to ethanol feed ratios give better performance; indeed, at H2O/EtOH = 3/1 and the same operating conditions the hydrogen yield is quite low, around 20%.64
On the contrary, Iulianelli et al.62,63 performed the ESR reaction at quite high steam to ethanol feed ratio (18.7/1), 400 °C catalyzed by Co–Al2O3, but also analyzing the effect of an increase of reaction pressure. They obtained a hydrogen yield ranging from ∼20% to more than 50% as well as a COx-free hydrogen recovery (defined as the COx-free hydrogen collected in the permeate side on the total hydrogen produced during the reaction) from 30% to around 90%, by varying the reaction pressure between 1.5–3.0 bar and keeping constant the permeate pressure at 1.0 bar. These results are due to the effect of higher pressures, which favour the aforementioned “shift effect” owing to an increase of the hydrogen permeation driving force (see eqn (15)). This involves a greater removal of hydrogen from the reaction side to the permeate side as a COx-free hydrogen stream, improving both conversion and hydrogen yield as well as the COx-free hydrogen recovery.
With the same intent of the authors reported above, Basile et al.64,65 also used a self-supported Pd–Ag MR42 for studying the ESR reaction to produce hydrogen. In one case,64 Basile et al. obtained around 20% of high purity hydrogen recovery working at 1.3 bar of reaction pressure, 400 °C and stoichiometric H2O/EtOH feed ratio. In the other case,65 although the experimental campaign was performed at higher temperature and pressure, both positive for improving the hydrogen permeation through the membrane, only around 25% hydrogen recovery as a COx-free stream was reached. This was probably due to the influence of a higher H2O/EtOH feed ratio (11/1), which diluted the retentate stream lowering the hydrogen partial pressure on this side, globally reducing the hydrogen permeation driving force and, as a consequence, the hydrogen recovery.
Papadias et al.66 investigated ESR reaction at a steam to carbon molar ratio varying between 3/1 and 12/1, using a supported Pd–Ag MR packed with an Rh–LaAl2O3 catalyst. At 700 °C and 6.9 bar, this MR gives around 65% hydrogen yield, while at 69 bar it drops to 42% owing to an increase of methane yield. In this case, by using a supported Pd-based membrane, the effect of an increase of pressure on the hydrogen permeation is less evident than using self-supported Pd-based membranes. Therefore, the Pd-based supported membrane not being full perm-selective to hydrogen, the aforementioned shift effect is less effective. Furthermore, higher reaction pressure gives a detrimental effect on ESR reaction by thermodynamics (ESR proceeds with an increase of moles number) and the conversion as well as the hydrogen yield is lowered.
Other authors investigated ESR reaction using supported Pd-based MRs with the purpose to reduce the presence of palladium in the membrane and, as a consequence, the cost of this alternative technology. For example, Gallucci et al.67 performed ESR reaction at medium water/ethanol feed ratio (4.5/1), using a supported Pd–Ag MR packed with a Ru–Al2O3 catalyst. The membrane is constituted of an asymmetric commercial TiO2–Al2O3 support onto which a Pd–Ag layer is deposited.12,31,34,42 As a best result of their work, at 600 °C and 1.3 bar, the authors obtained 35% hydrogen yield.
In another work, a membrane constituted by a Pd–Ag layer deposited onto a porous stainless steel (PSS) support was used by Lin and Chang 68 to perform ESR reaction in the MR. The membrane was prepared by electroless plating42,83 and the experimental tests were carried out at 450 °C, in the pressure range of 2.0–11.0 bar, using a Cu–Zn/Al2O3 catalyst. A sweep-gas was used for improving the flux of permeating hydrogen and both higher temperatures and pressures advantageously affect the hydrogen permeation driving force, favouring greater hydrogen recovery. As reported in Table 3, the best results were reached at 10.0 bar, with a hydrogen recovery of 80%. However, Lin et al. also studied the effect of oxygen addition during hydrogen production via the ESR reaction,69 finding that, by adding oxygen, CO2 selectivity is increased while CO selectivity remains almost the same. Hydrogen production is improved at higher pressures owing to the effect of a higher driving force, even though an increased oxygen input slightly lowers the hydrogen production. This is due to the fast oxidation reaction of hydrogen, which indirectly reduces the hydrogen permeation through the membrane.
Studying the oxidative ESR reaction using a self-supported Pd–Ag MR, Iulianelli et al.70 also confirmed that, by increasing the oxygen content on the reaction side (O2 to ethanol feed ratio > 1.3/1), both hydrogen yield and hydrogen recovery are lowered owing to the fast oxidation reaction of the hydrogen produced during the reaction. At O2 to ethanol feed ratio equal to 0.6/1, 400 °C and 2.5 bar, ∼18% hydrogen yield and 30% hydrogen recovery were achieved.
Oxygen addition to ethanol dehydrogenation reaction was studied also by Amandusson et al.71 using a laminated 25 μm PdAg membrane foil located within two reaction chambers. They performed the ethanol dehydrogenation at 350 °C and, as discussed above, observed that hydrogen is decreased with increasing the oxygen content in the feed. These authors pointed out that there is a delicate balance in oxygen/ethanol supply. Indeed, on the one hand at too low an oxygen content the carbonaceous layer produced during the dehydrogenation reaction of ethanol is not fully oxidised and the hydrogen permeation is lowered; on the other hand, at too high an oxygen content, hydrogen oxidation takes place, decreasing the hydrogen permeating through the membrane. In detail, the authors observed that when oxygen is not supplied the carbonaceous layer does not allow hydrogen permeation. At oxygen/ethanol feed ratio > 1, hydrogen permeation is lowered by the oxidation reaction of hydrogen, while at oxygen/ethanol feed ratio < 0.7, the carbonaceous layer is not fully oxidised, but a constant hydrogen permeation (and recovery) can be achieved. However, the best hydrogen yield achieved by the authors was around 40%.
Other researchers approached the ESR reaction using non-palladium-based MR with the scope of reducing the cost. For example, Lim et al.72 performed this reaction at 350 °C in a silica–alumina composite MR with a water/ethanol feed ratio ranging between 3/1 and 13/1. The membrane was produced by a chemical vapour deposition technique42,84 and it is not fully perm-selective to hydrogen permeation. Specifically, this membrane shows H2/CH4 gas selectivity equal to 350 at 350 °C with hydrogen permeance in the order of 10−8 mol m−2 s−1 Pa−1. At water/ethanol feed ratio equal to 3/1, more than 20% hydrogen yield was achieved. The authors concluded that the yield of hydrogen is improved 10–20% more than that of an FBR working at the same MR operating conditions, owing to the effect of the membrane on the reaction system.
An important contribution to hydrogen production from ESR reaction via MR technology was made by Yu et al.73 They used a non-palladium-based MR for collecting hydrogen with very low CO content (<1%). The membrane was synthesized as SiO2 supported onto PSS. The reaction is conducted on one side of the MR by means of an Ru–SiO2 catalyst and the stream permeated through the membrane is rich in hydrogen and CO, which is further converted via water gas shift reaction to decrease the CO content under 1% in the permeate stream. As shown in the table, at water/ethanol feed ratio equal to 5/1 and 600 °C, the hydrogen recovery and the hydrogen yield were around 85% and 50%, respectively.
As a general consideration, the data summarized in Table 3 indicate that the experimental results may vary greatly depending on the operating conditions adopted as well as the typology of inorganic membrane utilized. Therefore, a direct quantitative comparison among them is not possible, but only from a qualitative point of view.
Another important parameter taken into account by the researchers is the conversion of ethanol that describes the effect of the inorganic membrane on the process. Even in this case, it is not possible to compare directly the experimental results of the several studies present in the specialized literature because they are obtained at different operating conditions. Therefore, Fig. 8 summarizes qualitatively the most important results in terms of conversion against temperature on ethanol reforming reactions performed in MRs. Furthermore, some of the most significant results using FBRs packed with the most active catalysts for this reaction (Rh, Ni, Pt, Pd and Co-based) are reported for a qualitative comparison. Firstly, taking into account that ESR is an endothermic reaction, the ethanol conversion for both MRs and FBRs is favoured by a temperature increase. In the case of Pd-based MRs, the higher the temperature the higher the hydrogen permeating flux, which allows the equilibrium of the reaction to be shifted towards the reaction products, globally favouring a further conversion improvement. However, Fig. 8 points out an important aspect; by using FBR technology, ethanol conversions higher than 80% are achieved at T > 600 °C. Only in the case of FBRs packed with Co-based catalysts, the conversions exceed 80% at milder temperatures (450–500 °C). Otherwise, high conversions are reached by MR technology in the range of 300–400 °C, not depending on the catalyst used during the reaction.
 | ||
| Fig. 8 Ethanol conversion vs. reaction temperature for several MRs and FBRs studies present in the open literature. | ||
This ability makes possible energy saving for the MRs against the FBRs. Furthermore, full perm-selective Pd-based MRs seem to be an important choice in this context because they are able to perform the ESR reaction with complete conversion achieved at middle temperature (350–400 °C) with the further advantage of producing a high purity hydrogen stream to supply, for example, PEM fuel cells.
5. Modelling studies on ESR in MRs
A few studies can be found in the open literature regarding the modelling approach to ESR reaction to produce hydrogen via MR technology. In detail, Tosti et al.79 validated a kinetic expression applied to the specific case of an MR consisting of a dense self-supported Pd–Ag membrane tube. The modelling of the ESR reaction involves a finite element method where a simplified power law equation is considered for the reaction rate. The authors performed experimental tests on the Pd–Ag MR packed with different catalysts such as Ru-, Ni- and Pt-based to give the coefficients of the kinetic law. The study focuses particularly on a Damkohler–Peclet (DaPe) analysis useful for describing the behaviour of the Pd–Ag MR in terms of reaction and permeation kinetics. They investigated the influence of the DaPe number on the hydrogen yield from a modelling point of view, concluding that DaPe > 1 allows the permeation kinetics to be increased taking advantage in terms of hydrogen yield. Basile and co-workers80 used a theoretical model for simulating the ESR in a dense Pd–Ag MR, analyzing the influence of the different modes of sweep-gas supply with respect to the reactants (co-current and counter-current) at various operating conditions. They concluded that the counter-current mode is more efficient than the co-current for obtaining both higher hydrogen recovery as well as ethanol conversion. The better efficiency of the counter-current mode for recovering hydrogen and, as a consequence, improving the conversion owing to the aforementioned shift effect may account for the different hydrogen partial pressure distribution along the MR with respect to the co-current mode. Globally, this is reflected in a greater hydrogen permeation driving force that emphasizes the MR performances. In another study,81 the same authors compared the performances of a Pd–Ag MR with an FBR working at the same operating conditions, confirming that this kind of MR makes possible better ethanol conversions than the FBR with the further advantage of collecting a COx-free hydrogen stream.An energy efficiency analysis of both conventional and membrane processes for hydrogen production from ESR reaction was realized by Manzolini and Tosti.82 They applied Pd-based membrane fuel processors and PEM fuel cells for producing hydrogen from bio-ethanol. As a main result, these new systems allow the utilization of the renewable energy content of the bio-fuel at about 43% instead of 30% of pure bio-ethanol, which is widely diffused in conventional reciprocating engines. Otherwise, as a main drawback the authors pointed out that the membrane reactor/PEM fuel cell systems need a fuel processing unit to transfer the energy content of the ethanol into a hydrogen-rich gas stream, to be further converted into electricity in the fuel cell driving an electrical engine. This study compares three different fuel process systems consisting of: (a) a conventional high temperature steam reforming reactor and (b) two membrane processes where a water gas shift and a steam reforming MR are used, respectively. Dense self-supported Pd–Ag membrane tubes with a wall thickness of 50 μm are housed in the MR. Owing to their high hydrogen permeability and full hydrogen perm-selectivity, this system may directly supply the hydrogen produced into the PEM anode. According to the results of this study, the processes based on the water gas shift and the steam reformer MRs yield net electrical efficiencies as high as 39% and 41%, respectively. These results are around 7% and 9% higher than those of a conventional reformer. Furthermore, this study focused the steam to carbon (S/C) ratio as a critical parameter significantly affecting the system efficiency. At low S/C ratio, the energy efficiency increases greatly, even though the carbon deposition on the catalyst with its consequent deactivation could take place, globally reducing the system reliability and availability. In conclusion, relating to a simplified field-to-wheel analysis, this modelling study points out that the energy efficiency increases up to 50%, when the MRs are coupled to PEM fuel cells driving electrical engines with respect to the use of conventional reciprocating engines, particularly because the innovative membrane systems are capable of processing the humid bio-ethanol.
6. Future trends in ethanol reforming reactions performed in MRs
This review deals with MR technology combining two distinct sciences such as catalysis and inorganic membranes. In the scientific community, the difficulty is to establish which of them has more relevance in MR development. However, it is reasonable to understand that the exploitation of renewable sources is certainly a key factor in the route of hydrogen production via reforming reactions by MR technology, particularly to improve the hydrogen production units, e.g. fulfilling the requirements for the integration of fuel processors with PEM fuel cells. One of the issues that is rarely addressed in the open literature concerns this concept: while natural gas (and more in general derived fossil fuels) is essentially utilized for stationary applications, it is expected that alcohols such as ethanol or other more exotic fuels such as ammonia will have a major role in the future portable applications. A problem still existing in the area of ESR, oxidative ESR reactions and so on, performed in MRs is the impossibility of comparing the performances (in terms of conversion, hydrogen yield and hydrogen recovery) obtained in many scientific works, owing to the different operating conditions used in the experimental tests. Furthermore, although there are a number of studies on the ESR reaction performed in MRs cited in the literature, none of them focuses on cost analysis of these devices. This could account for the fact that MR technology still presents some deficiencies to be overcome before its implementation at larger scales. Future researches should be devoted to the preparation of defect-free inorganic membranes able to work for long periods at high temperatures and pressures as well as to develop membrane systems not based on palladium. Another important issue is the effect of impurities on metallic membranes. Generally, when using MRs housing hydrogen fully perm-selective membranes (i.e. dense Pd and Pd-alloy based membranes), hydrogen permeation may show some deviations from Sieverts' law owing to contaminant effects on the membrane surface. Once these problems are solved (i.e. synthesis of defect-free, stable and impurity-resistant membranes, no- and/or low-palladium content membranes development), the benefits resulting from the use of MRs rather than FBRs for performing ethanol reforming reactions at an industrial scale could become more realistic. Finally, better economic analyses based on the combination of ethanol as a renewable source and MR technology would be necessary for stimulating improvements in this research field.7. Conclusions
Hydrogen will play an important role as an energy carrier in future energy systems such as PEMFCs. Therefore, many industrial companies and the scientific community are paying a great deal of attention to produce hydrogen in a more technically, environmentally and economically attractive way, based on the idea of sustainable development. From a techno-economic point of view, today steam reforming natural gas and the exploitation of derived fossil fuels is currently the most favourable route for hydrogen production. Furthermore, hydrogen upgrading in refinery applications can be achieved by using PSA, membrane, or cryogenic separation processes. Inorganic membranes are excellent candidates for hydrogen purification, especially when incorporated into an MR, combining the reaction/separation process in a single unit. Meanwhile, ethanol as a bio-derived source is becoming a relevant candidate for producing hydrogen via reforming reaction and, combined to inorganic MR technology, could constitute an important goal in this area. In this review, the state of the art on ethanol reforming processes performed in MRs has been presented, paying particular attention to the effect of the different typology of inorganic membranes on the reaction performances in terms of hydrogen yield, hydrogen recovery and ethanol conversion. Pd-based MRs seem to be the dominant applications in this field, particularly because of the hydrogen perm-selectivity characteristics of Pd-based membranes.In summary, the future perspectives on performing the ethanol reforming in inorganic MRs are listed:
• development of low or non-palladium-based membranes useful for ethanol reforming in MRs. This task could represent a novel step in the viewpoint of MR cost reduction.
• Scale-up of ethanol reforming MRs is one of the most important issues. Developing low-cost, defect-free, effective membranes could be a chance for realistic application of MRs on an industrial scale.
• Future researches should be aimed at the improvement of membrane mechanical resistance during the ethanol reaction processes, at both relatively high reaction temperatures and pressures.
• Great attention should be paid to evaluating the effective balance between benefits and drawbacks of applying MR technology to the ethanol reforming process to produce hydrogen. Specifically, research should be devoted to studying the increase of operating or capital costs related to the use of relatively high reaction pressure and temperature in order to improve the hydrogen permeation driving force in hydrogen fully perm-selective MRs.
• More experimental studies on the lifetime of MRs utilized for carrying out the ethanol reforming processes should be undertaken in order to validate them as a possible alternative to the conventional systems at larger scales.
Acknowledgements
The authors would like to thank Mrs Simona Liguori of the Engineering Modelling Department of the University of Calabria (Italy) for her support to this review.References
- A. Heinzel, B. Vogel and P. Hiugner, Reforming of natural gas—hydrogen generation for small scale stationary fuel cell systems, J. Power Sources, 2002, 105, 202–207 CrossRef CAS.
- P. Moriarty and D. Honnery, Intermittent renewable energy: The only future source of hydrogen?, Int. J. Hydrogen Energy, 2007, 32, 1616–1624 CrossRef CAS.
- J. Wee, Applications of proton exchange membrane fuel cell systems, Renew. Sustainable Energy Rev., 2007, 11, 1720–1738 Search PubMed.
- M. M. Mulder, Basic Principles of Membrane Technology, Kluwer Academic, Dordrecht, 1996, ISBN: 0-7923-4247-X (HB) Search PubMed.
- G. Saracco and V. Specchia, Catalytic inorganic membrane reactors: present experience and future opportunities, Catal. Rev., 1994, 36, 305–384 CrossRef CAS.
- J. Shu, B. P. A. Grandjean, A. Van Neste and S. Kaliaguine, Catalytic palladium-based membrane reactors: a review, Can. J. Chem. Eng., 1991, 69, 1036–1060 CAS.
- G. Q. Lu, J. C. Diniz de Costa, M. Duke, S. Giessler, R. Socolow, R. H. Williams and T. Kreutz, Inorganic membranes for hydrogen production and purification: A critical review and perspective, J. Colloid Interface Sci., 2007, 314, 589–603 CrossRef CAS.
- T. L. Ward and T. Dao, Model of hydrogen permeation behaviour in palladium membranes, J. Membr. Sci., 1999, 153, 211–231 CrossRef CAS.
- G. J. Grashoff, C. E. Pilkington and C. W. Corti, The purification of hydrogen—A review of the technology emphasizing the current status of palladium membrane diffusion, Platinum Met. Rev., 1983, 27, 157–168 CAS.
- H. P. Hsieh, Inorganic membrane reactors—A review, AIChE Symp. Ser., 1989, 85, 53–67 CAS.
- F. A. Lewis, K. Kandasamy and B. Baranowski, The “Uphill” diffusion of hydrogen—Strain-gradient-induced effects in palladium alloy membranes, Platinum Met. Rev., 1988, 32, 22–26 CAS.
- K. Hou and R. Hughes, Preparation of thin and highly stable Pd/Ag composite membranes and simulative analysis of transfer resistance for hydrogen separation, J. Membr. Sci., 2003, 214, 43–55 CrossRef CAS.
- G. Maggio, S. Freni and S. Cavallaro, Light alcohols/methane fuelled molten carbonate fuel cells: a comparative study, J. Power Sources, 1998, 74, 17–23 CrossRef CAS.
- H. Song, L. Zhang, R. B. Watson, D. Braden and U. S. Ozkan, Investigation of bio-ethanol steam reforming over cobalt-based catalysts, Catal. Today, 2007, 129, 346–354 CrossRef CAS.
- M. Ni, D. Y. C. Leung and M. K. H. Leung, A review on reforming bio-ethanol for hydrogen production, Int. J. Hydrogen Energy, 2007, 32, 3238–3247 CrossRef CAS.
- M. Pfeffer, W. Wukovits, G. Beckmann and A. Friedl, Analysis and decrease of the energy demand of bioethanol-production by process integration, Appl. Therm. Eng., 2007, 27, 2657–2664 CrossRef.
- A. Haryanto, S. Fernando, N. Murali and S. Adhikari, Current status of hydrogen production techniques by steam reforming of ethanol: a review, Energy Fuels, 2005, 19, 2098–2106 CrossRef CAS.
- J. P. Breen, R. Burch and H. M. Coleman, Metal-catalysed steam-reforming of ethanol in the production of hydrogen for fuel cell applications, Appl. Catal., B, 2002, 39, 65–74 CrossRef CAS.
- F. Haga, T. Nakajima, K. Yamashita and S. Mishima, Effect of crystallite size on the catalysis of alumina-supported cobalt catalyst for steam reforming of ethanol, React. Kinet. Catal. Lett., 1998, 63, 253–259 CrossRef CAS.
- J. Llorca, N. Homs, J. Sales and P. R. de la Piscina, Efficient production of hydrogen over supported cobalt catalysts from ethanol steam reforming, J. Catal., 2002, 209, 306–317 CrossRef CAS.
- A. Kaddouri and C. Mazzocchia, A study of the influence of the synthesis conditions upon the catalytic properties of Co/SiO2 or Co/Al2O3 catalysts used for ethanol steam reforming, Catal. Commun., 2004, 5, 339–345 CrossRef CAS.
- M. S. Batista, R. K. S. Santos, E. M. Assaf, J. M. Assaf and E. A. Ticianelli, Characterization of the activity and stability of supported cobalt catalysts for the steam reforming of ethanol, J. Power Sources, 2003, 124, 99–103 CrossRef CAS.
- L. Dolgykh, I. Stolyarchuk, I. Denyega and P. Strizhak, The use of industrial dehydrogenation catalyst for hydrogen production from bioethanol, Int. J. Hydrogen Energy, 2006, 31, 1607–1610 CrossRef CAS.
- F. Frusteri, S. Freni, V. Chiodo, S. Donato, G. Bonura and S. Cavallaro, Steam and auto-thermal reforming of bio-ethanol over MgO and CeO2 Ni supported catalysts, Int. J. Hydrogen Energy, 2006, 31, 2193–2199 CrossRef CAS.
- M. Benito, J. L. Sanz, R. Isabel, R. Padilla, R. Arjona and L. Daza, Bio-ethanol steam reforming: Insights on the mechanism for hydrogen production, J. Power Sources, 2005, 151, 11–17 CrossRef CAS.
- V. Mas, R. Kipreos, N. Amadeo and M. Laborde, Thermodynamic analysis of ethanol/water system with the stoichiometric method, Int. J. Hydrogen Energy, 2006, 31, 21–28 CrossRef CAS.
- J. N. Keuler and L. Lorenzen, Comparing and modeling the dehydrogenation of ethanol in a plug-flow reactor and a Pd–Ag membrane reactor, Ind. Eng. Chem. Res., 2002, 41, 1960–1966 CrossRef CAS.
- W. J. Koros, Y. H. Ma and T. Shimidzu, Terminology for membranes and membrane processes, J. Membr. Sci., 1996, 120, i CrossRef.
- K. C. Khulbe, C. Y. Feng and T. Matsuura, Synthetic polymeric membranes, characterization by atomic force microscopy, Springer, 2007, ISBN:3540739939 Search PubMed.
- Y. Xia, Y. Lu, K. Kamata, B. Gates and Y. Yin, Macroporous materials containing three-dimensionally periodic structures, Chemistry of Nanostructured Materials, ed. P. Yang, World Scientific, 2003, pp. 69–100 Search PubMed.
- H. M. Van Veen, M. Bracht, E. Hamoen and P. T. Alderliesten, Feasibility of the application of porous inorganic gas separation membranes in some large-scale chemical processes, Fundamentals of inorganic membrane science and technology, ed. A. J. Burggraaf and L. Cot, Elsevier, 1996, vol. 14, pp. 641–681 Search PubMed.
- K. Briceño, R. G. Valls and D. Montanè, State of the art of carbon molecular sieves supported on tubular ceramics for gas separation applications, Asia-Pac. J. Chem. Eng., 2010, 5, 169–178 Search PubMed.
- J. Cejka, H. Van Bekkum, A. Corma and F. Schuth, Introduction to zeolite science and practice, studies in surface science and catalysis, Elsevier, 2007, ISBN: 978-0-444-53063-9 Search PubMed.
- A. Julbe, D. Farrusseng and C. Guizard, Porous ceramic membranes for catalytic reactors—overview and new ideas, J. Membr. Sci., 2001, 181, 3–20 CrossRef CAS.
- J. Zaman and A. Chakma, Inorganic membrane reactors, J. Membr. Sci., 1994, 92, 1–28 CrossRef CAS.
- G. Saracco, H. W. J. P. Neomagus, G. F. Versteeg and W. P. M. van Swaaij, High-temperature membrane reactors: potential and problems, Chem. Eng. Sci., 1999, 54, 1997–2017 CrossRef CAS.
- J. Coronas and J. Santamaria, Catalytic reactors based on porous ceramic membranes, Catal. Today, 1999, 51, 377–389 CrossRef CAS.
- K. Keizer, V. T. Zaspalis, R. S. A. De Lange, M. P. Harold and A. J. Burggraaf, Membrane reactors for partial oxidation and dehydrogenation reactions, Membr. Proc. Sep. Pur., ed. J. G. Crespo and Boddeker, Netherlands, 1994, pp. 415–429 Search PubMed.
- S. Adhikari and S. Fernand, Hydrogen membrane separation techniques, Ind. Eng. Chem. Res., 2006, 45, 875–881 CrossRef CAS.
- W. J. Koros and G. K. Fleming, Membrane-based gas separation, J. Membr. Sci., 1993, 83, 1–80 CrossRef CAS.
- A. Basile, Hydrogen production using Pd-based membrane reactors for fuel cells, Top. Catal., 2008, 51, 107–122 CrossRef CAS.
- F. Gallucci, S. Tosti and A. Basile, Synthesis, Characterization and applications of palladium membranes, Membrane science and technology, ed. R. Malada and M. Menendez, Elsevier, 2008, ch. 8, pp. 255–323 Search PubMed.
- M. D. Dolan, N. C. Dave, A. Y. Ilyushechkin, L. D. Morpeth and K. G. McLennan, Composition and operation of hydrogen-selective amorphous alloy membranes, J. Membr. Sci., 2006, 285, 30–55 CrossRef CAS.
- S. Tosti, L. Bettinali and V. Violante, Rolled thin Pd and Pd–Ag membranes for hydrogen separation and production, Int. J. Hydrogen Energy, 2000, 25, 319–325 CrossRef CAS.
- S. Wieland, T. Melin and A. Lamm, Membrane reactors for hydrogen production, Chem. Eng. Sci., 2002, 57, 1571–1576 CrossRef.
- A. S. Damle, Hydrogen production by reforming of liquid hydrocarbons in a membrane reactor for portable power generation—Experimental studies, J. Power Sources, 2009, 186, 167–177 CrossRef CAS.
- D. J. Edlund and W. A. Pledger, Thermolysis of hydrogen sulfide in a metal-membrane reactor, J. Membr. Sci., 1993, 77, 255–264 CrossRef CAS.
- D. J. Edlund and W. A. Pledger, Catalytic platinum-based membrane reactor for removal of H2S from natural gas streams, J. Membr. Sci., 1994, 94, 111–119 CrossRef CAS.
- A. Li, W. Liang and R. Hughes, The effect of carbon monoxide and steam on the hydrogen permeability of a Pd/stainless steel membrane, J. Membr. Sci., 2000, 165, 135–141 CrossRef CAS.
- H. Amandusson, L. G. Ekedahl and H. Dannetun, The effect of CO and O2 on hydrogen permeation through a palladium membrane, Appl. Surf. Sci., 2000, 153, 259–267 CrossRef CAS.
- B. A. McCool and Y. S. Lin, Nanostructured thin palladium–silver membranes: Effects of grain size on gas permeation properties, J. Mater. Sci., 2001, 36, 3221–3227 CrossRef CAS.
- R. Mallada and M. Menéndez, Inorganic membranes: synthesis, characterization and applications, Technology & Engineering, Elsevier, ISBN: 978-0-444-53070-7 2008 Search PubMed.
- S. Uemiya, State-of-art of supported metal membranes for gas separation, Sep. Purif. Rev., 1999, 28, 51–85 CrossRef CAS.
- D. L. McKinley and W. V. Nitro, Metal alloy for hydrogen separation and purification, US patent, 3.350.845, 1967 Search PubMed.
- T. Graham, On the occlusion of hydrogen gas by meteoric iron, Proc. R. Soc. London, 1866, 15, 502–503 Search PubMed.
- V. M. Gryaznov, Patent, USSR Author's Certificate, 274092, 1964.
- J. C. S. Booth, M. L. Doyle, S. M. Gee, J. Miller, L. A. Scholtz and P. A. Walker, Advanced hydrogen separation via thin supported Pd membranes, Proc. of the 11th World Hydrogen Energy Conf., Stuttgart, Germany, June 23–28 1996, pp. 867–878 Search PubMed.
- M. J. Cole, The generator of pure hydrogen for industrial applications, Platinum Met. Rev., 1981, 25, 12–13.
- J. Philpott, Hydrogen diffusion technology. Commercial applications of palladium membrane, Platinum Met. Rev., 1985, 29, 12–16.
- S. Tosti, A. Basile, F. Borgognoni, V. Capaldo, S. Cordiner, S. Di Cave, F. Gallucci, C. Rizzello, A. Cantucci and E. Traversa, Low-temperature ethanol steam reforming in a Pd–Ag membrane reactor. Part 1. Ru-based catalyst, J. Membr. Sci., 2008, 308, 250–257 CrossRef CAS.
- S. Tosti, A. Basile, F. Borgognoni, V. Capaldo, S. Cordiner, S. Di Cave, F. Gallucci, C. Rizzello, A. Cantucci and E. Traversa, Low-temperature ethanol steam reforming in a Pd–Ag membrane reactor. Part 2. Pt-based and Ni-based catalyst and general comparison, J. Membr. Sci., 2008, 308, 258–263 CrossRef CAS.
- A. Iulianelli and A. Basile, An experimental study on bio-ethanol steam reforming in a catalytic membrane reactor. Part I: Temperature and sweep-gas flow configuration, Int. J. Hydrogen Energy, 2010, 35, 3170–3177 CrossRef CAS.
- A. Iulianelli, S. Liguori, T. Longo, S. Tosti, P. Pinacci and A. Basile, An experimental study on bio-ethanol steam reforming in a catalytic membrane reactor. Part II: Reaction pressure, sweep factor and WHSV effects, Int. J. Hydrogen Energy, 2010, 35, 3159–3164 CrossRef CAS.
- A. Basile, F. Gallucci, A. Iulianelli and S. Tosti, CO-free hydrogen production by ethanol steam reforming in a Pd–Ag membrane reactor, Fuel Cells, 2008, 8, 62–68 CrossRef CAS.
- A. Basile, F. Gallucci, A. Iulianelli, M. De Falco and S. Liguori, Hydrogen production by ethanol steam reforming: experimental study of a Pd–Ag membrane reactor and traditional reactor behaviour, Int. J. Chem. React. Eng., 2008, 6, A30 Search PubMed.
- D. D. Papadias, S. H. D. Lee, M. Ferrandon and S. Ahmed, An analytical and experimental investigation of high-pressure catalytic steam reforming of ethanol in a hydrogen selective membrane reactor, Int. J. Hydrogen Energy, 2010, 35, 2004–2017 CrossRef CAS.
- F. Gallucci, A. Basile, S. Tosti, A. Iulianelli and E. Drioli, Methanol and ethanol steam reforming in membrane reactors: An experimental study, Int. J. Hydrogen Energy, 2007, 32, 1201–1210 CrossRef CAS.
- W. H. Lin and H.-F. Chang, A study of ethanol dehydrogenation reaction in a palladium membrane reactor, Catal. Today, 2004, 97, 181–188 CrossRef CAS.
- W. H. Lin, C. S. Hsiao and H.-F. Chang, Effect of oxygen addition on the hydrogen production from ethanol steam reforming in a Pd–Ag membrane reactor, J. Membr. Sci., 2008, 322, 360–367 CrossRef CAS.
- A. Iulianelli, T. Longo, S. Liguori, P. K. Seelam, R. L. Keiski and A. Basile, Oxidative steam reforming of ethanol over Ru–Al2O3 catalyst in a dense Pd–Ag membrane reactor to produce hydrogen for PEM fuel cells, Int. J. Hydrogen Energy, 2009, 34, 8558–8568 CrossRef CAS.
- H. Amandusson, L. G. Ekedahl and H. Dannetun, Alcohol dehydrogenation over Pd versus PdAg membranes, Appl. Catal., A, 2001, 217, 157–164 CrossRef CAS.
- H. Lim, Y. Gu and S. T. Oyama, Reaction of primary and secondary products in a membrane reactor: Studies of ethanol steam reforming with a silica–alumina composite membrane, J. Membr. Sci., 2010, 351, 149–159 CrossRef CAS.
- C. Y. Yu, D. W. Lee, S. J. Park, K. Y. Lee and K. H. Lee, Study on catalytic membrane reactor for hydrogen production from ethanol steam reforming, Int. J. Hydrogen Energy, 2009, 34, 2947–2954 CrossRef CAS.
- E. Gernot, F. Aupretre, A. Deschamps, F. Epron, P. Merecot, D. Duprez, C. Etievant, Production of hydrogen from bioethanol in catalytic membrane reactor, 16th Confèrence Mondiale de l'Hydrogène Energie (WHEC16), 13-16 June 2006-Lyon (France), http://www.ceth.fr/download/presse/art_ceth_3.pdf.
- J. P. Breen, R. Burch and H. M. Coleman, Metal-catalysed steam reforming of ethanol in the production of hydrogen for fuel cell applications, Appl. Catal., B, 2002, 39, 65–74 CrossRef CAS.
- D. K. Liguras, D. I. Kondarides and X. E. Verykios, Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts, Appl. Catal., B, 2003, 43, 345–354 CrossRef CAS.
- A. N. Fatsikostas and X. E. Verykios, Reaction network of steam reforming of ethanol over Ni-based catalysts, J. Catal., 2004, 225, 439–452 CrossRef CAS.
- P. Bichon, G. Haugom, H. J. Venvik, A. Holmen and E. A. Blekkan, Steam reforming of ethanol over supported Co and Ni catalysts, Top. Catal., 2008, 49, 38–45 CrossRef CAS.
- S. Tosti, A. Basile R. Borrelli, F. Borgognoni, S. Castelli, M. Fabbricino, F. Gallucci and C. Ricusati, Ethanol steam reforming of a Pd–Ag membrane reactor, Int. J. Hydrogen Energy, 2009, 34, 4747–4754 CrossRef CAS.
- F. Gallucci, M. DeFalco, S. Tosti, L. Marrelli and A. Basile, Co-current and counter-current configurations for ethanol steam reforming in a dense Pd–Ag membrane reactor, Int. J. Hydrogen Energy, 2008, 33, 6165–6171 CrossRef CAS.
- F. Gallucci, M. De Falco, S. Tosti, L. Marrelli and A. Basile, Ethanol steam reforming in a dense Pd–Ag membrane reactor: a modelling work. Comparison with the traditional system, Int. J. Hydrogen Energy, 2008, 33, 644–651 CrossRef CAS.
- G. Manzolini and S. Tosti, Hydrogen production from ethanol steam reforming: energy efficiency analysis of traditional and membrane processes, Int. J. Hydrogen Energy, 2008, 33, 5571–5582 CrossRef CAS.
- W.-H. Lin and H.-F. Chang, Characterizations of Pd–Ag membrane prepared by sequential electroless deposition, Surf. Coat. Technol., 2005, 194, 157–166 CrossRef CAS.
- S.-S. Kin and B.-K. Sea, Gas permeation characteristics of silica/alumina composite membrane prepared by chemical vapor deposition, Korean J. Chem. Eng., 2001, 18, 322–329 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
