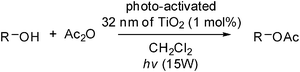
Oxidative, photo-activated TiO2 nanoparticles in the catalytic acetylation of primary alcohols†
Chien-Tien
Chen
*a,
Jun-Qi
Kao
b,
Cheng-Yuan
Liu
b and
Ling-Yu
Jiang
c
aDepartment of Chemistry, National Tsing Hua University, Hsinchu, Taiwan. E-mail: ctchen@mx.nthu.edu.tw
bDepartment of Chemistry, National Taiwan Normal University, Taipei, Taiwan
cSchool of Medicine, National Yang-Ming University, Taipei, Taiwan
First published on 31st January 2011
Abstract
A series of TiO2 particles of varying particle sizes from nano to micro scales was treated with three different oxidants (O2, TBHP, and aqueous H2O2) under irradiation conditions to form activated TiO2 particles bearing surface peroxo Ti(O2) species. The resultant, heterogeneous catalysts were tested for photo-catalytic acetylation of 2-phenylethanol by acetic anhydride in 8 different solvents. The best scenario involves the use of 32 nm grade of TiO2 nanoparticles and aqueous H2O2 for pre-activation and the use of CH2Cl2 as the reaction solvent. The 2-phenethyl acetate product can be completely formed in 10 h and isolated in essential quantitative yield. It was also found that catalysts derived from TiO2 nanoparticles are superior to those from ZrO2, Y2O3 and WO3 nanoparticles by using the oxidative, photo-activation protocol. The newly developed heterogeneous catalytic system can be smoothly applied to photocatalytic acetylation of several representative primary aliphatic and aromatic alcohols. The intermediacy of Ti–OH or Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O surface groups was proposed in the photo-catalytic acetylation conditions.
O surface groups was proposed in the photo-catalytic acetylation conditions.
The acylation of alcohols is important and is a commonly used transformation in organic synthesis.1 Conventionally, these reactions are catalyzed well by Lewis acids2 (e.g., AlCl3, TiCl4, La(O-i-Pr)3 and CoCl2) or Lewis bases3 (e.g., DMAP, phosphines and proazaphosphatrane). Trimethylsilyl (TMS) triflate4 and its metal salts derived from lithium,5magnesium,6bismuth,7indium,8tin,9scandium,10copper,11titanium,12zinc,13vanadium,14 and molybdenum15 have been found to be effective in catalyzing the acylation of alcohols with anhydrides in homogeneous media. Herein, we describe a new catalytic and heterogeneous protocol for acetylation of alcohols by TiO2 nanoparticles functionalized with active acetate surface groups.
It was demonstrated that infrared (IR) spectroscopy is a powerful method to study adsorbed species on TiO2 surfaces.16 By UV-irradiation of TiO2 powder in the presence of a hole scavenger in the gas phase, Szczepankiewicz et al.17 identified a new stretching band at 3716 cm−1 by diffuse reflectance IR Fourier transform spectroscopy (DRIFTS). The absorption band was assigned as O–H stretching for TiOH on the particle surface formed by electron capture at acidic TiOOH centers.18 Following this observation, we sought to functionalize the surface of TiO2 nanoparticles in the presence of a suitable oxidant under UV-irradiation conditions in order to generate putative Ti(IV)(O2) surface groups. Further treatment of the resultant nanoparticles by acetic anhydride under UV irradiation conditions would lead to the corresponding Ti–OAc or Ti(OAc)2 surface groups useful for subsequent nucleophilic acyl substitution by alcohols in catalytic fashion (vide infra).
Photo-activated TiO2 nanoparticles (32 nm, 1 mol%) was first used to test the potential acetylation of 2-phenylethanol in the presence of 1.5 equiv. of Ac2O. The reaction was somewhat complete in 48 h, leading to 2-phenethyl acetate in 88% yield. (entry 1, Table 1). Notably, the TiO2 nanoparticles cannot catalyze the acetylation of 2-phenylethanol at all without prior photo-activation (0% yield, 48 h). The result indicates the crucial role of Ti(OH) or Ti(OH)2 surface group in the catalytic reaction. Oxidative, photolytic activations of the TiO2 nanoparticles with three different oxidants t-butyl hydroperoxide (TBHP), H2O2 and O2 further increase the catalytic efficiency in the acetylation of 2-phenylethanol.19 The desired 2-phenethyl acetate can be isolated in 68% and 81% in 24 h when O2 and TBHP were used as oxidants, respectively. The optimal choice of the oxidant for the activation of TiO2 nanoparticles was aqueous H2O2. The resultant catalyst led to the complete consumption of 2-phenylethanol in 10 h and the corresponding 2-phenethyl acetate was obtained in > 99% yield (entry 4).
| Entry | Oxidant | t/h | Conversion (%) |
|---|---|---|---|
| a All reactions were carried out with 1 mol% of photo-activated TiO2 nano-catalysts and 1.5 equiv. of acetic anhydride in CH2Cl2. b Isolated yield. | |||
| 1 | none | 48 | 88 |
| 2 | O2 | 24 | 68 |
| 3 | TBHP | 24 | 81 |
| 4 | H2O2 | 10 | >99b |
To gain insights into the effects of solvents on the catalytic efficiency of the activated TiO2 nanoparticles, eight different solvents of varying polarity and coordination nature were examined. Among them, CH2Cl2 remains to be the best solvent of choice based on this newly developed catalytic protocol. The acetylations of 2-phenylethanol performed in non-polar, aromatic and aliphatic solvents like toluene and hexanes led to poor (26%) to moderate (45%) yields in 24 h under the optimal catalytic system (entries 2 and 3 in Table 2). Moderate conversions (45–48%) of the catalytic acetylation were attained by the use of ether type solvents of moderate coordination ability (e.g., ether and THF). In marked contrast, very poor performances (0–18% yields in 24 h) were observed when the catalytic acetylations were carried out in strong polar, coordinating solvents like acetone, EtOAc, and CH3CN.
In principle, the overall catalytic efficiency of the photo-activated TiO2 with oxidative pre-treatment highly depends on the particle size and the overall surface density of the putative Ti(IV)(O2) active sites on TiO2. Functionalized TiO2 catalysts derived from five different particle sizes were thus examined. Among them, the catalyst prepared from 32 nm grade of TiO2 showed the best catalytic efficiency (entry 2, Table 3) for the test acetylation in CH2Cl2. A turnover number of 99 mol acetate per mol of 32 nm TiO2 nanoparticles was found. Moderate conversions (69–74%) were observed by employing TiO2 with sizes ranging from 300 nm to ≥50 μm. Conversely, functionalized TiO2 catalyst derived from 9 nm grade tends to form aggregates during the acetylation,20 thus leading to the worst catalytic profile (81% yield, 72 h). A couple of catalysts based on other metal oxide nanoparticles were also examined (entries 6 and 7, Table 3). It was found that moderate catalytic efficiency (68–77% yields, 24 h) was achieved by using Y2O3 and WO3 nanoparticles (≤50–100 nm), respectively, when treated with aqueous H2O2 under irradiation.21 Besides, ZrO2 was also found suitable for the new catalytic protocol. The acetylation proceeds in 87% in 24 h (entry 8).
| Entry | Catalyst | t/h | Conversion (%) |
|---|---|---|---|
| a Specific surface area (SSA) about 131 ± 12 m2 g−1. b Anatase/rutile, 4/1, SSA about 45 ± 2 m2 g−1. c SSA about 6 m2 g−1. d SSA about 40 m2 g−1. e SSA about 5–20 m2 g−1. f SSA about 10 m2 g−1. | |||
| 1 | TiO2 (anatase, 9 nm)a | 72 | 81 |
| 2 | TiO2 (32 nm)b | 10 | >99 |
| 3 | TiO2 (rutile, 300 nm)c | 24 | 74 |
| 4 | TiO2 (rutile, 4,400 nm) | 24 | 69 |
| 5 | TiO2 (rutile, ≥ 50 mm) | 24 (48) | 71 (94) |
| 6 | Y2O3 (30–50 nm)d | 24 | 77 |
| 7 | WO3 (≤100 nm)e | 24 | 68 |
| 8 | ZrO2 (≤100 nm)f | 24 | 87 |
Several representative primary aliphatic and aromatic alcohols were selected for the optimized catalytic acetylation protocol. The acetylation of benzyl and trans-cinnamyl alcohol proceeded smoothly in 18–26 h under the standard reaction conditions in CH2Cl2. The desired acetylated products were isolated in quantitative yields (entries 1 and 2 in Table 4). Salicylic acid can also be completely acetylated in 16 h, leading to aspirin in 88% isolated yield.
| Entry | R–OH | t/h | Yield (%) |
|---|---|---|---|
| 1 |
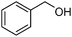
|
18 | 99 |
| 2 |
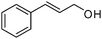
|
26 | 99 |
| 3 |
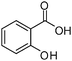
|
16 | 88 |
Based on the presumption that Ti(IV)(O2) peroxo surface groups are generated by the photo-activation of TiO2 in aqueous H2O2, photoreduction or reductive photocleavage of Ti(O2) under weakly acidic conditions (i.e. HOAc or Ac2O) would generate the active catalyst bearing Ti(OH) or Ti(OH)2 (Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) surface groups (Scheme 1a). Acetylation of the surface groups by acetic anhydride would proceed with generation of acetic acid by-product, leading to Ti–OAc or Ti(OAc)2 adducts as surface groups (Scheme 1b). Nucleophilic acyl substitution (addition followed by elimination) of the surface Ti–OAc or Ti(OAc)2 groups by a given alcohol would provide the corresponding acetate product along with regeneration of Ti(OH) or Ti
O) surface groups (Scheme 1a). Acetylation of the surface groups by acetic anhydride would proceed with generation of acetic acid by-product, leading to Ti–OAc or Ti(OAc)2 adducts as surface groups (Scheme 1b). Nucleophilic acyl substitution (addition followed by elimination) of the surface Ti–OAc or Ti(OAc)2 groups by a given alcohol would provide the corresponding acetate product along with regeneration of Ti(OH) or Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O surface groups and acetic acid, thus completing the catalytic cycle.
O surface groups and acetic acid, thus completing the catalytic cycle.
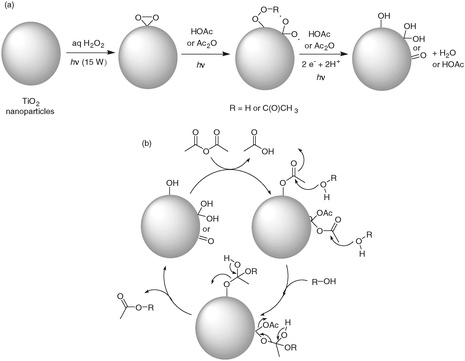 | ||
| Scheme 1 Proposed catalytic cycle on the acetylation of a given alcohol by photo-activated TiO2 nanoparticles with oxidative pre-treatment of aqueous H2O2. | ||
In conclusion, we have documented a new heterogeneous acetylation protocol for primary aliphatic and aromatic alcohols catalyzed by photo-activated TiO2 nanoparticles with oxidative pretreatment of aqueous H2O2 under very mild reaction conditions. The activated TiO2 nanoparticles and the remaining acetic anhydride/AcOH can be readily removed by sequential filtration and aqueous work up. Therefore, there is no need of chromatographic purification after completion of acetylation, auguring well for its practical application in industry.
Experimental
General
The method of Barbé was used to prepare TiO2 nanoparticles.22 The particle size was found about 32 nm with a surface area of about 45 m2 g−1. Titanium dioxide nanoparticles (average particle size 34 nm, BET specific surface area 45 m2 g−1, 80% anatase and 20% rutile) can also be purchased from Nanophase Technologies Corporation (Romeoville, IL, USA). Y2O3 nanoparticle (30–50 nm, SSA 40 m2 g−1) and WO3 (≤100 nm, SSA 5–20 m2 g−1) colloidal dispersion were purchased from SkySpring nanomaterials, Inc. ZrO2 nanoparticle (100 nm, SSA 10 m2 g−1) colloidal dispersion was obtained from Alfa Aesar company. The UV-lightbox was purchased from “Topbio” company in Taiwan. UV lamp (15 W) at 254 nm fitted with a long-range UV filter is used a light source.Representative experimental procedure for catalytic acetylation of a given alcohol
In a dry 25 mL quartz cell was placed 32 nm grade of TiO2 nanoparticles (0.8 mg, 0.01 mmol, 1 mol%) and H2O2 (30 wt%, 10 mL) was added. The heterogeneous mixture was kept under UV irradiation (254 nm, 15 W × 6 tubes) at ambient temperature for 30 min and then concentrated. The resultant yellowish solid was then suspended in CH2Cl2 (3 mL) in a 5 mL quartz cell followed by addition of acetic anhydride (140 μL, 1.5 mmol) and 2-phenylethanol (120 μL, 1 mmol). The reaction mixture was stirred at ambient temperature under UV irradiation (254 nm, 15 W × 6 tubes) for 10 h. After completion of the reaction as monitored by TLC analysis, the reaction mixture was filtered and then quenched by saturated aqueous NaHCO3 solution (5 mL). The organic layer was dried (MgSO4), filtered, and evaporated to give 2-phenylethyl acetate in quantitative yield.References
- T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, 3rd ed., John Wiley & Sons, New York, 1999 Search PubMed.
- (a) R. H. Baker and F. G. Bordwell, Organic Synthesis, Wiley, New York, 1995, Collect. Vol. III, p. 141 Search PubMed; (b) S. Chandrasekar, T. Ramchander and M. Takhi, Tetrahedron Lett., 1998, 39, 3263 CrossRef CAS; For a review, see: (c) K. L. Chandra, P. Saravanan, R. K. Singh and V. K. Singh, Tetrahedron, 2002, 58, 1369 CrossRef; (d) Z. Zhang, Z. Yang, H. Wong, J. Zhu, N. A. Meanwell, J. F. Kadow and T. Wang, J. Org. Chem., 2002, 67, 6226 CrossRef CAS; For the uses of N-acylbenzotriazoles and Lewis acids in acylation chemistry, see: (e) A. R. Katritzky, K. Suzuki, S. K. Singh and H.-Y. He, J. Org. Chem., 2003, 68, 5720 CrossRef CAS; (f) A. R. Katritzky, H.-Y. He and K. Suzuki, J. Org. Chem., 2000, 65, 8210 CrossRef CAS; (g) T. Okano, K. Miyamoto and J. Kiji, Chem. Lett., 1995, 246 CAS; (h) J. Iqbal and R. R. Srivastava, J. Org. Chem., 1992, 57, 2001 CrossRef CAS.
- For DMAP, see: (a) G. Hofle, V. Steglish and H. Vorbruggen, Angew. Chem., Int. Ed. Engl., 1978, 17, 569 CrossRef; (b) C. Grondal, Synlett, 2003, 1568 CrossRef; For functionalized DMAPs, see: (c) K. A. Wayman and T. Sammakia, Org. Lett., 2003, 5, 4105 CrossRef CAS; (d) T. Kurahashi, T. Mizutani and J. Yoshida, Tetrahedron, 2002, 58, 8669 CrossRef CAS; For leading references of phosphines, see: (e) E. Vedejs, N. S. Bennett, L. M. Conn, S. T. Diver, M. Gingvas, S. Lin, P. A. Oliver and M. J. Peterson, J. Org. Chem., 1993, 58, 7286 CrossRef CAS; (f) E. Vedejs and S. T. Diver, J. Am. Chem. Soc., 1993, 115, 3358 CrossRef CAS; For proazaphosphatrane, see: (g) J. G. Verkade and P. Ilankumar, J. Org. Chem., 1999, 64, 9063 CrossRef CAS; (h) B. D'Sa and J. G. Verkade, J. Org. Chem., 1996, 61, 2963 CrossRef CAS.
- (a) P. A. Procopiou, S. P. D. Baugh, S. S. Flack and G. G. A. Inglis, J. Org. Chem., 1998, 63, 2342 CrossRef CAS; (b) R. Kumaresvaran, A. Gupta and Y. D. Vankar, Synth. Commun., 1997, 27, 277 CrossRef.
- G. Bartoli, M. Bosco, E. Marcantoni, M. Massaccesi, S. Rinaldi and L. Sambri, Tetrahedron Lett., 2002, 43, 6331 CrossRef CAS.
- (a) A. K. Chakraborti, L. Sharma and R. S. Gulhane, Tetrahedron, 2003, 59, 7661 CrossRef CAS; (b) G. Bartoli, M. Bosco, R. Dalpozzo, E. Marcantoni, M. Massaccesi, S. Rinaldi and L. Sambri, Synlett, 2003, 39 CAS.
- (a) A. Orita, C. Tanahashi, A. Kakuda and J. Otera, Angew. Chem., Int. Ed., 2000, 39, 2877 CrossRef CAS; (b) A. Orita, C. Tanahashi, A. Kakuda and J. Otera, J. Org. Chem., 2001, 66, 8926 CrossRef CAS; (c) K. E. Peterson, R. C. Smith and R. S. Mohan, Tetrahedron Lett., 2003, 44, 7723 CrossRef CAS; For a review of Bi(III) in organic synthesis, see: (d) N. M. Leonard, L. C. Wieland and R. S. Mohan, Tetrahedron, 2002, 58, 8373 CrossRef CAS; (e) S. Antoniotti, Synlett, 2003, 1566 CrossRef.
- (a) K. K. Chauhan, C. G. Frost, I. Love and I. Waite, Synlett, 1999, 1743 CrossRef CAS; For a review on indium salt-promoted organic reactions, see: (b) F. Fringuelli, O. Piermatti, F. Pizzo and L. Vaccaro, Curr. Org. Chem., 2003, 7, 1661 CrossRef CAS.
- For Sn(OTf)2, see: (a) T. Mukaiyama, I. Shiina and M. Miyashira, Chem. Lett., 1992, 625 CAS ; For tin(IV) porphyrin perchlorate, see: S. Tangestaninejad, M. H. Habibi, V. Mirkhani and N. Moghadam, Synth. Commun., 2002, 32, 1337 Search PubMed.
- (a) K. Ishihara, M. Kubota, H. Kurihara and H. Yamamoto, J. Org. Chem., 1996, 61, 4560 CrossRef CAS; (b) K. Ishira, M. Kubota and H. Yamamoto, Synlett, 1996, 265 CrossRef CAS.
- (a) P. Sarvanan and V. K. Singh, Tetrahedron Lett., 1999, 40, 2611 CrossRef CAS; (b) C.-A. Tai, S. S. Kulkarni and S.-C. Hung, J. Org. Chem., 2003, 68, 8719 CrossRef CAS . For Cu(BF4)2, see: A. K. Chakraborti and R. S. Gulhane, Synthesis, 2004, 111 Search PubMed.
- For TiCl(OTf)3, see: (a) J. Izumi, I. Shiina and T. Mukaiyama, Chem. Lett., 1995, 141 CAS; For TiCl4/AgClO4, see: (b) M. Miyashita, I. Shiina and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1993, 66, 1516 CrossRef CAS.
- For Zn(ClO4)2-6H2O, see: G. Bartoli, M. Bosco, R. Dalpozzo, E. Marcantoni, M. Massaccesi and L. Sambri, Eur. J. Org. Chem., 2003, 4611 Search PubMed.
- C.-T. Chen, J.-H. Kuo, C.-H. Li, N. B. Barhate, S.-W. Hon, T.-W. Li, S.-D. Chao, C.-C. Liu, Y.-C. Li, I.-H. Chang, J.-S. Lin, C.-J. Liu and Y.-C. Chou, Org. Lett., 2001, 3, 3729 CrossRef CAS.
- C.-T. Chen, J.-H. Kuo, V. D. Pawar, Y. S. Munot, S.-S. Weng, C.-H. Ku and C.-Y. Liu, J. Org. Chem., 2005, 70, 1188 CrossRef CAS.
- R. Nakamura, A. Imanishi, K. Murkoshi and Y. Nakato, J. Am. Chem. Soc., 2003, 125, 7443 CrossRef CAS.
- S. H. Szczepankiewicz, J. A. Moss and M. R. Hoffmann, J. Phys. Chem. B, 2002, 106, 7654 CrossRef.
- R. Nakamura and S. Sato, Langmuir, 2002, 18, 4433 CrossRef CAS.
- For the use of H2O2 to improve the crystallinity of TiO2 (anatase phase) in layered compound, see: C. Ooka, H. Yoshida, S. Takeuchi, M. Maekawa, Z. Yamada and T. Hattori, Catal. Commun., 2004, 5, 49 Search PubMed.
- For the increasing aggregation of TiO2 nanoparticles (<10 nm) in the presence of organic acids, see: J. M. Pettibone, D. M. Cwiertny, M. Scherer and V. H. Grassian, Langmuir, 2008, 24, 6659 Search PubMed.
- (a) For the photocatalytic capability of self-assembled nanoporous WO3, see: Y. Guo, X. Quan, N. Lu, H. Zhao and S. Chen, Environ. Sci. Technol., 2007, 41, 4422 Search PubMed; (b) For the Photodegradation of phenol on Y2O3 surface, see: C. Karunakaran, R. Dhanalakshmi and P. Anilkumar, J. Hazard. Mater., 2009, 167, 664 Search PubMed; (c) For the photoactivity and photoselectivity of ZrO2, see: A. V. Emeline, G. N. Kuzmin, L. L. Basov and N. Serpone, J. Photochem. Photobiol., A, 2005, 174, 214 Search PubMed.
- C. J. Barbé, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover and M. Grätzel, J. Am. Ceram. Soc., 1997, 80, 3157 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0cy00005a |
| This journal is © The Royal Society of Chemistry 2011 |