An unusual 1D ladder-like silver(I) coordination polymer involving novel 1D → 3D five-folded interpenetrated and [3 + 2] catenaned features generated by Ag⋯O interactions†
Guo-Ping
Yang
,
Jun-Hong
Zhou
,
Yao-Yu
Wang
*,
Ping
Liu
,
Chun-Cheng
Shi
,
Ai-Yun
Fu
and
Qi-Zhen
Shi
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, Shaanxi Key Laboratory of Physico-Inorganic Chemistry, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069, Shaanxi, P. R. China. E-mail: wyaoyu@nwu.edu.cn.; Fax: +86-29-88303798
First published on 19th October 2010
Abstract
An unusual 1D ladder-like silver(I) complex has been synthesized and characterized. The polymer exhibits interesting plywood-like stacking arrays and a novel five-folded interpenetrated supramolecular network with [3 + 2] catenations.
The utilization of crystal engineering concepts has produced a considerable number of new coordination polymers (CPs) in the past two decades, the major interest in which is rapidly expanding not only because of their realistic and potential applications as functional materials,1 but also for their fascinating networks and structural topologies.2 Currently, topological investigation is a useful means to analyse structures in crystal chemistry and it is usually an effective way to identify molecular stacking trends which may benefit design and syntheses of the desired materials. As we know, one-dimensional (1D) infinite chain complexes have the simplest topology, but their stacking patterns are by no means always simple in crystals.3 Hitherto, several types of stacking modes have been observed (Scheme 1):4 parallel motifs, plywood-like arrays, warp-and-weft motifs, molecular braids and helices, polyrotaxaned motifs, and interpenetrated arrays. However, it still remains a great challenge for chemists to predict or control the 1D chain packing trends, although various weak supramolecular forces (H-bonding, π⋯π aromatic stacking interactions, and van der Waals forces.) are believed to play greatly important roles.1a,5 Therefore, much more work should be done on building other new coordination polymers to perfect crystal engineering theory.
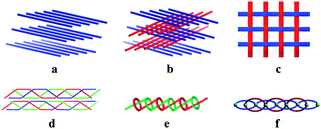 | ||
| Scheme 1 Several types of stacking patterns based on 1D chain polymers. | ||
In our previous work, some 1D molecular braids have been reported composed of suitable rigid/flexible tectons;4f–h and the 1D chains self-assembled by using H-bonding interlocks. Two isomers have also been isolated from the same mixed solution. One of the isomers contains two kinds of 1D chiral helical water chains supported by the host 1D chain template;6 and it was found that the stacking fashion of these water clusters should be attributed to plywood-like arrays. What's more, silver(I) ion has been commonly employed to prepare new functional solids due to its various coordination numbers, and also, the potential Ag–Ag interactions to have a positive influence on the resulting complexes.7 As the continuation of our work, we present herein an unusual 1D ladder-like silver(I) complex, [Ag3(cis-pda)(bipy)3]n·0.5n(trans-pda)·5nH2O (1) [H2pda = m-phenylenediacetic acid, bipy = 4,4′-bipyridine], which shows a 3D plywood-like array with the ⋯ABAB⋯ sequence. Two different formations (cis- and trans-) of the pda ligands exist in 1, which have not been observed simultaneously in typical documented polymers up to now. Moreover, a 1D fishbone-like guest tape was fused by the decameric water clusters and trans-pda ligands in the crystal lattice. However, once the Ag⋯O interactions were considered, a novel five-folded interpenetrated network was further assembled by the host 1D chains and guest trans-pda ligands, exhibiting an unknown topology with the vertex symbol of (42.6)2(42.6.143). Most significantly, a unique [3 + 2] catenation of the complex has been documented for the first time.
The hydrothermal reaction of H2pda and bipy with silver(I) acetate in the mixed CH3OH–H2O medium at 130 °C for three days generates the needle shaped colorless crystalline products [Ag3(cis-pda)(bipy)3]n·0.5n(trans-pda)·5nH2O (1). The as-synthesized samples were confirmed by elemental analysis, IR spectrum and single crystal X-ray analysis. The phase purity of bulk products was also identified by the powder X-ray diffraction (Fig. S1†), suggesting that the as-synthesized bulk material is the same as the determined structure. The X-ray crystal structure analyses unambiguously revealed that 1 crystallizes in the monoclinic space groupC2/c (Table S1†).‡ The asymmetric unit of 1 contains three crystallographically independent silver(I) ions, one and a half pda ligands, three distorted bipy ligands, and five guest water molecules. As shown in Fig. 1a, Ag1 ion adopts an approximate T-shaped coordination geometry and is three-coordinated by two nitrogen atoms from two discrete bipy ligands and one carboxylic oxygen atom from one pda ligand; Ag2 and Ag3 ions are all of linear geometry and two-coordinated by the nitrogen atoms from different bipy ligands. The bond lengths of Ag–N are in the normal range from 2.163(7) to 2.227(7) Å and Ag–O is 2.547(7) Å (Table S2†), which are typical values for silver(I) complexes. Interestingly, there are two kinds of ligand-supported Ag–Ag contacts in 1. The distances between the silver(I) centers are below the sum of silver van der Waals radii (3.44 Å);8 therefore, the resulting network exhibits a novel 1D ladder-like sheet based on such Ag–Ag interactions and bridging bipy ligands (Fig. S2†).
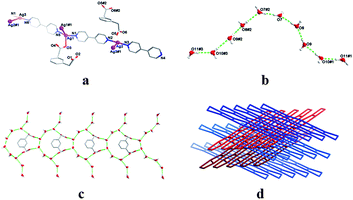 | ||
| Fig. 1 (a) the asymmetric unit of 1; (b) decameric water cluster; (c) 1D guest fishbone-like tape; (d) 3D plywood-like arrays. | ||
Remarkably, pda ligands display two distinct conformations simultaneously which are cis monodentate mode and free trans fashion in 1 (Fig. S3†). Generally, pda ligands often adopt a much more stable trans coordination mode with metal ions; there are only few cases where they act as a distorted cis mode with metal centers.9 Thus far, the phenomenon of the pda ligands in 1 was not observed in the typical reported frameworks. Of further interest, there exists a guest discrete decameric water cluster, notated as D10 array,10 in the crystal lattice (Fig. 1b). Such a water motif is constructed from the five guest water molecules (O7, O8, O9, O10 and O11) and their equivalents by H-bond interatcions (Table S3†). The average O⋯O distance of 2.840 Å within the decamer is much longer than the value of 2.759 Å in ice Ih at −90 °C, but very close to the distance in the liquid water;11 and the average value of the O⋯O⋯O angles is 128.52°, which deviates largely from the value found in the liquid water.12 Moreover, the decamers and free transpda ligands self-recognize each other by H-bonding interlocks (O7⋯O6 = 2.785(11) Å, O9⋯O5 = 2.786(11) Å) into an 1D extending fishbone-like guest tape when viewed along the a axis (Fig. 1c). When the guest molecules were removed from the crystal lattice, calculation with PLATON program indicated that the effective void volume for the inclusion is 1965.5 Å3 per unit cell volume, which is 22.5% of the crystal space (8733.0 Å3).
The packing of the 1D polymers often occurs with a parallel orientation of all chains into a high-dimensional motif; less commonly they can extend along two different directions on the alternate neighboring layers.4m Herein, another appealing feature of 1 is the 1D ladder-like chains running in two different growth directions and parallel on each layer but nonparallel on the alternate layers, showing the plywood-like arrays with ⋯ABAB⋯ sequence (Fig. 1d). The paralleled ladders are linked with each other through supramolecular interlocks involving strong π⋯π stacking with the nearest phenyl separation of 3.445(4) Å (Fig. S4†), resulting two sets of 2D wavelike sheets in the bc plane. Moreover, the free trans-pda ligands play a key role not only in forming the aforementioned guest tapes but also in condensing the 1D chains into a 3D open framework as the linkers based on Ag⋯O interactions with the distance of 2.655(9) Å (Fig. S5†).7e From the topological view, if the Ag1 and Ag3 ions are considered as 3-connected nodes, the Ag2 ions are considered as 4-connected nodes, and the trans-pda dianions and bipy ligands are considered as the spacers, the whole 3D structure exhibits an unprecedented binodal (3,4)-connected topology with the vertex symbol (42.6)2(42.6.143) (Fig. 2a). What's more, in order to reduce the large void cavities and stabilize the framework, the potential voids were incorporated by the four identical networks, thus giving an unusual 3D five-fold interpenetrated framework (Fig. 2b). To further study the structure carefully, it was found that the network shows extremely fascinating polycatenated character, i.e. each single network is offset by 1/2 compared with the other four equivalent networks;4b so finally the three smallest parallel subunit rings interlock with the other two parallel meshes to result in a unique [3 + 2] catenations (Fig. 2c, 2d, and S6†). Herein, 1 is entirely distinguished from the sole known 2D five-folded interpenetrated polymer, namely [Cd(bib)(L)]n (bib = 1,4-(bis(1H-imidazol-1-yl)butane, H2L = 4,4′-(2,2′-oxybis(ethane-2,1-diyl)bis(oxy))dibenzoic acid),13 showing an uneven five catenaned property in which the connection mode is similar to the Olympic Rings pattern. Importantly, infinite catenation of coordination polymers represents a new type of entanglement in that the whole catenated array displays a special “dimensional expansion” phenomenon;4m to our knowledge, herein 1 shows a novel 3D five-fold interpenetrated motif with the unknown topology and unique [3 + 2] catenation based on the Ag⋯O interactions that has not been observed previously for the coordination framework solids.
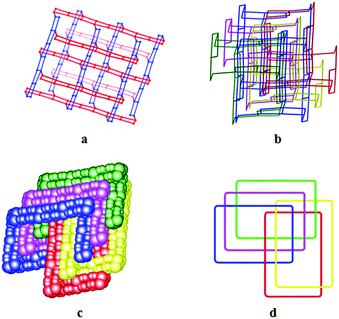 | ||
| Fig. 2 (a) the single net of 1; (b) five-folded interpenetrated network; (c) space-filling mode of catenations; (d) schematic view of catenations. | ||
Complex 1 is stable in air and insoluble in the common solvents. Thermogravimetric analysis of 1 showed loss of the lattice water molecules and pda ligands (obsd 29.4%) within the temperature range of 47–223 °C (calcd 32.3%) (Fig. S7†). Beyond the temperature, the host 1D products begin to collapse and lose the bipy ligands, and finally the residual sample is silver powder. Luminescent polymers are of great interest because of their various potential applications.14 Therefore, the luminescence of 1 was also investigated in the solid state at room temperature. As shown in Fig. 3, 1 showed two emission peaks at ca. 412 nm and a shoulder at ca. 452 nm. The intense emission is blue-shifted compared with that of H2pda ligands (λem = 435 nm) which would be assigned to the ligand-to-metal charge transfer (LMCT) contribution, and the shoulder band should be attributed to the intraligand emission from the bipy ligands. At the same time, probably the Ag–Ag and π⋯π stacking interactions also contribute to this luminescence.3a Usually, silver(I) complexes might emit weak luminescence at the lower temperature, and therefore, 1 is an unusual case of room temperature luminescent silver(I) coordination polymers.15
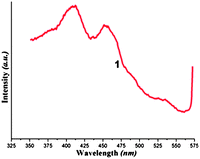 | ||
| Fig. 3 The emission spectrum of 1. | ||
In summary, we have obtained a new 1D silver(I) complex which shows a special 3D plywood-like stacking array. There exists a fishbone-like motif built on decameric water clusters and free trans-pda ligands. Once Ag⋯O interactions were considered, a novel five-fold interpenetrated framework was condensed by the host 1D chains and trans-pda ligands. Especially, it is significant that a unique [3 + 2] catenation has been documented for the first time. Further work is under way to isolate novel complexes with functional properties based on the H2pda ligand in our group.
This work was supported by the National Natural Science Foundation of China (No. 20771090), State Key Program of National Natural Science of China (No. 20931005), Natural Science Foundation of Shaanxi Province (No. 2009JZ001), and Specialized Research Found for the Doctoral Program of Higher Education (No. 20096101110005).
Notes and references
- (a) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS; (b) J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (c) G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC; (d) C. Janiak, Dalton Trans., 2003, 2781 RSC; (e) X. L. Hong, J. F. Bai, Y. Song, Y. Z. Li and Y. Pan, Eur. J. Inorg. Chem., 2006, 3659 CrossRef CAS.
- (a) D. S. Liu, G. S. Huang, C. G. Huang, X. H. Huang, J. Z. Chen and X. Z. You, Cryst. Growth Des., 2009, 9, 5117 CrossRef CAS; (b) L. F. Ma, L. Y. Wang, M. Du and S. R. Batten, Inorg. Chem., 2010, 49, 365 CrossRef CAS; (c) M. L. Tong, X. L. Chen and S. R. Batten, J. Am. Chem. Soc., 2003, 125, 16170 CrossRef CAS; (d) C. S. Liu, J. J. Wang, L. F. Yan, Z. Chang, X. H. Bu, E. C. Sanuso and J. Ribas, Inorg. Chem., 2007, 46, 6299 CrossRef CAS.
- (a) X. Q. Lü, Y. Q. Qiao, J. R. He, M. Pan, B. S. Kang and C. Y. Su, Cryst. Growth Des., 2006, 6, 1910 CrossRef; (b) S. Lidin, M. Jacob and S. Andersson, J. Solid State Chem., 1995, 114, 36 CrossRef CAS; (c) A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouga, N. V. Zyk and M. Schröder, Coord. Chem. Rev., 2001, 222, 155 CrossRef CAS.
- (a) C. D. Wu and W. B. Lin, Angew. Chem., Int. Ed., 2005, 44, 1958 CrossRef CAS; (b) C. Y. Su, A. M. Goforth, M. D. Smith and H. C. Loye, Chem. Commun., 2004, 2158 RSC; (c) G. P. Yang, Y. Y. Wang, L. F. Ma, J. Q. Liu, Y. P. Wu, W. P. Wu and Q. Z. Shi, Eur. J. Inorg. Chem., 2007, 3892 CrossRef CAS; (d) Y. H. Li, C. Y. Su, A. M. Goforth, K. D. Shimizu, K. D. Gray, M. D. Smith and H. C. Loye, Chem. Commun., 2003, 1630 RSC; (e) Y. Cui, H. L. Ngo and W. Lin, Chem. Commun., 2003, 1388 RSC; (f) X. J. Luan, Y. Y. Wang, D. S. Li, P. Liu, H. M. Hu, Q. Z. Shi and S. M. Peng, Angew. Chem., Int. Ed., 2005, 44, 3864 CrossRef CAS; (g) X. J. Luan, X. H. Cai, Y. Y. Wang, D. S. Li, C. J. Wang, P. Liu, H. M. Hu, Q. Z. Shi and S. M. Peng, Chem.–Eur. J., 2006, 12, 6281 CrossRef CAS; (h) J. Q. Liu, Y. Y. Wang, P. Liu, Z. Dong, Q. Z. Shi and S. R. Batten, CrystEngComm, 2009, 11, 1207 RSC; (i) L. Carlucci, G. Ciani, A. Gramaccioli, D. M. Peoserpio and S. Rizzato, CrystEngComm, 2000, 2, 154 RSC; (j) X. M. Gao, D. S. Li, J. J. Wang, F. Fu, Y. P. Wu, H. M. Hu and J. W. Wang, CrystEngComm, 2008, 10, 479 RSC; (k) S. G. Li, B. Wu, Y. J. Hao, Y. Y. Liu and X. J. Yang, CrystEngComm, 2010, 12, 2001 RSC; (l) X. L. Wang, C. Qin, E. B Wang and L. Xu, Cryst. Growth Des., 2006, 6, 2061 CrossRef CAS.
- L. Brammer, Chem. Soc. Rev., 2004, 33, 476 RSC.
- P. Liu, G. P. Yang, Y. Y. Wang, G. L. Wen, C. Y. Guo and Q. Z. Shi, Inorg. Chem. Commun., 2009, 12, 263 CrossRef CAS.
- (a) D. J. Eisler and R. J. Puddephatt, Inorg. Chem., 2005, 44, 4666 CrossRef CAS; (b) W. H. Bi, R. Cao, D. F. Sun, D. Q. Yuan, X. Li and M. C. Hong, Inorg. Chem. Commun., 2003, 6, 1426 CrossRef CAS; (c) L. Pan, E. B. Woodlock, X. T. Wang, K. C. Lam and A. L. Rheingold, Chem. Commun., 2001, 1762 RSC; (d) M. L. Tong, J. X. Shi and X. M. Chen, New J. Chem., 2002, 26, 814 RSC; (e) J. C. Jin, Y. Y. Wang, W. H. Zhang, A. S. Lermontov, E. K. Lermontova and Q. Z. Shi, Dalton Trans., 2009, 10181 RSC.
- (a) M. Bertelli, L. Carlucci, G. Ciani, D. M. Proserpio and A. Sironi, J. Mater. Chem., 1997, 7, 1271 RSC; (b) Q. M. Wang and T. C. W. Mak, J. Am. Chem. Soc., 2000, 122, 7608 CrossRef CAS.
- (a) Y. F. Zhou, R. H. Wang, B. L. Wu, R. Cao and M. C. Hong, J. Mol. Struct., 2004, 697, 73 CrossRef CAS; (b) H. F. Zhu, W. Y. Sun, T. Okamura and N. Ueyama, Inorg. Chem. Commun., 2003, 6, 168 CrossRef CAS; (c) M. Kato, A. K. Sah, T. Tanase and M. Mikuriya, Inorg. Chem., 2006, 45, 6646 CrossRef CAS; (d) M. L. Zhang, D. S. Li, J. J. Wang, F. Fu, M. Du, K. Zou and X. M. Gao, Dalton Trans., 2009, 5355 RSC.
- L. Infantes, J. Chisholm and S. Motherwell, CrystEngComm, 2003, 5, 480 RSC.
- (a) D. Eisenberg and W. Kauzmann, The Structure and Properties of Water, Oxford University Press, Oxford, 1969 Search PubMed; (b) A. H. Narten, W. E. Thiessen and L. Blum, Science, 1982, 217, 1033 CrossRef CAS.
- F. Li, T. H. Li, W. Su, S. Y. Gao and R. Cao, Eur. J. Inorg. Chem., 2006, 1582 CrossRef CAS.
- Y. Q. Lan, S. L. Li, J. S. Qin, D. Y. Du, X. L Wang, Z. M. Su and Q. Fu, Inorg. Chem., 2008, 47, 10600 CrossRef CAS.
- (a) Q. Wu, M. Esteghamatian, N. X. Hu, Z. D. Popovic, G. Enright, Y. Tao, M. D'Iorio and S. Wang, Chem. Mater., 2000, 12, 79 CrossRef CAS; (b) J. E. McGarrah, Y. J. Kim, M. Hissler and R. Eisenberg, Inorg. Chem., 2001, 40, 4510 CrossRef CAS.
- X. Liu, G. C. Guo, M. L. Fu, X. H. Liu, M. S. Wang and J. S. Huang, Inorg. Chem., 2006, 45, 3679 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, crystal data with CIF file, selected bond distances and angles, H-bonding parameters table, IR, EA, XRPD, and additional plots of the structures. CCDC reference number 789922. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ce00601g |
| ‡ Crystal data for 1: C45H46N6O11Ag3, monoclinic, space groupC2/c, a = 39.052(6) Å, b = 9.8693(13) Å, β = 120.12(5), c = 26.197(4) Å, V = 8733(2) Å3, Z = 8, ρc = 1.780 g cm−3. Refinement of 587 parameters on 7687 independent reflections out of 21186 measured reflections (Rint = 0.0587) led to R1 = 0.0678, wR2 = 0.1953 and S = 1.008. CCDC Number: 789922. |
| This journal is © The Royal Society of Chemistry 2011 |
