Small molecules induce mesocrystal formation: nanoparticle aggregation directed by self-assembling calixarenes†
Andrew
Baynton
a,
Tomoko
Radomirovic
a,
Mark I.
Ogden
a,
Colin L.
Raston
b,
William R.
Richmond
a and
Franca
Jones
*a
aCurtin University of Technology, Department of Chemistry, GPO Box U1987, Perth, WA, Australia 6845. E-mail: F.Jones@curtin.edu.au; Fax: +61 8 9266 4699; Tel: +61 8 9266 7677
bCentre for Strategic Nano-Fabrication, School of Biomedical, Biomolecular and Chemical Sciences, The University of Western Australia, Crawley, WA 6009, Australia
First published on 8th October 2010
Abstract
Calixarenes have been shown to induce mesocrystal formation of barium sulfate, despite being relatively low molecular weight additives. Scanning probe microscopy has shown that a possible mechanism is the self-assembling properties of the calixarene resulting in steric stabilization of the nanoparticles, comparable to that typically requiring polymeric additives.
Non-classical crystallization involves the assembly of nanoparticles with sufficient order that the resulting mesocrystal is virtually indistinguishable from a single crystal using standard techniques such as powder XRD.1 This phenomenon is regarded as being significant in better understanding biomineralisation processes, where proteins exhibit exquisite control of crystal growth.2,3 Synthetic polyelectrolytes, typically block copolymers, have been reported to induce the formation of mesocrystals in a wide range of inorganic systems, with extension to an organic material recently reported.4,5 The impact of a random copolymer upon the crystallization of calcium carbonate has been studied in detail, with the realisation that single crystals are virtually absent under all of the conditions studied, with non-classical crystallization dominating the system.6 These results highlight the significance of nanoparticle-mediated crystallization, and suggest that this mechanism of crystal growth may be widespread. An important aspect of the mechanism of formation of mesocrystals is the stabilisation of the nanoparticles so that they have time to aggregate in a non-random fashion. Recent literature highlights that this stabilisation can occur in many different ways, through emulsions/micelle formation, magnetism, and charge stabilisation just to name a few.7 In fact, for small molecules, the stabilisation mechanism is often simply assumed to be charge stabilisation without further investigation.7d
Previously we reported on the use of the calix[4]arene framework, which usually exists in the cone conformation, to control the orientation of moieties known to be significant in modifying crystal growth. Functionalizing the lower (phenolic) rim of the calixarene with aspartic acid, for example, produced a potent growth inhibitor.8 In the present study we focus on functionalizing the divergent upper rim with water solubilising sulfonic acid/sulfonate or phosphonic acid/phosphonate groups, as molecules 1 and 2, respectively, and assess the impact on the crystal growth of barium sulfate (Scheme 1). Barium sulfate has been shown to form mesocrystals, with their shape depending on parameters such as pH and the type of functional groups attached to the di-block copolymer additive.5
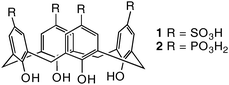 | ||
| Scheme 1 Calixarene molecules investigated in this work. | ||
Based on the typical behaviour of sulfonate and phosphonate derivatives as crystal growth inhibitors, we expected 2 to have a greater inhibitory effect than 1, and this is indeed the case. The phosphonate, 2, is able to inhibit precipitation (involving barium chloride and sodium sulfate at a supersaturation ratio of ∼25) completely over a 3 hour time period with as little as 0.013–0.014 mM, whereas even 0.2 mM of 1 only inhibits barium sulfate precipitation by 34% relative to the control (ESI, Fig. S1†). Introduction of the additives also results in changes in crystal morphology (Fig. 1 and ESI, Fig. S2†). Both calixarene additives induce an initial change from the ‘pillow’ (Fig. 1A) to ‘square tablet’ like particles, although again 2 is more potent than 1 (ESI, Fig. S2†). As the concentration of calixarene is increased, rod-shaped particles are formed, which become rougher, smaller and flanged at the extremities with increasing concentrations of the additive. Remarkably, dumbbell shaped particles eventually form, which can aggregate together. Similar changes were observed with 1 but at higher concentrations (ESI, Fig. S3†).
![Barium sulfate particles formed in the presence of p-phosphonatocalix[4]arene 2 at concentrations of (A) 0.003 µM, (B) 0.0034 mM, (C) 0.0067 mM, and (D) 0.0134 mM.](/image/article/2011/CE/c0ce00579g/c0ce00579g-f1.gif) | ||
| Fig. 1 Barium sulfate particles formed in the presence of p-phosphonatocalix[4]arene 2 at concentrations of (A) 0.003 µM, (B) 0.0034 mM, (C) 0.0067 mM, and (D) 0.0134 mM. | ||
Larger (4 L) batch experiments were performed to obtain solids for bulk characterization; in this case a fibre-like morphology was obtained (Fig. 2). Both particle shapes (dumbbell and fibre) are comparable to those observed for mesocrystals formed in the presence of di-block co-polymers.5 The formation of possible mesocrystals in this system is all the more remarkable when the concentration is considered; ∼0.01 mM when 2 is present versus ∼0.1 mM for the block co-polymers.5
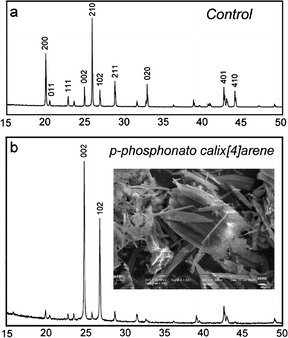 | ||
| Fig. 2 XRD patterns of barium sulfate particles formed on a 4 L batch scale, in the presence of (a) 0 mM (control) and (b) 0.0134 mM 2. | ||
The most striking differences between the control XRD pattern and that with 2 present (Fig. 2) lie in the changes to relative intensities of specific peaks. The pattern of the product obtained in the presence of 2 reveals that the (002) and (102) reflections display intensities much greater than all other peaks in the pattern. This is characteristic of a strong preferred orientation effect, consistent with the presence of particles with an anisotropic morphology. Line width analysis of multiple peaks in each pattern provided average crystallite sizes of 215 nm for the control sample and 160 nm for the material formed with 2. Transmission electron microscopy (TEM) on ultramicrotomed samples was therefore used to determine the faces present (Fig. 3).
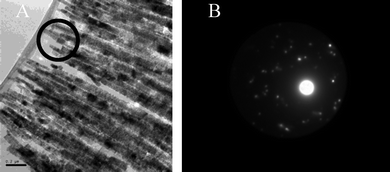 | ||
| Fig. 3 Barium sulfate particles formed in the presence of (A) 0.0134 mM 2 (4 L batch), and (B) SAED of the area within the highlighted circle in (A). | ||
The thin sections of particles viewed under the TEM show conclusively that these fibres are made up of smaller particles, and their selected area electron diffraction (SAED) pattern shows significant if imperfect alignment, thereby confirming mesocrystal formation. In addition, the indexing of this zone as the (100) (see ESI, Fig. S4†) leads to the conclusion that the fibres are elongated in the (001) direction. Presumably, as found in previous studies, this is due to selective adsorption onto specific faces.9 Given that calixarene molecules can induce mesocrystal formation, it was necessary to consider how these relatively small molecules can stabilize the nanoparticles to the same extent as block copolymers.5 The calixarenes used in this work are not surfactants nor do these molecules phase separate or form structures at an oil–water or air–water interface thus negating these as possible mechanisms for mesocrystal formation. Two possibilities appear likely, namely, charge stabilization or self-assembly of the organic moiety at the crystal surface such that it achieves steric stabilization of the particles comparable to that of polymeric additives.
The zeta potential of the precipitated barium sulfate in the presence of 2 (0.0134 mM) was measured at −17 mV, which, while being slightly negative, it is still within the range of zeta potentials (±30 mV) where the system is expected to be unstable and would spontaneously coagulate.10 Thus, charge stabilization is unlikely to be a factor in barite mesocrystal formation in the presence of 2. Stabilization of some kind must be operating in order to avoid random aggregative processes. We therefore investigated the possibility of the calixarene self-assembly hypothesis viaatomic force microscopy (AFM).
Atomic resolution imaging of the barium sulfate crystal grown in the presence of the sulfonated calixarene at 26.71 mM (Fig. 4) showed similar spacings to that expected for pure barium sulfate, with the parameters for a unit cell being 9.0 and 5.5 Å (compared to 8.91 and 5.47 Å for pure barite).
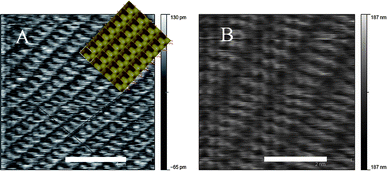 | ||
| Fig. 4 Atomic resolution AFM images of the barite crystal surface in the presence of (A) sulfonated calixarene 1 (simulated‡ image for pure barium sulfate overlaid) and (B) phosphonated calixarene 2 (size bars represent 4 and 2 nm respectively). | ||
In the case of the phosphonated calixarene, this is altered significantly in that the distance between the dark ridges is now 12.0 Å while the other axis is 6.2 Å apart. Thus, the surface imaged in the presence of the phosphonated calixarene does not represent pure barium sulfate. The formation of self-assembled bi-layered structures is well established for 1, with the calixarenes in an alternate up-down arrangement with the sulfonate groups equally decorating the surfaces of the bi-layers.12 Recently, this type of arrangement has been established for 2,13 at different degrees of deprotonation of the phosphonic acid groups, with calcium counter ions interposed between bi-layers.14 Indeed, for the pristine phosphonic acid, association into nano-rafts prevails in solution, presumably with the same interlocking of the cavitands as in the bi-layers in the solid state,13,14 which is through hydrogen bonding and π-stacking. Such an arrangement of 2 on the surface of the nanoparticles is possible in the present study, in providing steric stabilization of the nanoparticles for controlled aggregation and formation of the mesocrystals, with the potential for the calixarenes to bind to barium, like in the aforementioned calcium complex.14 Comparison of the structural data from these complexes shows the metal-free bilayers have unit cellsa = b = 11.9381 Å and c = 14.0678 Å. Thus, the AFM image is consistent with such a structure where the c direction would be normal to the image. However, two factors suggest this is not the case. Firstly, it is unlikely that a purely organic absorbed layer would be robust enough to be imaged in contact mode by AFM. Secondly, the simulated AFM images of the phosphonated bilayer are not consistent with the AFM image obtained (see ESI, Fig. S5†). It is most likely that the AFM image shows an ionic absorbed layer involving the calixarene in combination with barium and/or sulfate ions. The calcium complexes also involve bilayer formation and have lattice parameters that are comparable to the AFM data with one structure having a b axis of ∼12.85 Å.13 The calcium complexes known to form with the phosphonated calixarene were taken and simulated atomic resolution AFM surfaces were constructed (see ESI, Fig. S6†). These results show that a similar surface structure can be seen particularly for the (001) face of form 2.13 Noting that we expect the parameters observed in the bulk structures would be somewhat modified for a surface absorbed structure we thus, propose that the AFM image is consistent with barium (and possibly sulfate) ions interacting with the phosphonated calixarene in a bilayer type arrangement on the crystal surface.
The stabilisation of the barium sulfate particles could then be due to the bilayer-type structure sterically stabilising the nanoparticles during mesocrystal formation. It is also possible that the bilayer changes surface properties of the barium sulfate (such as local charge density) such that oriented attachment can occur.15 Clearly, however, the presence of the calixarene is critical and formation of the bilayer appears to be involved. Future work will focus on further elucidating the role of the bilayer in mesocrystal formation.
In summary, we note that these calixarenes are small molecules that are not surfactants and that the concentration of calixarene required to induce mesocrystal formation is significantly lower than for other molecules studied thus far: for example, in the case of EDTA7d where ∼50 mM is the concentration used. In this instance mesocrystal formation is observed with as little as ∼0.01 mM. These results demonstrate the broad potential for non-classical crystallization induced by low molecular weight additives, and highlights that it should not be assumed that small molecules can only act by charge stabilising the nanoparticles prior to self-assembly. Given that small molecules of the type used in the present study can be readily and systematically modified, there is potential for using this approach in gaining control over the formation of mesocrystals, and this is of fundamental importance in ultimately developing their applications.
Acknowledgements
We would like to thank John Murphy, Centre for Microscopy and Microanalysis (UWA) for ultramicrotomed samples, and the Australian Research Council for financial support.References
- (a) M. Niederberger and H. Cölfen, Phys. Chem. Chem. Phys., 2006, 8, 3271–3287 RSC; (b) F. C. Meldrum and H. Cölfen, Chem. Rev., 2008, 108, 4332–4432 CrossRef CAS; (c) H. Cölfen and M. Antonietti, Mesocrystals and Non Classical Crystallization, John Wiley & Sons, Chichester, UK, 2008 Search PubMed.
- D. Gebauer, A. Verch, H. G. Borner and H. Cölfen, Cryst. Growth Des., 2009, 9, 2398–2403 CrossRef CAS.
- R. Q. Song, A. W. Xu, M. Antonietti and H. Cölfen, Angew. Chem., Int. Ed., 2009, 48, 395–399 CrossRef CAS.
- (a) L. Börger, H. Cölfen and M. Antonietti, Colloids Surf., A, 2000, 163, 29–38 CrossRef CAS; (b) H. Cölfen and M. Antonietti, Langmuir, 1998, 14, 582 CrossRef; (c) H. Cölfen, Curr. Opin. Colloid Interface Sci., 2003, 8, 23–31 CrossRef CAS.
- (a) L. Qi, H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2000, 39, 604–607 CrossRef CAS; (b) L. Qi, H. Cölfen and M. Antonietti, Chem. Mater., 2000, 12, 2392–2403 CrossRef CAS.
- R. Q. Song, H. Cölfen, A. W. Xu, J. Hartmann and M. Antonietti, ACS Nano, 2009, 3, 1966–1978 CrossRef CAS.
- (a) M. Li, H. Schnablegger and S. Mann, Nature, 1999, 402(6760), 393–395 CrossRef CAS; (b) A. Ahniyaz, Y. Sakamoto and L. Bergstrom, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17570–17574 CrossRef CAS; (c) V. M. Yuwono, N. D. Burrows, J. A. Soltis and R. L. Penn, J. Am. Chem. Soc., 2010, 132(7), 2163–2165 CrossRef CAS; (d) I. C. Romero-Ibarra, G. Rodríguez-Gattorno, M. F. García-Sánchez, A. Sánchez-Solis and O. Manero, Langmuir, 2010, 26(10), 6954–6959 CrossRef CAS.
- F. Jones, M. Mocerino, M. I. Ogden, A. Oliveira and G. M. Parkinson, Cryst. Growth Des., 2005, 5, 2336–2343 CrossRef CAS.
- (a) T. Wang and H. Cölfen, Langmuir, 2006, 22, 8975–8985 CrossRef CAS; (b) T. Wang, A. Reinecke and H. Cölfen, Langmuir, 2006, 22, 8986–8994 CrossRef CAS.
- T. Mäurer and B. Kraushaar-Czarnetzki, Helv. Chim. Acta, 2001, 84, 2550–2556 CrossRef CAS.
- S. D. Fleming and A. L. Rohl, Z. Kristallogr., 2005, 220(5–6), 580–584 CrossRef CAS.
- L. Ling, Y. Alias, A. N. Sobolev and C. L. Raston, Cryst. Growth Des., 2009, 9, 4497–4503 CrossRef CAS.
- T. E. Clark, M. Makha, A. N. Sobolev, H. Rohrs, J. L. Atwood and C. L. Raston, Chem.–Eur. J., 2008, 14, 3931–3938 CrossRef CAS.
- A. D. Martin, A. N. Sobolev, M. A. Spackman and C. L. Raston, Cryst. Growth Des., 2009, 9, 3759–3764 CrossRef CAS.
- M. Alimohammadi and K. A. Fichthorn, Nano Lett., 2009, 9(12), 4198–4203 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, SEM images of barium sulfate formed in the presence of 1, SAED attribution to [100] zone, simulated AFM surfaces for calcium complexes of 2. See DOI: 10.1039/c0ce00579g |
| ‡ Simulation of the (001) barium sulfate surface using the iso-surfaces functionality in GDIS.11 |
| This journal is © The Royal Society of Chemistry 2011 |
