Room temperature synthesis of zinc hydroxystannate hollow core-shell microspheres and their hydrothermal growth of hollow core-shell polyhedral microcrystals†
Hai
Fan
*,
Shiyun
Ai
and
Peng
Ju
College of Chemistry and Material Science, Shandong Agricultural University, Taian, Shandong 271018, People's Republic of China. E-mail: fanhai@sdau.edu.cn; Fax: +86-538-8242251; Tel: +86-538-8242853
First published on 25th August 2010
Abstract
In this study, a room temperature NH3 bubble templating technique is developed to prepare zinc hydroxystannate [ZnSn(OH)6] hollow core-shell microspheres. Then, via a hydrothermal recrystallizing process, ZnSn(OH)6 hollow core-shell microcrystals with polyhedral morphology can be successfully obtained. It was found that the concentration of NH4F plays a crucial role in the morphology of the product. The optimal condition for the formation of hollow core-shell ZnSn(OH)6 microcrystals is that the concentration of NH4F is at 0.25 mol L−1 while the concentration of NaOH is fixed at 0.25 mol L−1. Reaction time has also a great influence on the morphology of the product. With the reaction time prolonged, hollow core-shell ZnSn(OH)6 microspheres would gradually crystallize into hollow core-shell ZnSn(OH)6 polyhedral microcrystals. The samples have been characterized by means of X-ray power diffraction (XRD), field emission scanning electron microscopy (FESEM), X-ray photoelectron spectra (XPS), selected area electron diffraction (SAED) and transmission electron microscopy (TEM). The formation mechanism of the ZnSn(OH)6 hollow core-shell microcrystals with polyhedral morphology has been discussed based on the experimental results.
Introduction
ZnSn(OH)6 is well-documented as a nontoxic flame-retardant and smoke suppressant additive for a wide range of plastics, rubbers paints, and other polymeric materials.1–3 More importantly, ZnSn(OH)6 thermal decomposition products including amorphous ZnSnO3, crystalline SnO2 and Zn2SnO4 can be used in lithium ion battery anodes, gas/vapor sensors and photocatalysts.4–7 It is well known that the shape and size of inorganic materials have great effects on their widely varying properties,8,9 therefore, to improve the properties of ZnSn(OH)6 and its decomposed materials for practical and potential applications, much effort has been devoted to the study of this material. In the past, the method to prepare ZnSn(OH)6 materials has mainly been coprecipitation of hydroxides.10 Other techniques, such as ion exchange and sono-chemical methods have also been used.11,12 However, the resultant materials were micrometre-sized powders comprised of aggregated nanosized crystallites. Recently, an hydrothermal technique has been applied to controlled synthesis of varies materials due to its mildness, simplicity, low cost, mass-production and shape-controllability. Using this method, ZnSn(OH)6 nanocubes and microcubes have been prepared. For example, free-standing ZnSn(OH)6 nanocubes were prepared hydrothermally using a mixture of ZnCl2,Na2SnO3·3H2O, and polyvinylpyrrolidone.4ZnSnO3 and ZnSn(OH)6 microcube films were obtained by hydrothermal method on zinc oxide-seeded indium tin oxide substrates.13ZnSn(OH)6 micro- and nanocubes were grown on pure tin substrates via a seedless hydrothermal synthesis method.14 Till now, it has been a great challenge to control the synthesis of ZnSn(OH)6 crystals with special morphology. To the best of our knowledge, ZnSn(OH)6 hollow core-shell microspheres and polyhedral microcrystals have seldom been reported.The design and construction of hollow or core-shell micro- or nanostructures have attracted much attention due to their low densities, large surface areas, and high surface permeability, which may have potential applications in photonic devices, drug delivery, active material encapsulation, ionic intercalation, surface functionalization, robust catalysts/carriers, and size-selective reactions.15 Generally, there are two major methods most widely used to prepare this class of materials: hard- and soft-templating syntheses.16 In the hard-template assisted synthesis, colloidal particles, fibers, anodic alumina membranes, polycarbonate membranes, and sacrificial metallic cores are commonly utilized. After removal of the templates, inorganic nanomaterials with hollow interiors can be obtained. In the soft-template assisted synthesis, on the other hand, ligands, surfactants, organogelators, and polymers have been widely used. However, in most cases, the pure product was obtained only after the complete removal of the templates, which makes the experiments become more complicated. Recently, gas bubbles, as an important soft template, have been widely used to prepare hollow microstructures, since this method is very simple, convenient and avoids the introduction of impurities. For example, Li and co-workers17 prepared ZnSe hollow spheres by using N2 bubbles as soft template. Lu and co-workers18 synthesized ZnS hollow spheres by taking H2S bubbles as the aggregation centers. Wang et al.19 prepared Cu3P hollow spheres templated by H3P bubbles. However, a few studies have been conducted in relation to the synthesis of ZnSn(OH)6 core-shell microspheres and polyhedral microcrystals templated by NH3 bubbles.
Herein, we report a facile NH3 bubble templating hydrothermal technique for the growth of ZnSn(OH)6 hollow core-shell microcrystals with polyhedral morphology. ZnSn(OH)6 hollow core-shell microspheres can be successfully prepared templated by the NH3 bubbles. Then, via a recrystallizing process, ZnSn(OH)6 hollow core-shell polyhedral microcrystals can also be obtained. This simple method may have wide application in the control synthesis of core-shell and hollow micro/nanostructures.
Experimental
General methods
Zn(Ac)2·2H2O, SnCl4·5H2O, NH4F, and NaOH were purchased and used as received without further purification. The phase and the crystallographic structure of the products were determined by X-ray diffraction (XRD) using a Japan Rigaku D/max-γA rotating-anode X-ray diffractometer equipped with graphite monochromatized Cu-Kα radiation (λ = 1.54178 Å). The morphology and size of the products were observed by using field emission scanning electronic microscopy (FESEM), which was performed on a JEOL JSM 6700 scanning electron microanalyzer. Transmission electron microscopy (TEM) images obtained using a Hitachi H-800 transmission electron microscope were used to observe the morphologies of the as-prepared products. For the high-resolution transmission electron microscopy (HRTEM) observations, a JEOL-2010 transmission electron microscope was used with an accelerating voltage of 200 kV. Selected area electron diffraction (ED) was also conducted using the microscope. The X-ray photoelectron spectra (XPS) were collected by using an ESCALab MKII X-ray photoelectron spectrometer with non-monochromatized Mg-Kα X-ray as the excitation source.Preparation
In a typical procedure for synthesizing ZnSn(OH)6 hollow core-shell microcrystals, Zn(Ac)2·2H2O (0.8 g), SnCl4·5H2O (0.5 g) were put into a beaker together and dissolved by a suitable volume of deionized water. Then, a suitable volume of NH4F solution (1.0 mol L−1) was added to the above solution to let the concentration of NH4F in the solution be at 0.25 mol L−1. After that, a NaOH solution (1.0 mol L−1) was added drop-wise into the above mixed solution slowly and under magnetic stirring at room temperature until the concentration of NaOH in the final solution was 0.25 mol L−1. To obtain ZnSn(OH)6 hollow core-shell microspheres, the above solution was filtered and washed with absolute ethanol and distilled water several times respectively, and dried in vacuum at 50 °C for 4 h. To obtain ZnSn(OH)6 hollow core-shell polyhedral microcrystals, the final solution was transferred into a 60-mL Teflon-lined stainless steel autoclave, which was filled with distilled water up to 80% of the total volume. The autoclave was sealed and maintained at 150 °C for 25 h, and then allowed to cool to room temperature naturally. The final product was filtered and washed with absolute ethanol and distilled water several times respectively, and dried under vacuum at 50 °C for 4 h.Results and discussion
Fig. 1a,b shows the XRD patterns of the products obtained at room temperature and at 150 °C for 25 h, respectively. All the diffraction peaks can be readily indexed to the cubic ZnSn(OH)6 with calculated lattice parameters a = 7.80 Å, which is in good agreement with the literature values (JCPDS 74-1825). No other peaks for impurities were detected. Compared with Fig. 1a and 1b, it can be seen obviously that the relative intensity of the diffraction peaks in Fig. 1b is much higher than in Fig. 1a, which indicates that the crystallinity of the product obtained at 150 °C for 25 h is much higher than the product obtained at room temperature.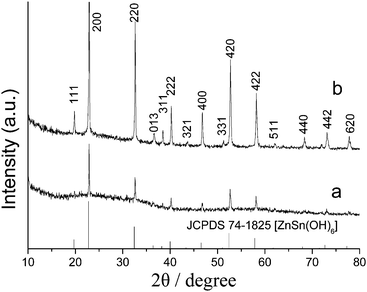 | ||
| Fig. 1 XRD patterns of the products obtained at room temperature (a) and at 150 °C for 25 h (b). | ||
Important information about the chemical composition of the products can be further provided by X-ray photoelectron spectroscopy (XPS) measurements, as shown in Fig. 2. The binding energies obtained in the XPS analyses were corrected for specimen charging by referencing the C1s peak to 284.60 eV. Fig. 2a shows the survey XPS spectrum of ZnSn(OH)6 products. It clearly revealed that mainly Zn, Sn and O elements existed in the product except C contamination. There is one major peak with binding energy at 1022.9 eV (Fig. 2b), which corresponds to the binding energy of Zn2p3/2.20 The peaks at 486.6 and 495.1 eV shown in Fig. 2c correspond to the binding energies of Sn3d3/2 and Sn3d5/2, respectively.21 The peak at 531.4 eV shown in Fig. 2d corresponds to the binding energy of O1s. The quantification of peaks gave a ratio of Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn
Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O of 10.80
O of 10.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.45
11.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77.75, which is almost consistent with the stoichiometry of ZnSn(OH)6. The oxygen ratio is a little higher than the stoichiometry of the product, which may due to the oxygen absorption on the surface of the product. No hydrogen signal could be obtained due to the detection limit of XPS analysis.
77.75, which is almost consistent with the stoichiometry of ZnSn(OH)6. The oxygen ratio is a little higher than the stoichiometry of the product, which may due to the oxygen absorption on the surface of the product. No hydrogen signal could be obtained due to the detection limit of XPS analysis.
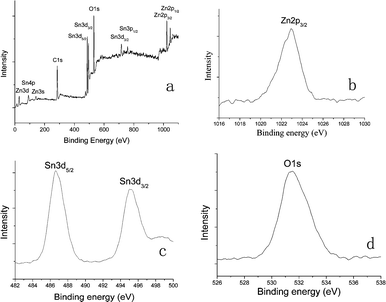 | ||
| Fig. 2 XPS spectra of ZnSn(OH)6 hollow core-shell microcrystals: (a) Survey spectrum; (b) Zn region; (c) Sn region; (d) O region. | ||
Fig. 3a shows the FESEM image of ZnSn(OH)6 hollow core-shell microspheres obtained at room temperature. As it can be seen, many core-shell microspheres could be obtained in the product. From the magnified image of one microsphere in Fig. 3b, we can clearly see the hollow core-shell structure of the product with a core diameter of 350 nm and a shell width of 200 nm. Furthermore, there are some circular empty spaces between the core and the shell with distance of about 100 nm. Fig. 3c shows the FESEM image of the product obtained at 150 °C for 25 h. It was shown that the product was composed of many regular polyhedral microcrystals with hollow core-shell structure. The magnified morphology in Fig. 3d clearly indicates the polyhedral structure of the product. Each crystal has six octangle faces, eight hexagonal faces, twelve quadrangle faces, forty-eight vertices and seventy-two edges.
 | ||
| Fig. 3 FESEM images of ZnSn(OH)6 hollow core-shell microspheres (a,b) and hollow core-shell polyhedral microcrystals (c,d). | ||
The structure and morphology of ZnSn(OH)6 hollow core-shell microspheres and microcrystals were further observed by TEM and HRTEM, in which the sample was treated by ultrasonic dispersion in ethanol for 5 min and then put onto a copper mesh. The TEM image of the microspheres clearly shows the hollow core-shell structure (ESI,† Fig. S1a). No obvious lattice fringes could be observed in the HRTEM image of the microspheres (ESI,† Fig. S1b). This may be due to the poor crystalline quality as a result of the low growth temperature, which is consistent with what obtained from the XRD patterns. The TEM image (ESI,† Fig. S1c) of the microcrystals confirms that they consist of mainly core-shell crystals with a shell width of about 150 nm and an average outer diameter of 1.6 μm. Both the SAED pattern (ESI,† Fig. S1d) and corresponding HRTEM image (ESI†, Fig. S1e) recorded from the edge of the crystals clearly demonstrated the cubic ZnSn(OH)6 phase of the product. The lattice fringes are observed in the HRTEM image with a d spacing of about 5.52 Å, corresponding to the {110} plane of the cubic ZnSn(OH)6.
The morphology of the products was found to be greatly influenced by the concentration of NH4F. When no NH4F was added in our experiment, irregular nanoparticles could be observed in the product (ESI,† Fig. S2a). When the concentration of NH4F increased to 0.05 mol L−1, microspheres could be obtained, but a few could be observed as hollow or core-shell structures (ESI,† Fig. S2b). With the concentration of NH4F at 0.25 mol L−1, typical ZnSn(OH)6 core-shell microcrystals could be obtained. As the concentration was further increased up to 0.5 mol L−1, nanoparticles instead of microcrystals could be found in the product (ESI,† Fig. S2c). As can clearly be seen, most of the spheres have disassembled into nanoparticles, although a few sphere shells could be observed, they are still assembled of nanoparticles with large pore size in them. Therefore, to obtain core-shell ZnSn(OH)6 microcrystals, the optimal condition should be that the concentration of NH4F is at 0.25 mol L−1 while the concentration of NaOH in the final solution is fixed at 0.25 mol L−1.
The reaction time on the influence of the morphology of ZnSn(OH)6 crystals has been investigated. When the products were obtained at room temperature, hollow core-shell microspheres could be obtained in the product, as shown in Fig. 3a. When the reaction time was prolonged to 5 h at 150 °C, it can be clearly seen that the shell layer of the hollow core-shell microspheres has gradually crystallized to form polyhedral structure (Fig. 4a,b). While increasing the reaction time to 15 h, the surfaces of the crystals become regular (Fig. 4c,d). Further increasing to more than 25 h, ZnSn(OH)6 microcrystals with regular polyhedral morphology has been formed.
 | ||
| Fig. 4 FESEM images of the products obtained at 150 °C for 5 h (a, b) and 15 h (c, d). | ||
The reaction temperature aslo has great influence on the morphology of the products. When the reaction temperature is at 100 °C (ESI†, Fig. S3a), the products obtained are still consisted of microspheres. While the temperature is up to 120 °C (ESI†, Fig. S3b), some of the products have grown into polyhedral microcrystals. At 150 °C, most of the products have formed the polyhedral microcrystals (Fig. 3c,d). Up to 150 °C, the morphology keeps almost unchanged. From the products obtained at 180 °C (ESI†, Fig. S3c), it can be clearly seen that regular polyhedral microcrystals can be obtained.
Based on the above experimental results, a possible reaction process demonstrating the synthesis of ZnSn(OH)6 can be proposed as follows:
| Zn2+ + 4NH3 = Zn(NH3)42+ | (1) |
| Zn(NH3)42+ + Sn4+ + 6OH− = 4NH3↑ + ZnSn(OH)6 | (2) |
After the NH4F solution was added, NH4+ would be released into the reaction solution. As the NaOH solution was added drop-wise to the above solution, NaOH would react with NH4+ to form NH3, which would immediately react with Zn2+ to form the Zn(NH3)42+ complexes, as shown in eqn (1). Subsequent addition of the NaOH solution would result in the reaction shown in eqn (2). ZnSn(OH)6 would immediately form and NH3 gas would be released at the same time.
A possible formation mechanism of the hollow core-shell ZnSn(OH)6 microcrystals was also discussed. A soft template based on the NH3 bubbles was proposed for the synthesis of the hollow core-shell ZnSn(OH)6 microcrystals, as shown in Scheme 1. Firstly, ZnSn(OH)6 nanoparticle aggregates were formed, meanwhile, NH3 gas would be produced and form NH3 bubbles around the surface ZnSn(OH)6 nanoparticle aggregates to minimize interfacial energy. Then, ZnSn(OH)6 nanoparticles continue to aggregate around the surface of NH3 bubbles, driven by the minimization of interfacial energy. Therefore, hollow core-shell ZnSn(OH)6 microspheres would be formed immediately after the initial materials were mixed. The release of NH3 gas can be confirmed by the NH3 gas smell given out when adding NaOH to NH3F included in the reaction solution. A suitable volume of NH3 gas is necessary to obtain hollow core-shell ZnSn(OH)6 microspheres. Keeping the concentration of NaOH solution unchanged, without the addition of NH4F, no NH3 gas could be obtained, therefore, no hollow core-shell structure could be observed. When the concentration of NH4F is 0.05 mol L−1, little NH3 gas could be produced, thus few hollow core-shell structures could be observed. By increasing the concentration of NH4F to 0.25 mol L−1, most of the products obteined are hollow core-shell microcrystals. A further increase up to 0.5 mol L−1, resulted in a large volume of NH3 gas, which would prevent the nanoparticles to assemble into microspheres, therefore, only nanoparticles could be obtained. When the reaction temperature was increased to 150 °C, the nanoparticles that consist of microspheres would spontaneously grow into larger crystals with the reaction time prolonged via an Ostwald ripening process,22,23 in which many small crystals form initially in a system but slowly disappear except for a few that grow larger, at the expense of the small crystals. In a cluster of nanoparticles, fewer and larger crystals may form that have smaller surface-to-volume ratios compared to the smaller particles, thus reducing the energy of the entire system. With the reaction time prolonged, the surface of the core-shell microspheres would recrystallize into a single crystalline shell, and the polyhedral morphology would gradually developed due to its crystal nature. It is known that cubic ZnSn(OH)6 crystals usually expose {100} faces which possess low surface energy.13,14 However, the crystal morphology depends not only on the intrinsic crystal structure but also on the synthesis conditions.24 In our experiment, because core-shell microspheres have been formed initially, in the following dissolving-recrystallizing process, the low concentration of the growth nutrients would be insufficient for the {100} faces to grow perfectly. To further lower the energy of the system, nanoparticles on the crystals would dissolve and recrystallize in situ to form other faces, such as {110} and {111} faces. Meanwhile, the nanoparticle assembles of the core parts also experienced the Ostwald ripening process. However, during the process, the size of the core parts does not changed much. This can be confirmed by the FESEM images of the products obtained at 150 °C for different reaction times. Therefore, hollow core-shell ZnSn(OH)6 microcrystals with polyhedral morphology were formed in the end.
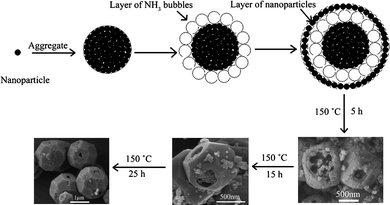 | ||
| Scheme 1 Schematic illustration describing the formation of ZnSn(OH)6 hollow core-shell polyhedral microcrystals. | ||
Control experiments were further conducted using other ammonium salts such as ammonium carbonate instead of NH4F with the other conditions unchanged. The product obtained shows that hollow core-shell ZnSn(OH)6 microspheres can also be obtained (ESI†, Fig. S4a,b). When no ammonium carbonate was added in the reaction solution, no hollow core-shell could be observed, as shown in Fig. S4c. This further supports the formation mechanism that the in situ produced NH3 gas bubbles act as soft template to the formation of hollow core-shell ZnSn(OH)6 microspheres.
Conclusion
In summary, ZnSn(OH)6 hollow core-shell microspheres have been successfully prepared via a facile NH3 bubble templating method. Then, via a hydrothermal recrystallizing process, ZnSn(OH)6 hollow core-shell polyhedral microcrystals have also been obtained. The optimal condition for the formation of hollow core-shell ZnSn(OH)6 microcrystals is that the concentration of NH4F is at 0.25 mol L−1 while the concentration of NaOH is fixed at 0.25 mol L−1. A possible mechanism is proposed based on our experiment results. This method is simple and easily controllable, and may be used to prepare other hollow or core-shell micro/nanostructures.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 20775044) and the Natural Science Foundation of Shandong province, China (Y2006B20).Notes and references
- P. A. Cusack, M. S. Heer and A. W. Monk, Polym. Degrad. Stab., 1991, 32, 177 CrossRef CAS.
- F. Andre, P. A. Cusack, A. W. Monk and R. Seangprasertkij, Polym. Degrad. Stab., 1993, 40, 267 CrossRef CAS.
- P. A. Cusack and P. R. J. Hornsby, J. Vinyl Addit. Technol., 1999, 5, 21 Search PubMed.
- Z. Y. Yuan, F. Huang, J. T. Sun and Y. H. Zhou, Chem. Lett., 2002, 408 CrossRef CAS.
- Z. Lu and Y. Tang, Mater. Chem. Phys., 2005, 92, 5 CrossRef CAS.
- W. J. Moon, J. H. Yu and G. M. Choi, Sens. Actuators, B, 2001, 80, 21 CrossRef.
- W. W. Wang, Y. J. Zhu and L. X. Yang, Adv. Funct. Mater., 2007, 17, 59 CrossRef CAS.
- A. P. Alivisatos, Science, 1996, 271, 933 CrossRef CAS.
- M. A. El-Sayed, Acc. Chem. Res., 2001, 34, 257 CrossRef CAS.
- M. Inagaki, T. Kuroishi, Y. Yamashita and M. Z. Urata, Z. Anorg. Allg. Chem., 1985, 527, 193 CrossRef CAS.
- D. Kovacheva and K. Petrov, Solid State Ionics, 1998, 109, 327 CrossRef CAS.
- H. Jena, K. V. Govindan Kutty and T. R. N. Kutty, Mater. Chem. Phys., 2004, 88, 167 CrossRef CAS.
- Y. J. Zhang, M. Guo, M. Zhang, C. Y. Yang, T. Ma and X. D. Wang, J. Cryst. Growth, 2007, 308, 99 CrossRef CAS.
- G. Wrobel, M. Piech, S. Dardona, Y. Ding and P. X. Gao, Cryst. Growth Des., 2009, 9, 4456 CrossRef CAS.
- H. C. Zeng, Int. J. Nanotechnol., 2007, 4, 329 Search PubMed.
- H. C. Zeng, J. Mater. Chem., 2006, 16, 649 RSC.
- Q. Peng, Y. J. Dong and Y. D. Li, Angew. Chem., Int. Ed., 2003, 42, 3027 CrossRef CAS.
- F. Gu, C. Z. Li, S. F. Wang and M. K. Lu, Langmuir, 2006, 22, 1329 CrossRef CAS.
- X. J. Wang, F. Q. Wan, J. Liu, Y. J. Gao and K. Jiang, J. Alloys Compd., 2009, 474, 233 CrossRef CAS.
- L. Zou, X. Xiang, M. Wei, F. Li and D. G. Evans, Inorg. Chem., 2008, 47, 1361 CrossRef CAS.
- D. Deng and J. Y. Lee, Chem. Mater., 2008, 20, 1841 CrossRef CAS.
- W. Z. Ostwald, Phys. Chem., 1897, 22, 289 CAS.
- W. Z. Ostwald, Phys. Chem., 1900, 34, 495.
- X. Y. Chen, M. H. Qiao, S. H. Xie, K. N. Fan, W. Z. Zhou and H. Y. He, J. Am. Chem. Soc., 2007, 129, 13305 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM, SAED, HRTEM and FESEM images. See DOI: 10.1039/c0ce00050g |
| This journal is © The Royal Society of Chemistry 2011 |
