DOI:
10.1039/C0CE00547A
(Communication)
CrystEngComm, 2011,
13, 52-54
A chirally helical coordination polymer self-assembled with the ligand 2-(2-carboxyphenyl)imidazo(4,5-f)(1,10)phenanthroline: crystal structure, SHG response and tunable photoluminescence†
Received
19th August 2010
, Accepted 8th October 2010
First published on 28th October 2010
Abstract
A chirally helical coordination polymer [Zn(H2O)(ONCP)Cl]n self-assembled with ligand 2-(2-carboxyphenyl)imidazo(4,5-f)(1,10)phenanthroline displays second harmonic generation efficiency, which is approximately two times as much as that of potassium dihydrogen phosphate (KDP), and tunable photoluminescence images form yellow-green to white based on the variation of excitation light.
The rational design and construction of new coordination polymers based upon assembly of metal ions and multifunctional organic ligands is an interesting research field due to their intriguing structural diversities and potential applications in functional materials.1 Especially, the construction of chiral coordination polymers has aroused a great deal of attention because of their utility in chiral synthesis, asymmetric heterogeneous catalysis, enantioselective separation, second-order non-linear optics, and so on.2 Abundant chiral coordination polymers have been synthesized, including introduction of chiral organic ligands, chiral metal compounds, or spontaneous solution from achiral materials without any chiral auxiliaries.3 Apparently, the topological structures of resulting coordination polymer are mostly dependent on geometry of metal centers and bonding mode of bridging ligands.4 We have employed a designed long-conjugated ligand HNCP (2-(4-carboxyphenyl)imidazo(4,5-f)(1,10)phenanthroline) with phenanthroline nitrogen and carboxylate oxygen donors in either end to link tetrahedrally connected Mn(II) centers, creating a robust 7-fold interpenetration diamondoid network.5 By using another new designed ligand O-HNCP (2-(2-carboxyphenyl)imidazo(4,5-f)(1,10)-phenanthroline) (Scheme 1), a helical coordination polymer [Zn(H2O)(ONCP)Cl]n (1) with chiral space groupP21 was successfully self-assembled by ONCP− anions with end phenanthroline nitrogen and side carboxylate oxygen donors alternately bridging Zn(II) ions. The non-linear optical property of 1 and tunable photoluminescence from green-yellow to white based on the variation of excitation light are discussed in this communication.
 |
| | Scheme 1 Structure and synthetic route of O-HNCP. | |
The compound 1 was synthesized hydrothermally by treating ZnCl2 and designed ligand O-HNCP at 140 °C.‡ All major peaks of experimental powder X-ray pattern (XRPD) match quite well with that of the simulated XRPD (Fig. S1 see ESI), indicating reasonable crystalline phase purity of compound 1.† Homochiral crystalline packing of right-handed or left-handed helices was revealed by X-ray single crystal diffraction investigations.† As shown in Fig. 1, the Zn(II) ion is pentacoordinate to one chlorine anion in an apical position and to the four N2O2 atoms from two phenanthroline nitrogen atoms, one coordination water and one carboxylate oxygen atom. Adopting a μ2-ηN2:ηO1 mode in N,N-chelating and O-monodentate fashion, ONCP− anions, in which the phenyl ring and imidazole ring rotate around C–C bond in opposite direction with the dihedral angle of about 31°, alternately bridge Zn(II) ions to form 1D left-handed or right-handed helical chains along the 21 screw axes in the crystals of compound 1 (Fig. 2). Strikingly, four randomly selected crystals from the same batch all showed the chiral space groupP21 and nearly zero Flack parameters χ (0.04(2), 0.065(14), 0.02(3), 0.020(17), respectively), indicating homochirality in the crystals.6 In other words, we structurally identified both right-(P) and left-(M) handed helices of two different crystals from same batch, demonstrating a case of conglomeration (racemic mixture of chiral crystals).7 The homochirality in the crystals of 1 could be explained by strong supramolecular interactions between the single-helical chains. As shown in Fig. 3 and 4, the strong hydrogen bonding interactions between N–H group and chlorine atom, coordination water and chlorine atom or uncoordinated carboxylate oxygen atom make the single-helical chains array uniformly in crystallographic bc-plane, and π–π attraction between phenyl ring and pyridine ring, in offset face-to-face mode with the parallel distance of about 3.5 Å, make the single-helical chains array uniformly in crystallographic ab-plane. Thus, compound 1 possesses a chiral supramolecular architecture due to the existence of supramolecular interactions between the single-helical chains, through which the same chirality is preserved. Most coordination helices in the 3D crystal framework are formed by the self-assembly of achiral or racemic ligands with metal ions and therefore yield racemates of right-handed and left-handed helices.8 In a few cases, the use of enantiopure ligands leads to the formation of chiral helices.9 The compound 1 represents spontaneous resolution of chiral helices through supramolecular interactions.7,10 Second-order non-linear optical effect for microcrystalline 1 was investigated by optical second harmonic generation (SHG) on the basis of the principles proposed by Kurtz and Perry.11 The intensity of green light (frequency-doubled output: 532 nm) produced by microcrystalline 1 is approximately two times as much as that produced by KDP power. The SHG response is consist with the uniform array of single-helical chains along crystallographic b axis in the crystals of 1.
 |
| | Fig. 1 ORTEP drawing with 30% probability ellipsoids of enantiomorph in the left-handed (a) and right-handed (b) helices of compound 1. Symmetry code: #1 − x + 1, y + 1/2, −z + 1; #2 −x, y − 1/2, −z + 1. | |
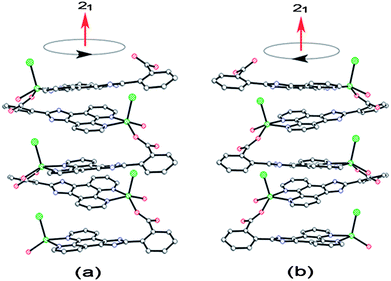 |
| | Fig. 2 1D right-handed (a) and left-handed (b) helical chains. | |
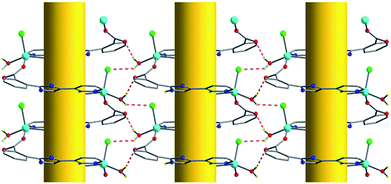 |
| | Fig. 3 H-bonding interactions (red dish lines) between single-helical chains, viewed from the crystallographic a-axis, golden cylinders representing 21 screw axes. | |
 |
| | Fig. 4 π–π attraction between single-helical chains, viewed from the crystallographic c-axis. | |
Another important feature of 1 is the strong yellow-green luminescence. Fig. 5 shows the excitation and emission spectra of free ligand O-HNCP and compound 1 in the solid state. Compound 1 exhibits strong yellow-green emission with broad band in the range of 450–800 nm and the strongest wavelength λmax at 550 nm. The excitation peak is located in the range of 380–420 nm, which is blue shifted compared with the free ligand. In addition, the luminescence lifetime is measured to be 22 ns, indicating its fluorescence characteristic. Notably, compound 1 exhibits different photoluminescence images to the naked eye upon variation of excitation light. When excited at 380 nm, compound 1 exhibits characteristic yellow-green emission light. Upon excitation in the range of 400–420 nm, the yellow-green emission light mixes with the purplish-blue excitation light, creating bright white image. The yellow-green emission of compound 1 is intraligand fluorescence emission in nature. But the excitation peak of 1 differ from the free ligand because of the interaction of Zn(II) ion and ONCP− ligand, resulting in tunable photoluminescence images from yellow-green to white based on the variation of excitation light.
![Solid-state PL spectra of ligand O-HNCP and [Zn(H2O)(ONCP)Cl]n. Inset: PL images of the sample of [Zn(H2O)(ONCP)Cl]n excited by 380 nm, 400 nm and 420 nm light, respectively.](/image/article/2011/CE/c0ce00547a/c0ce00547a-f5.gif) |
| | Fig. 5 Solid-state PL spectra of ligand O-HNCP and [Zn(H2O)(ONCP)Cl]n. Inset: PL images of the sample of [Zn(H2O)(ONCP)Cl]n excited by 380 nm, 400 nm and 420 nm light, respectively. | |
In conclusion, the helical chain [Zn(H2O)(ONCP)Cl]n is self-assembled by using designed ligand O-HNCP and strong supramolecular interactions make the right-handed or left-handed helices array uniformly along crystallographic b-axis, resulting in chiral space groupP21. The special excitation and emission character of the title compound creates tunable photoluminescence images form yellow-green to white based on variation of excitation light, which suggest that the title compound may be used as potential phosphor for a white-light-emitting diode (white LED). In the past two decades, the photoluminescence of metal–organic frameworks have received intense interest due to their fascinating structures and plentiful charge-transfer types. But studies on the photoluminescence of single-component coordination compounds for the use of phosphors on white LEDs are still in their infancy.12 Taking advantage of complementary colour theory to search single-component coordination compounds for the application on white LEDs is a noticeable research field.
Acknowledgements
We gratefully acknowledge the financial support of Natural Science Foundation of China (no. 20973147), 973 Project (2007CB805307) and Project of Fujian Province (2007F3115).
Notes and references
-
(a) J. Y. Lu, Coord. Chem. Rev., 2003, 246, 327 CrossRef CAS;
(b) S. L. James, Chem. Soc. Rev., 2003, 32, 276 RSC;
(c) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS;
(d) O. R. Evans and W. Lin, Acc. Chem. Res., 2002, 35, 511 CrossRef CAS;
(e) S. Subramanian and M. J. Zaworotko, Coord. Chem. Rev., 1994, 137, 357 CrossRef CAS;
(f) A. M. Beatty, Coord. Chem. Rev., 2003, 246, 131 CrossRef CAS;
(g) D. Braga, L. Maini, M. Polito, E. Tagliavini and F. Grepioni, Coord. Chem. Rev., 2003, 246, 53 CrossRef CAS;
(h) M. Hong, Cryst. Growth Des., 2007, 7, 10 CrossRef CAS;
(i) Z. Ma, R. Hopson, C. Cai, S. Han and B. Moulton, Cryst. Growth Des., 2010, 10, 2376 CrossRef CAS.
-
(a) M. E. Davis, Acc. Chem. Res., 1993, 26, 111 CrossRef CAS;
(b) A. Müller, F. Peters, M. T. Pope and D. Gatteschi, Chem. Rev., 1998, 98, 239 CrossRef;
(c) E. Q. Gao, Y. F. Yue, S. Q. Bai, Z. He and C. H. Yan, J. Am. Chem. Soc., 2004, 126, 1419 CrossRef CAS;
(d) D. A. J. Judd, J. H. Nettles, N. Nevins, J. P. Snyder, D. C. Liotta, J. Tang, J. Ermolieff, R. F. Schinazi and C. L. Hill, J. Am. Chem. Soc., 2001, 123, 886 CrossRef CAS.
-
(a) C. Piguet, G. Bernardinelli and G. Hopfgartner, Chem. Rev., 1997, 97, 2005 CrossRef CAS;
(b) J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kimoon, Nature, 2000, 404, 982 CrossRef CAS;
(c) R. G. Xiong, J. L. Zuo, X. Z. You, B. F. Abrahams, Z. P. Bai, C. M. Che and H. K. Fun, Chem. Commun., 2000, 2061 RSC;
(d) P. A. Maggard, C. L. Stern and K. R. Poeppelmeier, J. Am. Chem. Soc., 2001, 123, 7742 CrossRef CAS;
(e) I. Katsuki, Y. Motoda, Y. Sunatsuki, N. Matsumoto, T. Nakashima and M. Kojima, J. Am. Chem. Soc., 2002, 124, 629 CrossRef CAS;
(f) T. Ezuhara, K. Endo and Y. Aoyama, J. Am. Chem. Soc., 1999, 121, 3279 CrossRef CAS;
(g) K.-Z. Shao, Y.-H. Zhao, Y. Xing, Y.-Q. Lan, X.-L. Wang, Z.-M. Su and R.-S. Wang, Cryst. Growth Des., 2008, 8, 2986 CrossRef CAS;
(h) Y.-H. Xu, Y.-Q. Lan, S.-X. Wu, K.-Z. Shao, Z.-M. Su and Y. Liao, CrystEngComm, 2009, 11, 1711 RSC;
(i) W. Liu, Y. Song, Y. Z. Li, Y. Zou, D. Dang, C. Ni and Q. Meng, Chem. Commun., 2004, 2348 RSC.
-
(a) O. M. Yaghi, H. Li, C. David, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS;
(b) C. J. Kepert, T. J. Prior and M. J. Rosseinsky, J. Am. Chem. Soc., 2000, 122, 5158 CrossRef CAS;
(c) B. L. Chen, M. Eddaoudi, S. T. Hyde, M. O'Keeffe and O. M. Yaghi, Science, 2001, 291, 1021 CrossRef CAS;
(d) H. K. Chae, D. Y. Siberioperez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523 CrossRef CAS;
(e) D. Sun, S. Ma, Y. Ke, T. M. Petersen and H.-C. Zhou, Chem. Commun., 2005, 2663 RSC;
(f) Y. Wei, Y. Yu and K. Wu, Cryst. Growth Des., 2008, 8, 2087 CrossRef CAS;
(g) Y. Wei, Y. Yu, R. Sa, Q. Li and K. Wu, CrystEngComm, 2009, 11, 1054 RSC.
- Y. Wei, Y. Yu and K. Wu, Cryst. Growth Des., 2007, 7, 2262 CrossRef CAS.
-
(a) H. D. Flack and G. Bernardinelli, Acta Crystallogr., Sect. A: Found. Crystallogr., 1999, 55, 908 CrossRef;
(b) H. D. Flack and G. Bernardinelli, J. Appl. Crystallogr., 2000, 33, 1143 CrossRef CAS.
-
(a) V. Balamurugan and R. Mukherjee, CrystEngComm, 2005, 7, 337 RSC;
(b) U. Siemeling, I. Scheppelmann, B. Neumann, A. Stammler, H.-G. Stammler and J. Frelek, Chem. Commun., 2003, 2236 RSC;
(c) K. Biradha, C. Seward and M. J. Zaworotko, Angew. Chem., Int. Ed., 1999, 38, 492 CrossRef CAS;
(d) X.-D. Zheng and T.-B. Lu, CrystEngComm, 2010, 12, 324 RSC.
-
(a) Y. Wang, J. Yu, M. Guo and R. Xu, Angew. Chem., Int. Ed., 2003, 42, 4089 CrossRef CAS;
(b) J. Hou, M. Li, Z. Li, S. Zhan, X. Huang and D. Li, Angew. Chem., Int. Ed., 2008, 47, 1711 CrossRef CAS;
(c) Y. Sun, J. Zhang, Y. Chen and G. Yang, Angew. Chem., Int. Ed., 2005, 44, 5814 CrossRef CAS;
(d) W.-G. Lu, J.-Z. Gu, L. Jiang, M.-Y. Tan and T.-B. Lu, Cryst. Growth Des., 2008, 8, 192 CrossRef CAS;
(e) Y. Qi, F. Luo, S. R. Batten, Y.-X. Che and J.-M. Zheng, Cryst. Growth Des., 2008, 8, 2806 CrossRef CAS.
-
(a) H. An, E. Wang, D. Xiao, Y. Li, Z. Su and L. Xu, Angew. Chem., Int. Ed., 2006, 45, 904 CrossRef CAS;
(b) N. Ohata, H. Masuda and O. Yamauchi, Angew. Chem., Int. Ed. Engl., 1996, 35, 531 CrossRef CAS;
(c) O. Mamula, A. V. Zelewsky, T. Bark and G. Bernardinelli, Angew. Chem., Int. Ed., 1999, 38, 2945 CrossRef CAS;
(d) S.-P. Wu and C.-H. Lee, CrystEngComm, 2009, 11, 219 RSC.
-
(a) Q. Ye, X.-S. Wang, H. Zhao and R.-G. Xiong, Tetrahedron: Asymmetry, 2005, 16, 1595 CrossRef CAS;
(b) F. Dai, H. He, X. Zhao, Y. Ke, G. Zhang and D. Sun, CrystEngComm, 2010, 12, 337 RSC.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798 CrossRef CAS.
-
(a) Y.-C. Liao, C.-H. Lin and S.-L. Wang, J. Am. Chem. Soc., 2005, 127, 9986 CrossRef CAS;
(b) W. Ki and J. Li, J. Am. Chem. Soc., 2008, 130, 8114 CrossRef CAS;
(c) M.-S. Wang, S.-P. Guo, Y. Li, L.-Z. Cai, J.-P. Zou, G. Xu, W.-W. Zhou, F.-K. Zheng and G.-C. Guo, J. Am. Chem. Soc., 2009, 131, 13572 CrossRef CAS;
(d) M.-S. Wang, G.-C. Guo, W.-T. Chen, G. Xu, W.-W. Zhou, K.-J. Wu and J.-S. Huang, Angew. Chem., Int. Ed., 2007, 46, 3909 CrossRef CAS;
(e) P. Coppo, M. Duati, V. N. Kozhevnikov, J. W. Hofstraat and L. Cola, Angew. Chem., Int. Ed., 2005, 44, 1806 CrossRef CAS;
(f) R.-S. Liu, V. Drozd, N. Bagkar, C.-C. Shen, I. Baginskiy, C.-H. Chen and C. H. Tan, J. Electrochem. Soc., 2008, 155, P71 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of experiment, experimental and simulated XRPD, thermogravimetric analysis for the title compound. CCDC reference numbers 772274 and 789384. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ce00547a |
| ‡ Preparation: the synthetic route of O-HNCP is the same as that of HNCP, except that 2-carboxybenzaldehyde is substituted for 4-carboxybenzaldehyde. Preparation of [Zn(H2O)(ONCP)Cl]n (1): hydrothermal treatment of ZnCl2 (0.3 mmol), O-HNCP (0.4 mmol) and H2O (25 mL) in an autoclave at 140 °C for 3 d gave pale yellow crystals of 1 (yield: 82% based on O-HNCP). Anal. calcd for Zn1C20H13N4O3: C 52.43, H 2.86, N 12.23, O 10.48%. Found: C 52.38, H 2.90, N 12.27, O 10.54%. Crystal data for left-handed helix of compound 1: Zn1C20H13N4O3, Mr = 458.16, monoclinic, space groupP21, a = 9.287(3) Å, b = 7.0017(19) Å, c = 13.168(4) Å, β = 91.941(4)°, V = 855.7(4) Å3, Z = 2, ρc = 1.778 g cm−3, μ = 1.625 mm−1, reflections collected 6538, refinement of 3566 reflections, 270 parameters, and 4 restraints yielded wR2 = 0.0974 for all data and a conventional R1 = 0.0292 based on 3215 reflections with I > 2σ(I), Flack parameter value χ = 0.065(14). Crystal data for right-handed helix of compound 1: Zn1C20H13N4O3, Mr = 458.16, monoclinic, space groupP21, a = 9.291(5) Å, b = 7.006(3) Å, c = 13.182(6) Å, β = 91.935(7)°, V = 857.5(7) Å3, Z = 2, ρc = 1.775 g cm−3, μ = 1.622 mm−1, reflections collected 6621, refinement of 3327 reflections, 270 parameters, and 4 restraints yielded wR2 = 0.1136 for all data and a conventional R1 = 0.0336 based on 2897 reflections with I > 2σ(I), Flack parameter value χ = 0.020(17). |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 




![Solid-state PL spectra of ligand O-HNCP and [Zn(H2O)(ONCP)Cl]n. Inset: PL images of the sample of [Zn(H2O)(ONCP)Cl]n excited by 380 nm, 400 nm and 420 nm light, respectively.](/image/article/2011/CE/c0ce00547a/c0ce00547a-f5.gif)
