Covalent assistance to supramolecular synthesis: directing the supramolecular assembly of co-crystals by in situ modification of hydrogen bonding functionality†
Andreas
Lemmerer
*a,
Joel
Bernstein
*b and
Volker
Kahlenberg
c
aMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Johannesburg, 2050, South Africa. E-mail: andreas.lemmerer@gmail.com; Fax: +27 11 717 6749; Tel: +27 11 717 6723
bDepartment of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, 84105, Israel. E-mail: yoel@bgu.ac.il; Fax: +972 8 647 2943; Tel: +972 8 647 2455
cInstitute of Mineralogy and Petrography, University of Innsbruck, Innsbruck, Austria. Fax: +43 512 507 2926; Tel: +43 512 507 5503
First published on 1st November 2010
Abstract
Nicotinic acid hydrazide (niazid) readily co-crystallizes with carboxylic acids in methanol to form a 2-D sheet structure that utilizes all three H bond donors on the carbohydrazide functional group. In acetone solution niazid undergoes a condensation reaction with the solvent, which replaces the amine group with a hydrocarbon group leaving only one hydrogen bond donor on the modified niazid molecule, now containing a N-acylhydrazone functional group. The resulting reduced hydrogen bonding functionality leads to a new supramolecular assembly when co-crystallized with the same dicarboxylic acids.
Supramolecular synthesis makes use of intermolecular interactions to assemble molecules, and the understanding of how these weaker interactions (compared to covalent bonds) can be controlled has been the subject of great interest in the last decade.1,2 In fact, the ultimate goal is that the same level of understanding that now exists in covalent bond formation can be attained in non-covalent bond formation.3 The similarity of crystal engineering to organic synthesis has been noted by Desiraju and many concepts and strategies have been successfully transferred. Akin to Corey's synthon concept in synthetic organic chemistry, a supramolecular synthon has been defined1a as one of the principal synthetic tools for the supramolecular chemist,4 preeminent being the hydrogen bond, which is the most energetic and directional of intermolecular interactions.5 There has been a considerable measure of success in identifying hydrogen bonding interactions that occur reliably and robustly in a number of different environments and they are used to create specific assemblies.6–10
Supramolecular synthesis and organic synthesis have been combined in assisting the latter by supramolecular assistance to organic synthesis,11 where the molecules to be covalently reacted are positioned to do so in a desired or controlled manner by templating or by self-assembly.11d In this report we propose the reverse concept, namely covalent assistance to supramolecular synthesis. The realisation of this strategy is based on the assumption that organic synthesis and supramolecular synthesis can be performed in situ in one reaction vessel if certain functional groups are present.12 For instance, when co-crystallizing the antituberculosis drug isonicotinic acid hydrazide (isoniazid) with 3-hydroxybenzoic acid, the choice of solvent can be a product defining factor (Scheme 1). If acetone or 2-butanone is used, the –NH2group of the carbohydrazide functional group undergoes a Schiff-based condensation reaction with the solvent,13 and replaces the two H atoms with an N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond to either a propy-2-ylidene or butan-2-ylidene group respectively. Nonetheless, consistent with the previously noted affinity of carboxylic acids and pyridine,7,8 the 3-hydroxybenzoic acid molecule hydrogen bonds to the pyridine of the new molecule, containing now a N-acylhydrazone group instead of the carbohydrazide functionality, to produce co-crystals 1 and 2. The covalent reaction followed by the supramolecular reaction described for isoniazid is not dissimilar in concept to those of prodrugs, where a drug molecule undergoes a change in structure in vivo, prior to participating in a supramolecular event.14
C double bond to either a propy-2-ylidene or butan-2-ylidene group respectively. Nonetheless, consistent with the previously noted affinity of carboxylic acids and pyridine,7,8 the 3-hydroxybenzoic acid molecule hydrogen bonds to the pyridine of the new molecule, containing now a N-acylhydrazone group instead of the carbohydrazide functionality, to produce co-crystals 1 and 2. The covalent reaction followed by the supramolecular reaction described for isoniazid is not dissimilar in concept to those of prodrugs, where a drug molecule undergoes a change in structure in vivo, prior to participating in a supramolecular event.14
 | ||
| Scheme 1 The two simultaneous covalent and supramolecular synthesis steps carried out in the co-crystallisation of isoniazid and 3-hydroxybenzoic acid.12 Note that the R22(7) heterosynthon and C(4) homosynthon are retained in both reactions resulting in almost identical crystal packing (see Fig. 6 in ref. 12). | ||
This raises the possibility of performing two types of supramolecular synthesis: (i) one in which no covalent reaction takes place, in other words a conventional supramolecular synthesis between two components, and (ii) a covalent assisted supramolecular synthesis, where one of the supramolecular reactants undergoes a deliberate covalent reaction with a second component, before co-crystallizing with a third component, as shown in Scheme 2. We demonstrate this new concept and its potential utility by performing four co-crystallizations with nicotinic acid hydrazide (niazid) and the dicarboxylic acids succinic, adipic and sebacic acid (Scheme 3).‡
 | ||
| Scheme 2 The two types of supramolecular synthesis between niazid and adipic acid: conventional supramolecular synthesis in co-crystal 3 forms 2-D sheets, whereas the covalent-assisted supramolecular synthesis features a covalent reaction with niazid and subsequent co-crystallization with adipic acid to form 1-D ribbons (4). | ||
 | ||
| Scheme 3 Co-crystal components: supramolecular reagent niazid, acetone, and dicarboxylic acids used in co-crystallization experiments. | ||
Analogous to traditional bond synthesis, it is possible to prepare supramolecular reaction schemes for the hydrogen bonding interactions used in supramolecular synthesis, including the different “reagent” hydrogen bond homosynthons and heterosynthons, and the solvent of crystallizations used (Scheme 2). For example, we have shown previously for isoniazid12 that the hydrogen bonded homosynthon C(4)15 connects the isoniazid molecules into chains, and that the ring-type R22(7)15 heterosynthons connect the carboxylic acid containing molecule to the pyridine of the isoniazid. By analogy, this isomeric modification might be expected to lead to the same synthons in the co-crystallizing experiments with nicotinic acid hydrazide (niazid), and in this discussion we limit ourselves to these two supramolecular synthons, while recognizing that other hydrogen bonded synthons are possible in this situation.
In a conventional supramolecular synthesis, i.e. the dissolution of two compounds with similar solubilities, and co-crystallization by slow evaporation, one obtains a co-crystal with a specific assembly of molecules depending on the primary hydrogen bonded interactions.16 Co-crystal 3 was obtained in such a manner, where 1 equivalent of adipic acid was dissolved with two equivalents of niazid in methanol.§ The ratio of the two starting materials was determined by the two expected primary hydrogen bond interactions, namely the two carboxylic acids hydrogen bonding to the single pyridine of two niazid molecules. Indeed, the crystallographic asymmetric unit of the resulting crystal structure reflects this stoichiometry, containing one niazid and half an adipic acid molecule. The crystal packing consists of flat 2-D sheets, in which the two molecules lie approximately co-planar. The expected R22(7) heterosynthon is formed between the two adipic acid and one niazid molecule lying on a crystallographic center of symmetry (which requires the pyridine ring and carboxylic acid to be co-planar), while a C(4) homosynthon through the amide group connects adjacent niazid molecules within the plane (Fig. 1a). The 2-D sheets are stacked in a parallel fashion via a combination of inversion centers and translation, and are connected by the two amine protons, protruding above and below the sheet (Fig. 1b). Hence, in 3, all three H bond donors are used, with the two amine protons linking the layering of the 2-D sheets.
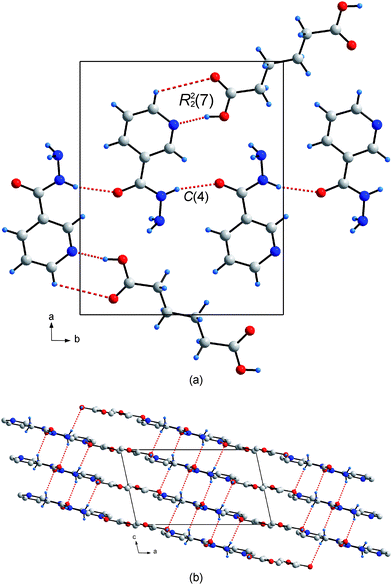 | ||
| Fig. 1 (a) The primary heterosynthon and homosynthon between the niazid and adipic acid molecule resulting in a planar relative arrangement. The planar sheets are stacked plane-to-plane and connected by hydrogen bonds of the amine protons to the O atoms of adjacent sheets. | ||
The second supramolecular synthesis carried out is novel. The conventional supramolecular synthesis, as described for 3, is assisted by a covalent reaction. The two starting materials were dissolved in acetone rather than methanol to facilitate the Schiff-base condensation reaction between the solvent acetone and the niazid molecule as described above. The amine of the carbohydrazide reacts with the carbonyl of the acetone, to form N′-(propan-2-ylidene)nicotinohydrazide (p-niazid). The original carbohydrazide functional group is converted to a N-acetylhydrazone group. This reaction, which effectively replaces the two amine protons with a propyl-2-ylidene group is followed in situ by the supramolecular synthesis involving a co-crystallization of one adipic acid molecule with two p-niazid molecules (co-crystal 4) from the same (unreacted) acetone solvent. The primary hydrogen bonding is similar to 3, where the adipic acid hydrogen bonds via the R22(7) heterosynthon to the pyridine, and the C(4) homosynthon (i.e., the organic reaction did not affect the amide functionality) is still observed, connecting adjacent p-niazid molecules.17 Hence, one of the three original hydrogen bond donors is unchanged and participates in the same C(4) synthon as in the original co-crystal 3. It is the removal of the two amine protons that has the greatest effect. The overall crystal packing of the hydrogen bonded assembly of the resulting co-crystal is significantly changed. In the modified situation, a ribbon structure is formed, with the adipic acid molecules forming the backbone of the ribbon and the p-niazid molecules on the periphery (Fig. 2a). Ultimately, the hydrogen bonded assembly can be described as a herringbone shaped arrangement of ribbons, where adjacent ribbons are connected via C–H⋯N hydrogen bonds from the propyl-2-ylidiene group to the N atom in a second C(4) chain along the c-axis (Fig. 2b).
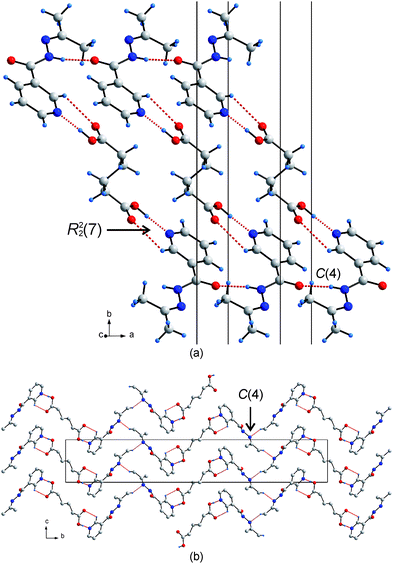 | ||
| Fig. 2 (a) The primary heterosynthon and homosynthon between the p-niazid and adipic acid molecule resulting in a ribbon arrangement. (b) The 1-D ribbons align in a herringbone fashion and are connected via a C–H⋯N hydrogen bond to form a C(4) chain. | ||
What do the co-crystals 3 and 4 signify in terms of crystal engineering? The idea behind a covalent assisted supramolecular synthesis is similar to a supramolecular assisted covalent synthesis. In the latter case, molecules are positioned in an arrangement that will allow for an organic reaction to take place, for example in the positioning of double bonds on adjacent molecules to allow solid state photodimerizations to occur.18 In the former and current case, we are altering the supramolecular assembly by changing the chemical identity of our supramolecular reagent which removes some of its hydrogen bonding functionality. In the co-crystals presented here, we first carried out a supramolecular synthesis that makes use of all three hydrogen bond donors on the carbohydrazide functional group of the niazid. In that supramolecular synthesis, the primary hydrogen bond synthons were observed to be R22(7) and C(4). Thereafter, we wanted to perform the “same” supramolecular synthesis, retaining the R22(7) and C(4) synthons, but remove the influence of the amine protons and their hydrogen bonds. It turns out that those hydrogen bonds indeed have a vital influence on the resulting three-dimensional architecture of the co-crystal, giving a 2-D structure in 3 and a ribbon motif in 4. Thus we were able to engineer the hydrogen bonded assembly and influence the outcome of the supramolecular synthesis of 4 but still retain the primary R22(7) and C(4) synthons.19 To be of potential utility such a hydrogen bonding protecting group must be of use in a number of different experiments or environments. This has been demonstrated in the co-crystals of 1 and 2, where two different protecting groups, a prop-2-ylidene and butan-2-ylidiene, were used but gave similar hydrogen bonding interactions.12 Further evidence of this general utility is shown in the co-crystals 5 and 6. Co-crystal 5 was prepared in an identical manner to 3, where succinic acid was co-crystallized with niazid from methanol. The crystal structure shows a topologic identity with 3 (Fig. 3a), with a puckered rather than flat, 2-D sheet. Similarly, in the co-crystal of sebacic acid with p-niazid 6 the amine protons were replaced by the prop-2-ylidene group with the resulting structure essentially isomorphous to 4 (Fig. 3b).20
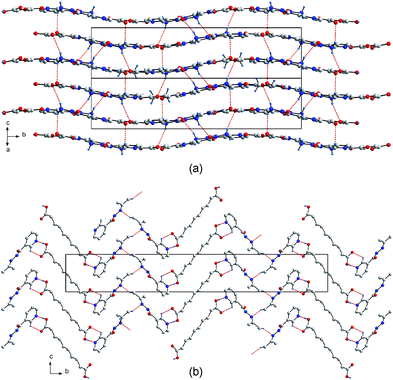 | ||
| Fig. 3 The similar crystal architecture of co-crystals 5 (a) and 6 (b), mimicking those of 3 and 4 respectively. | ||
Thus, we have demonstrated the utility of a strategy to control the supramolecular assembly of co-crystals by employing traditional organic bond synthesis to selectively remove specific elements of the hydrogen bond functionality, yet retain the desired supramolecular synthon formation of the co-crystals. As stated by Fyfe and Stoddart,11b “…supramolecular chemistry—the chemistry of the intermolecular bond—has established itself in the realm of the synthetic chemist…”. Conversely, as is presented here, organic chemistry can be a vital asset to the supramolecular chemist in engineering new materials.
This work was supported in part by Grant No. 2004118 from the United States–Israel Binational Science Foundation (Jerusalem). A.L. thanks the South African National Research Foundation for a postdoctoral scholarship (SFP2007070400002) and the Oppenheimer Memorial Trust for financial support. The authors thank Dr Dmitry Mogilyanski (Ben-Gurion University of the Negev) and Dr Daniel Többens (University of Innsbruck) for powder diffraction work.
Notes and references
- (a) G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS; (b) G. R. Desiraju, Curr. Opin. Solid State Mater. Sci., 1997, 2, 451 CrossRef CAS; (c) M. D. Hollingsworth, Science, 2002, 295, 2410 CAS; (d) G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 2 CrossRef.
- D. N. Reinhoudt, J. F. Stoddart and R. Ungaro, Chem.–Eur. J., 1998, 4, 1349 CrossRef.
- (a) C. B. Aakeröy and D. J. Salmon, CrystEngComm, 2005, 7, 439 RSC; (b) C. B. Aakeröy, J. Desper, E. Elisabeth, B. A. Helfrich, B. Levin and J. F. Urbina, Z. Kristallogr., 2005, 220, 325 CrossRef.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS.
- (a) C. B. Aakeröy and K. R. Seddon, Chem. Soc. Rev., 1993, 22, 397 RSC; (b) A. D. Burrows, Struct. Bonding, 2004, 108, 55 CAS; (c) G. R. Desiraju, Acc. Chem. Res., 2002, 35, 565 CrossRef CAS.
- (a) K. D. M. Harris, N. M. Stainton, A. M. Callan and R. A. Howie, J. Mater. Chem., 1993, 3, 947 RSC; (b) J. C. MacDonald and G. M. Whitesides, Chem. Rev., 1994, 94, 2383 CrossRef CAS; (c) J. A. Zerkowski, J. C. MacDonald, C. T. Seto, D. A. Wierda and G. M. Whitesides, J. Am. Chem. Soc., 1994, 116, 2382 CrossRef CAS; (d) J. J. Kane, R.-F. Liao, J. W. Lauher and F. W. Fowler, J. Am. Chem. Soc., 1995, 117, 12003 CrossRef CAS; (e) S. Palacin, D. N. Chin, E. E. Simanek, J. C. MacDonald, G. M. Whitesides, M. T. McBride and G. T. R. Palmore, J. Am. Chem. Soc., 1997, 119, 11807 CrossRef CAS; (f) J. C. MacDonald, P. C. Dorrestein, M. M. Pilley, M. M. Foote, J. L. Lundburg, R. W. Henning, A. J. Schultz and J. L. Manson, J. Am. Chem. Soc., 2000, 122, 11692 CrossRef CAS; (g) J. M. A. Robinson, D. Philip, K. D. M. Harris and B. M. Kariuki, New J. Chem., 2000, 24, 799 RSC; (h) J. C. MacDonald, P. C. Dorrestein and M. M. Pilley, Cryst. Growth Des., 2001, 1, 29 CrossRef CAS; (i) S. Ahn, J. P. Reddy, B. M. Kariuki, S. Chatterjee, A. Ranganathan, V. R. Pedireddi, C. N. R. Rao and K. D. M. Harris, Chem.–Eur. J., 2005, 11, 2433 CrossRef CAS; (j) L. S. Reddy, N. J. Babu and A. Nangia, Chem. Commun., 2006, 1369 RSC.
- A. Lemmerer, N. B. Báthori and S. A. Bourne, Acta Crystallogr., Sect. B: Struct. Sci., 2008, 64, 780 CrossRef.
- (a) T. R. Shattock, K. K. Arora, P. Vishweshwar and M. J. Zaworotko, Cryst. Growth Des., 2008, 8, 4533 CrossRef CAS; (b) R. D. B. Walsh, M. W. Bradner, S. G. Fleischmann, L. A. Morales, N. Rodriquez-Hornando and M. J. Zaworotko, Chem. Commun., 2003, 186 RSC.
- (a) M. C. Etter, Acc. Chem. Res., 1990, 23, 120 CrossRef CAS; (b) C. B. Aakeröy and A. M. Beatty, Aust. J. Chem., 2001, 54, 409 CrossRef CAS.
- (a) C. B. Aakeröy, A. M. Beatty and B. A. Helfrich, Angew. Chem., Int. Ed., 2001, 40, 3240 CrossRef CAS; (b) C. B. Aakeröy, A. M. Beatty and B. A. Helfrich, J. Am. Chem. Soc., 2002, 124, 14425 CrossRef; (c) C. B. Aakeröy, A. M. Beatty and B. A. Helfrich, Cryst. Growth Des., 2003, 3, 159 CrossRef; (d) P. Vishweshwar, A. Nangia and V. M. Lynch, CrystEngComm, 2003, 5, 164 RSC; (e) B. R. Bhogala, S. Basavoju and A. Nangia, CrystEngComm, 2005, 7, 551 RSC.
- (a) G. M. Whitesides, E. E. Simanek, J. P. Mathias, C. T. Seto, D. N. Chin, M. Mammen and D. M. Gordon, Acc. Chem. Res., 1995, 28, 37 CrossRef CAS; (b) M. C. T. Fyfe and J. F. Stoddart, Acc. Chem. Res., 1997, 30, 393 CrossRef CAS; (c) S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898 CrossRef; (d) J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, Wiley-VCH, Weinheim, 1995 Search PubMed.
- A. Lemmerer, J. Bernstein and V. Kahlenberg, CrystEngComm, 2010, 12, 2856 RSC.
- M. J. Hearn, M. H. Cynamon, M. F. Chen, R. Coppins, J. Davis, H. J.-O. Kang, A. Noble, B. Tu-Sekine, M. S. Terrot, D. Trombino, M. Thai, E. R. Webster and R. Wilson, Eur. J. Med. Chem., 2009, 44, 4169 CrossRef CAS.
- Prodrugs: Challenges and Rewards, ed. V. Stella, R. Borchardt, M. Hageman, R. Oliyai, H. Maag and J. Tilley, Springer, 2007 Search PubMed.
- J. Bernstein, R. E. Davis, L. Shimoni and N.-L. Chang, Angew. Chem., Int. Ed. Engl., 1995, 34, 1555 CrossRef CAS.
- We are excluding proton transfer to form a molecular salt, which can be considered a simple example of a covalent reaction, where the breaking and forming of bonds involving H atoms creates a salt and a stronger hydrogen bonded interaction compared to the analogues neutral co-crystal.
- The crystal structure of the parent compound N′-(propan-2-ylidene)nicotinohydrazide (p-niazid) (7) has C(4) chains. 7: 0.500 g of nicotinic acid hydrazide (3.65 mmol) was dissolved in 5 ml of acetone, heated and stirred for 1 hour in a sealed vial, and colourless needle-like crystals were obtained by cooling, mp 141–142 °C.
- (a) L. R. MacGillivray, J. Org. Chem., 2008, 73, 3311 CrossRef CAS; (b) T. Friščić and L. R. MacGillivray, Aust. J. Chem., 2006, 59, 613 CrossRef CAS; (c) D.-K. Bučar and L. R. MacGillivray, J. Am. Chem. Soc., 2007, 129, 32 CrossRef CAS; (d) L. R. MacGillivray, G. S. Papaefstathiou, T. Friščić, T. D. Hamilton, D.-K. Bučar, Q. Chu, D. B. Varshney and I. G. Georgiev, Acc. Chem. Res., 2008, 41, 280 CrossRef CAS; (e) T. Odani and A. Matsumoto, CrystEngComm, 2002, 4, 467 RSC; (f) M. Pattabiraman, A. Natarajan, R. Kaliappan, J. T. Mague and V. Ramamurthy, Chem. Commun., 2005, 4542 RSC; (g) C. Avendaño and A. Briceño, CrystEngComm, 2009, 11, 408 RSC.
- The observed crystal structures do not necessarily represent the only possible results, as there are now different functional groups present as well as the unpredictability of the crystallization process. Additional crystallization experiments could possibly result in polymorphic co-crystals or different stoichiometric co-crystals.
- The single crystal structure of 6 showed evidence of modulation, which will be discussed in a separate publication. H. Krüger, A. Lemmerer, V. Kahlenberg and J. Bernstein, Acta Crystallogr., Sect. B, 2010 Search PubMed , to be submitted.
Footnotes |
| † Electronic supplementary information (ESI) available: Cif files, PXRDs and details of crystal structure refinements. CCDC reference numbers 753783–753786. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ce00347f |
| ‡ Single crystal diffraction data were collected on an Oxford Diffraction Gemini R Ultra diffractometer equipped with a Ruby CCD-detector with Mo-Kα radiation (λ = 0.71073 Å, graphite monochromator, mono-capillary collimator) for all compounds at 173 K using an Oxford Cryostream 700 cooler. Powder X-ray diffraction confirms the single-crystal structure is representative of the bulk material. All experimental data and software used are given in the ESI†. |
| § Co-crystal 3: 0.100 g nicotinic acid hydrazide (0.729 mmol) and 0.053 g adipic acid (0.363 mmol) were dissolved in 5 ml of hot methanol and crystals grown by slow evaporation, blocks, mp 126 °C. Co-crystal 4: 0.100 g nicotinic acid hydrazide (0.729 mmol) and 0.043 g succinic acid (0.364 mmol) were dissolved in 5 ml of hot acetone and crystals grown by slow evaporation, blocks, mp 101–103 °C. Co-crystal 5: 0.100 g nicotinic acid hydrazide (0.729 mmol) and 0.043 g succinic acid (0.364 mmol) were dissolved in 5 ml of methanol and crystals grown by slow evaporation, blocks, mp 152–153 °C. Co-crystal 6: 0.200 g nicotinic acid hydrazide (1.46 mmol) and 0.144 g succinic acid (0.712 mmol) were dissolved in 5 ml of hot acetone and crystals grown by slow evaporation, blocks, mp 94–97 °C. All crystals were colourless. |
| This journal is © The Royal Society of Chemistry 2011 |
