Syntheses and crystal structures of dinuclear, trinuclear [2 × 1 + 1 × 1] and tetranuclear [2 × 1 + 1 × 2] copper(II)–d10 complexes (d10 ⇒ ZnII, CdII, HgII and AgI) derived from N,N′-ethylenebis(3-ethoxysalicylaldimine)†
Malabika
Nayak
a,
Sohini
Sarkar
a,
Susanta
Hazra
a,
Hazel A.
Sparkes
b,
Judith A. K.
Howard
b and
Sasankasekhar
Mohanta
*a
aDepartment of Chemistry, University of Calcutta, 92 A. P. C. Road, Kolkata, 700 009, India. E-mail: sm_cu_chem@yahoo.co.in; Fax: +91-33-23519755
bDepartment of Chemistry, University of Durham, University Science Laboratories, South Road, Durham, UK DH1 3LE
First published on 27th August 2010
Abstract
Syntheses, characterization and crystal structures of heterotetranuclear [2 × 1 + 1 × 2] co-crystals [{CuIILZnII(H2O)2}{CuIIL}2](ClO4)2 (1) and [{CuIILCdII(H2O)2(CH3CN)}{CuIIL}2](ClO4)2 (2), dinuclear compound [CuIILHgII(CH2COCH3)](ClO4) (3) and heterotrinuclear [2 × 1 + 1 × 1] co-crystal [{CuIILAgI(H2O)}{CuIIL}](ClO4) (4) derived from N,N′-ethylenebis(3-ethoxysalicylaldimine) (H2L) are reported herein. Compounds 3 and 4 crystallize in the orthorhombic Pbca and monoclinic P21/c systems, respectively, while the space group of compounds 1 and 2 is monoclinic C2/c. The structure of 3 consists of a monophenoxo-bridged CuIIHgII dinuclear core in which the mercury(II) centre is dicoordinated by the bridging phenoxo oxygen atom and the CH2 carbon atom of the mono-deprotonated acetone anion. The coordination environment of HgII in this compound is almost linear. In the CuII2AgI compound 4, one diphenoxo-bridged [CuIILAgI(H2O)]+ cation is co-crystallized with one mononuclear [CuIIL] moieties. On the other hand, the structures of the CuII3MII compounds 1 (M = Zn) and 2 (M = Cd) consist of one diphenoxo-bridged dinuclear CuIIMII unit and two mononuclear [CuIIL] moiety; the composition of the dinuclear unit being [CuIILZnII(H2O)2]2+ and [CuIILCdII(H2O)2(CH3CN)]2+ for 1 and 2, respectively. The AgI (in 4), ZnII (in 1) and CdII (in 2) ions in the diphenoxo-bridged dinuclear cores are tri-, tetra- and heptacoordinated, respectively. The metal ions are coordinated to two bridging phenoxo oxygen atoms, with the silver(I) and zinc(II) centres being additionally coordinated to one and two water molecules, respectively. While in the case of cadmium(II), the additional five coordination positions are occupied by two ethoxy oxygen atoms, two water oxygen atoms and one acetonitrile nitrogen atom. The coordination environment of silver(I) in 4 is pyramidal with a scalene O3 triangle as the base, whereas those of zinc(II) and cadmium(II) in 1 and 2 are distorted tetrahedral and distorted pentagonal bipyramidal, respectively. The coordinated water molecule in 4 and each of the two coordinated water molecules in 1 and 2 are encapsulated into the O4 compartment of a [CuIIL] moiety resulting in the [2 × 1 + 1 × 1] (for 4) or [2 × 1 + 1 × 2] (for 1 and 2) cocrystallization and self-assemblies. Clearly, the encapsulation of the coordinated water molecule(s) is the governing force for the formation of dinuclear-mononuclear co-crystals in 1, 2 and 4. In addition all four compounds 1–4 have π–π stacking interactions which form dimers between pairs of adjacent molecules in the structure of 3 consisting of CuIIHgII dimers, while one-dimensional stacks are formed in the CuII3ZnII (1), CuII3CdII (2) and CuII2AgI (4) complexes. The compositions of the title compounds are compared with the related systems derived from the same ligand. The [2 × 1 + 1 × 1] co-crystal, 4, is a new type of system in terms of the number of components. Again, in spite of the tri-, tetra- and hepta-coordinated nature of the second metal ion in the dinuclear cores, co-crystallization in 1, 2 and 4, respectively, are new observations. The accommodation/coordination of varieties of metal ions by the O4 compartment of H2L has also been highlighted in the present investigation.
Introduction
Utilization of crystal engineering principles to design self-assemblies resulting from supramolecular interactions, namely, strong and weak hydrogen bonds, C–H⋯π, π–π stacking, halogen–halogen, sulfur–sulfur, gold–gold interactions, etc., is a frontier research area.1–16 Supramolecular interactions between the same molecules result in the formation of dimers and oligomers as well as extended networks.1–7 Similarly, the attractive forces between different moieties may produce multicomponent crystals, known as co-crystals.8–19 It is worth noting that, in comparison to the large number of organic co-crystals,8–10 there are only a few multicomponent compounds containing only metal complexes as the components.11–17,19During the last few years, we have been exploring the chemistry of mono-, di- and oligonuclear complexes derived from the compartmental Schiff base ligand 3-ethoxysalicylaldehyde-diamine, particularly N,N′-ethylenebis(3-ethoxysalicylaldimine) (H2L; Scheme 1).11–18,20 In the mononuclear complexes of copper(II) and nickel(II), [CuIIL⊂(H2O)]11 and [NiIIL⊂(H2O)],12 respectively, derived from this ligand, one water molecule is encapsulated in the O4 compartment by forming bifurcated hydrogen bonds with the phenoxo and ethoxy oxygen atoms. The copper(II)/nickel(II)–lanthanides(III)20 and copper(II)–bismuth(III)15 complexes are dinuclear, while copper(II)–barium(II)15 and copper(II)–lead(II)15 compounds are trinuclear and tetranuclear, respectively. On the other hand, copper(II)/nickel(II)–3d11,12 and copper(II)–sodium(I)14 complexes are tetranuclear [2 × 1 + 1 × 2] co-crystals of a dinuclear and two mononuclear units. Interestingly, the nickel(II)–sodium(I) compound is a unique example of a three component co-crystal of metal complexes; one trinuclear, one dinuclear and two mononuclear moieties are co-crystallized in this heptanuclear [3 × 1 + 2 × 1 + 1 × 2] co-crystal.16 In all of these co-crystals, encapsulation of two coordinated water molecules in the O4 compartments of two mononuclear [CuII/NiIIL] moieties takes place. Clearly, the tendency of a water molecule to be encapsulated in the O4 compartment of the mononuclear units is the governing factor to generate both the inclusion products and above mentioned co-crystals. On the other hand, the copper(II)–uranyl(VI) system is a hexanuclear [2 × 1 + 1 × 4] co-crystal of one diaqua-bridged diuranyl(VI) unit and four [CuIIL⊂(H2O)] moieties.15 This CuII4UVI2 system is also an example of a self-assembly of 3d and 5f complexes resulted from C–H⋯π interactions. Similarly a copper(II)–potassium(I) complex is an interesting example of an octanuclear [3 × 1 + 5 × 1] co-crystal of a double-decker and a triple-decker systems.14 As illustrated, the coordination compounds derived from H2L tend to show structural diversity. We anticipated therefore that new types of compositions/co-crystals may be obtained in the heterometallic complexes involving some other metal ion combinations, copper(II)–zinc(II), copper(II)–cadmium(II), copper(II)–mercury(II) and copper(II)–silver(I), for example. As the coordination number or geometry of these d10 metal ions should be different than those of the 3d or alkali metal ions, we were interested in exploring the nature and number of components in these copper(II)–d10 complexes. Accordingly, we reacted perchlorate salts of zinc(II), cadmium(II), mercury(II) and silver(I) with [CuIIL⊂(H2O)]. We report herein the syntheses, characterization and crystal structures of the products obtained there from.
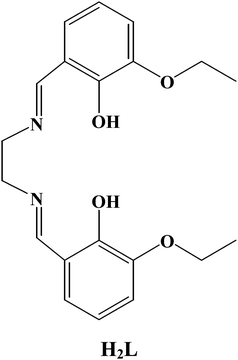 | ||
| Scheme 1 Chemical structure of H2L. | ||
Experimental
Materials and physical measurements
All the reagents and solvents were purchased from commercial sources and used as received. The mononuclear inclusion product [CuIIL⊂(H2O)] was synthesized by the reported procedure.11 Elemental (C, H and N) analyses were performed on a Perkin-Elmer 2400 II analyzer. IR spectra were recorded from KBr disks of the samples over the range 400–4000 cm−1 on a Bruker-Optics Alpha-T spectrophotometer.Syntheses
An acetone solution (10 mL) of silver(I) perchlorate hydrate (0.104 g, 0.5 mmol) was added dropwise to a suspension of [CuIIL⊂(H2O)] (0.218 g, 0.5 mmol) in acetonitrile (20 mL). After stirring for 10 min, the dark red solution was filtered to remove any suspended particles and the filtrate kept at room temperature was allowed to evaporate slowly. After a few days, a red crystalline compound containing diffraction quality single crystals deposited, this was collected by filtration and washed with cold acetone. Yield: 0.199 g (75%). Anal. calcd for C40H46N4O13ClCu2Ag: C 45.27, H 4.37, N 5.28. Found: C 45.41, H 4.42, N 5.17. IR (cm−1, KBr): ν(H2O), 3434 m; ν(C–H), 3055 w, 2976 w, 2920 w, 2878 w; ν(C = N), 1629 vs; ν(ClO4), 1085 s, 619 w.
Data for 1: colour: red. Yield: 0.220 g (85%). Anal. calcd for C60H70N6O22Cl2Cu3Zn: C 46.37, H 4.54, N 5.40. Found: C 46.50, H 4.63, N 5.32. IR (cm−1, KBr): ν(H2O), 3452 m; ν(C–H), 3057 w, 2980 w, 2928 w, 2879 w; ν(C = N), 1630 vs; ν(ClO4), 1087 s, 621 w.
Data for 2: colour: red. Yield: 0.213 g (78%). Anal. calcd for C62H73N7O22Cl2Cu3Cd: C 45.34, H 4.48, N 5.97. Found: C 45.50, H 4.60, N 5.85. IR (cm−1, KBr): ν(H2O), 3425 m; ν(C–H), 3056 w, 2980 w, 2928 w, 2879 w; ν(acetonitrile), 2279 w; ν(C = N), 1632 vs; ν(ClO4), 1084 s, 620 w.
Data for 3: colour: red. Yield: 0.310 g (80%). Anal. calcd for C23H27N2O9ClCuHg: C 35.64, H 3.51, N 3.61. Found: C 35.78, H 3.62, N 3.50. IR (cm−1, KBr): ν(C–H), 3057 w, 2979 w, 2928 w; ν(acetone), 1671 w; ν(C = N), 1632 vs; ν(ClO4), 1080 s, 627 w.
Safety note. The perchlorate salts of a metal complex with an organic ligand are potentially explosive and should be handled with care.
Crystal structure determinations of 1–4
Crystallographic data for 1–4 are summarized in Table 1. X-Ray diffraction data for 1 and 2 were collected at 120 K on an Oxford Diffraction Gemini S Ultra diffractometer using graphite monochromated Mo-Kα radiation (λ = 0.71073 Å). The data were collected and integrated using Oxford Diffraction CrysAlis software. While X-ray diffraction data for 3 and 4 were collected at 120 K on a Bruker APEX CCD diffractometer. These data were collected using the SMART software, with subsequent data processing carried out in SAINT.21a,21b All of the structures were solved by direct methods in SHELXS-9721c and refined by full matrix least squares on F2 in SHELXL-97.21d In 2, one of the carbon atoms [C(32)] of the acetonitrile was disordered over 3 positions, due to the presence of a 2-fold rotation only 2 of these positions are in the asymmetric unit; one of which is on the 2-fold axis, the 3rd positions is generated by the 2-fold rotation axis from the disordered carbon not sitting on the 2-fold rotation axis. The occupancies of these sites were determined by fixing their isotropic temperature factors to be the same but allowing them to refine and setting the sum of their occupancies to be 0.5 (i.e. giving a fully occupied carbon atom after the application of the 2-fold axis), the occupancies refined to 0.214(10) (C32A) and 0.287(10) (C32B) and were subsequently fixed at 0.21 (C32A) and 0.29 (C32B), respectively. The anisotropic temperature factors of the C32 atoms were constrained to be the same and refined, but had to be modelled to be approximately isotropic to allow a stable refinement. All of the hydrogen atoms, apart from the solventwater hydrogen atoms in 1, 2 and 4, were positioned geometrically (aromatic C–H 0.95 Å, ethyl C–H 0.99 Å and methyl C–H 0.98 Å) and refined using a riding model with the isotropic displacement parameters fixed at Uiso(H) = 1.2 times Ueq of the parent carbon atom for the aromatic and ethyl hydrogens and Uiso(H) = 1.5 times Ueq of the parent carbon atom for the methyl hydrogens. The refinements converged to an R1 value [I > 2σ(I)] of 0.0387, 0.0222, 0.0191 and 0.0360 for 1–4, respectively.| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| a R 1 = [∑||Fo| − |Fc||/∑|Fo|]. b wR2 = [∑w(Fo2 − Fc2)2/∑wFo4]1/2. | ||||
| Formula | C60H70N6O22Cl2Cu3Zn | C62H73N7O22Cl2Cu3Cd | C46H54N4O18Cl2Cu2Hg2 | C80H92N8O26Cl2Cu4Ag2 |
| FW/g mol−1 | 1554.11 | 1642.19 | 1550.10 | 2122.42 |
| Crystal colour | Red | Red | Red | Red |
| Crystal system | Monoclinic | Monoclinic | Orthorhombic | Monoclinic |
| Space group | C2/c | C2/c | Pbca | P21/c |
| a/Å | 13.3706(3) | 27.3303(8) | 12.8064(5) | 14.3298(6) |
| b/Å | 22.8263(4) | 14.4792(2) | 19.7143(8) | 13.3337(6) |
| c/Å | 21.5225(5) | 20.0508(6) | 20.4235(9) | 22.3930(10) |
| β/° | 108.810(3) | 124.977(4) | 90 | 104.0450(10) |
| V/Å3 | 6217.9(2) | 6501.4(3) | 5156.3(4) | 4150.7(3) |
| Z | 4 | 4 | 4 | 2 |
| T/K | 120(2) | 120(2) | 120(2) | 120(2) |
| 2θ/° | 5.36−58.4 | 5.04–50.04 | 3.98–52.74 | 3.58–52.74 |
| μ(Mo-Kα)/mm−1 | 1.565 | 1.457 | 6.931 | 1.618 |
| ρ c/g cm−3 | 1.660 | 1.678 | 1.997 | 1.698 |
| F(000) | 3196 | 3356 | 3016 | 2160 |
| Absorption-correction | Analytical | Analytical | Multi-scan | Multi-scan |
| Index ranges | −16 < h < 17 | −32 <h < 30 | −16 <h < 15 | −17 <h < 17 |
| −30 < k < 28 | −16 <k < 17 | −24 <k < 24 | −16 <k < 16 | |
| −29 <l < 24 | −20 <l < 23 | −25 <l < 25 | −27 <l < 27 | |
| Reflections collected | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 495 495 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 844 844 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 297 297 |
| Independent reflections | 7408 | 5738 | 5269 | 8483 |
| R int | 0.0487 | 0.0217 | 0.0433 | 0.0573 |
| R 1 a/wR2b [I > 2σ(I)] | 0.0387/0.0712 | 0.0222/0.0590 | 0.0191/0.0466 | 0.0360/0.0718 |
| R 1 a/wR2b [For all Fo2] | 0.0844/0.0766 | 0.0286/0.0600 | 0.0245/0.0493 | 0.0604/0.0784 |
Results and discussion
Description of crystal structures of [{CuIILZnII(H2O)2}{CuIIL}2](ClO4)2 (1), [{CuIILCdII(H2O)2(CH3CN)}{CuIIL}2](ClO4)2 (2), [CuIILHgII(CH2COCH3)](ClO4) (3) and [{CuIILAgI(H2O)}{CuIIL}](ClO4) (4)
The structures of 1–4 are shown in Fig. 1–4, respectively. The structure of the CuIIHgII (3) compound consists of one monophenoxo-bridged dinuclear [CuIILHgII(CH2COCH3)]+ cation, in which CH2COCH3 implies a mono-deprotonated acetone anion, and one perchlorate anion, whereas the structure of the CuII2AgI (4) compound consists of one diphenoxo-bridged [CuIILAgI(H2O)]+ cation, one mononuclear [CuIIL] moieties and one perchlorate anion. On the other hand, in the structures of the CuII3MII compounds 1 (M = Zn) and 2 (M = Cd), the components are one diphenoxo-bridged dinuclear CuIIMII unit, two mononuclear [CuIIL] moieties and two perchlorate anions; the composition of the dinuclear unit being [CuIILZnII(H2O)2]2+ and [CuIILCdII(H2O)2(CH3CN)]2+ for 1 and 2, respectively.2 (1). Thermal ellipsoids: 90% for Cu; 95% for Zn; 50% for C; 60% for N and O; 10% for H. Hydrogen atoms, except those of water molecules, are omitted. The perchlorate anions and the ethoxy carbon atoms are also not shown for clarity. Symmetry code: A, −x, y, 0.5 − z.](/image/article/2011/CE/c0ce00158a/c0ce00158a-f1.gif) | ||
| Fig. 1 Crystal structure of [{CuIILZnII(H2O)2}{CuIIL}2](ClO4)2 (1). Thermal ellipsoids: 90% for Cu; 95% for Zn; 50% for C; 60% for N and O; 10% for H. Hydrogen atoms, except those of water molecules, are omitted. The perchlorate anions and the ethoxy carbon atoms are also not shown for clarity. Symmetry code: A, −x, y, 0.5 − z. | ||
2 (2). Thermal ellipsoids: 94% for Cu; 99% for Cd; 85% for C, N and O; 15% for H Hydrogen atoms, except those of water molecules, are omitted. The perchlorate anions and the ethoxy carbon atoms are also not shown for clarity. Symmetry code: C, −x, y, 1.5 − z.](/image/article/2011/CE/c0ce00158a/c0ce00158a-f2.gif) | ||
| Fig. 2 Crystal structure of [{CuIILCdII(H2O)2(CH3CN)}{CuIIL}2](ClO4)2 (2). Thermal ellipsoids: 94% for Cu; 99% for Cd; 85% for C, N and O; 15% for H Hydrogen atoms, except those of water molecules, are omitted. The perchlorate anions and the ethoxy carbon atoms are also not shown for clarity. Symmetry code: C, −x, y, 1.5 − z. | ||
 (3). Thermal ellipsoids: 94% for Cu; 99% for Hg; 85% for C, N and O. Hydrogen atoms and the perchlorate anions are not shown for clarity.](/image/article/2011/CE/c0ce00158a/c0ce00158a-f3.gif) | ||
| Fig. 3 Crystal structure of [CuIILHgII(CH2COCH3)](ClO4) (3). Thermal ellipsoids: 94% for Cu; 99% for Hg; 85% for C, N and O. Hydrogen atoms and the perchlorate anions are not shown for clarity. | ||
 (4). Thermal ellipsoids: 85% for Cu; 90% for Ag; 45% for C; 50% for N and O; 30% for H. Hydrogen atoms, except those of the water molecule are omitted. The perchlorate anions and the ethoxy carbon atoms are also not shown for clarity.](/image/article/2011/CE/c0ce00158a/c0ce00158a-f4.gif) | ||
| Fig. 4 Crystal structure of [{CuIILAgI(H2O)}{CuIIL}](ClO4) (4). Thermal ellipsoids: 85% for Cu; 90% for Ag; 45% for C; 50% for N and O; 30% for H. Hydrogen atoms, except those of the water molecule are omitted. The perchlorate anions and the ethoxy carbon atoms are also not shown for clarity. | ||
The copper(II) ions in the mononuclear [CuIIL] moieties in 1, 2 and 4 and in the dinuclear CuIIMI/II units in 1–4 occupy the salen type N2O2 compartment, while the second metal ion (ZnII, CdII, HgII or AgI) in 1–4 is present in the open and larger O4 compartment of the dinucleating compartmental ligand L2−. However, whilst the cadmium(II) centre in 2 is coordinated to the two phenoxo and two ethoxy oxygen atoms, all the four oxygen atoms of the O4 compartment are not coordinated to the second metal centres in other three compounds (vide infra). The selected bond lengths and angles of the coordination environments of the copper(II) centres and the second metal ions in 1–4 are summarized in Tables 2 and 3, respectively.
| Compound | Bond lengths | Bond angles | ||
|---|---|---|---|---|
| 1 | Cu(1)–O(2) | 1.9204(18) | N(1)–Cu(1)–O(3) | 177.05(9) |
| Cu(1)–O(3) | 1.8977(19) | O(2)–Cu(1)–N(2) | 174.62(10) | |
| Cu(1)–N(1) | 1.919(2) | N(1)–Cu(1)–O(2) | 93.36(9) | |
| Cu(1)–N(2) | 1.935(2) | N(1)–Cu(1)–N(2) | 84.71(10) | |
| Cu(2)–O(7) | 1.8882(18) | N(2)–Cu(1)–O(3) | 92.68(10) | |
| Cu(2)–N(3) | 1.907(2) | O(2)–Cu(1)–O(3) | 89.37(8) | |
| N(3)–Cu(2)–O(7A) | 177.14(10) | |||
| N(3)–Cu(2)–N(3A) | 86.91(16) | |||
| N(3)–Cu(2)–O(7) | 95.78(10) | |||
| O(7)–Cu(2)–O(7A) | 81.55(11) | |||
| 2 | Cu(1)–O(2) | 1.9176(15) | N(1)–Cu(1)–O(3) | 176.19(7) |
| Cu(1)–O(3) | 1.9240(16) | O(2)–Cu(1)–N(2) | 176.14(7) | |
| Cu(1)–N(1) | 1.9512(19) | N(1)–Cu(1)–O(2) | 92.39(7) | |
| Cu(1)–N(2) | 1.9379(18) | N(1)–Cu(1)–N(2) | 83.80(8) | |
| Cu(2)–O(7) | 1.8888(15) | N(2)–Cu(1)–O(3) | 92.55(7) | |
| Cu(2)–N(3) | 1.9079(18) | O(2)–Cu(1)–O(3) | 91.24(6) | |
| N(3)–Cu(2)–O(7C) | 177.98(7) | |||
| N(3)–Cu(2)–N(3C) | 86.90(11) | |||
| N(3)–Cu(2)–O(7) | 94.56(7) | |||
| O(7)–Cu(2)–O(7C) | 84.00(9) | |||
| 3 | Cu(1)–O(2) | 1.9390(18) | N(1)–Cu(1)–O(3) | 175.40(9) |
| Cu(1)–O(3) | 1.9226(18) | O(2)–Cu(1)–N(2) | 175.77(9) | |
| Cu(1)–N(1) | 1.948(2) | N(1)–Cu(1)–O(2) | 91.60(9) | |
| Cu(1)–N(2) | 1.917(2) | N(1)–Cu(1)–N(2) | 85.32(10) | |
| N(2)–Cu(1)–O(3) | 94.88(9) | |||
| O(2)–Cu(1)–O(3) | 87.97(8) | |||
| 4 | Cu(1)–O(2) | 1.886(2) | N(1)–Cu(1)–O(3) | 178.05(11) |
| Cu(1)–O(3) | 1.905(2) | O(2)–Cu(1)–N(2) | 177.31(11) | |
| Cu(1)–N(1) | 1.932(3) | N(1)–Cu(1)–O(2) | 92.96(11) | |
| Cu(1)–N(2) | 1.928(3) | N(1)–Cu(1)–N(2) | 85.62(13) | |
| Cu(2)–O(7) | 1.886(2) | N(2)–Cu(1)–O(3) | 93.13(11) | |
| Cu(2)–O(8) | 1.909(2) | O(2)–Cu(1)–O(3) | 88.34(9) | |
| Cu(2)–N(3) | 1.919(3) | N(3)–Cu(2)–O(8) | 176.14(10) | |
| Cu(2)–N(4) | 1.927(3) | O(7)–Cu(2)–N(4) | 179.31(10) | |
| N(3)–Cu(2)–O(7) | 94.76(10) | |||
| N(3)–Cu(2)–N(4) | 85.85(11) | |||
| N(4)–Cu(2)–O(8) | 93.81(10) | |||
| O(7)–Cu(2)–O(8) | 85.60(9) | |||
| Compounds | Bond distances | Bond angles/Bridge angle | ||
|---|---|---|---|---|
| 1 | Zn(1)–O(5) | 1.960(2) | O(5)–Zn(1)–O(7) | 108.09(8) |
| Zn(1)–O(7) | 2.0333(18) | O(5)–Zn(1)–O(5A) | 131.16(12) | |
| O(7)–Zn(1)–O(5A) | 110.29(8) | |||
| O(7)–Zn(1)–O(7A) | 74.67(10) | |||
| Cu(2)–O(7)–Zn(1) | 101.89(8) | |||
| 2 | Cd(1)–O(5) | 2.2895(16) | O(5)–Cd(1)–O(5C) | 168.86(8) |
| Cd(1)–O(6) | 2.6049(14) | O(6)–Cd(1)–O(6C) | 164.04(7) | |
| Cd(1)–O(7) | 2.2576(14) | O(5)–Cd(1)–O(6) | 89.13(5) | |
| Cd(1)–N(4) | 2.285(3) | O(5)–Cd(1)–O(6C) | 92.42(5) | |
| O(5)–Cd(1)–O(7) | 86.92(6) | |||
| O(5)–Cd(1)–O(7C) | 83.85(6) | |||
| O(5)–Cd(1)–N(4) | 95.57(4) | |||
| O(6)–Cd(1)–O(7) | 64.04(5) | |||
| O(6)–Cd(1)–O(7C) | 131.90(5) | |||
| O(6)–Cd(1)–N(4) | 82.02(3) | |||
| O(7)–Cd(1)–O(6C) | 131.90(5) | |||
| O(7)–Cd(1)–O(7C) | 68.09(7) | |||
| O(7)–Cd(1)–N(4) | 145.95(4) | |||
| N(4)–Cd(1)–O(5C) | 95.57(4) | |||
| N(4)–Cd(1)–O(6C) | 82.02(3) | |||
| Cu(2)–O(7)–Cd(1) | 103.95(6) | |||
| 3 | Hg(1)–O(2) | 2.1173(18) | C(21)–Hg(1)–O(2) | 175.03(9) |
| Hg(1)–C(21) | 2.070(3) | Cu(1)–O(2)–Hg(1) | 106.93(8) | |
| 4 | Ag(1)–O(5) | 2.308(3) | O(5)–Ag(1)–O(7) | 131.32(8) |
| Ag(1)–O(7) | 2.362(2) | O(5)–Ag(1)–O(8) | 156.31(8) | |
| Ag(1)–O(8) | 2.393(2) | O(7)–Ag(1)–O(8) | 65.67(7) | |
| Cu(2)–O(7)–Ag(1) | 101.45(9) | |||
| Cu(2)–O(8)–Ag(1) | 99.65(9) | |||
In total in the structures of 1–4, there are seven crystallographically different copper(II) ions in the salen type N2O2 compartment. Considering all these seven metal centres, the Cu–N/O bond distances lie in the range 1.886(2)–1.9512(19) Å. However, in all cases, a Cu–N bond length is slightly longer than its trans Cu–O bond distance. The ranges of cisoid angles in the seven CuN2O2 environments are very close and deviate by a maximum of ca. 8° from the ideal value; considering all of the seven metal centres, the cisoid angles vary between 81.55(11) and 95.78(10)°. For all the seven cases, the transoid angles also deviate only little from the ideal value and lie in the range 174.62(10)–179.31(10)°. Again, for the seven environments, the ranges of the deviation of the constituent atoms and displacement of the metal centre from the corresponding least-squares N2O2 plane are 0.003–0.052 Å and 0.000–0.064 Å, respectively. All these structural parameters indicate that the CuN2O2 environments in 1–4 deviate only a small amount from an ideal square planar geometry.
The mercury(II) ion in the monophenoxo-bridged dinuclear compound [CuIILHgII(CH2COCH3)](ClO4) (3) is dicoordinated; in addition to one of the bridging phenoxo oxygen atom [O(2)], the metal centre also coordinates to the carbon atom [C(21)] of the CH2 moiety of the mono-deprotonated acetone anion. One phenoxo oxygen atom, O(3), and both the ethoxy oxygen atoms, O(1) and O(4), of the O4 compartment remain uncoordinated. The coordination geometry of the HgII environment is almost linear as evidenced by the O(2)–Hg(1)–C(21) angle of 175.03(9)°. The Hg(1)–O(2) bond distance [2.1173(18) Å] is slightly longer than the Hg(1)–C(21) bond length [2.070(3) Å]. The copper(II)⋯mercury(II) distance and copper(II)–O(phenoxo)–mercury(II) bridge angle in the dinuclear core are 3.261 Å and 106.93(8)°, respectively. It is relevant to note that Hg–C bonds involving a mono-deprotonated acetone anion, as observed in 3, have been reported previously in few compounds.22
The silver(I) centre, Ag(1), in the diphenoxo-bridged [CuIILAgI(H2O)]+ cation of [{CuIILAgI(H2O)}{CuIIL}](ClO4) (4) is tricoordinated by the two bridging phenoxo oxygen atoms, O(7) and O(8), and one water oxygen atom, O(5). Both the ethoxy oxygen atoms, O(6) and O(9), of the O4 compartment remain uncoordinated. The two metal–phenoxo oxygen atom bond distances [Ag(1)–O(7) = 2.362(2) Å, Ag(1)–O(8) = 2.393(2) Å] are slightly different, by ca. 0.03 Å, while the metal–water bond distance [Ag(1)–O(5) = 2.308(3) Å] is slightly shorter than the two metal–phenoxo bond lengths. The O–Ag–O angles involving the two pairs of water and phenoxo oxygen atoms are obtuse [O(5)–Ag(1)–O(7) = 131.32(8)°, O(5)–Ag(1)–O(8) = 156.31(8)°], while the bond angle involving two phenoxo oxygen atoms is 65.67(7)°. The silver(I) ion is displaced by 0.28 Å from the O(5)O(7)O(8) triangular plane. Clearly the coordination environment of silver(I) can not be approximated to any of the tricoordinated symmetries, C3v, C3h and D3h. The AgO3 environment is actually irregular pyramidal in which the scalene triangle O(5)O(7)O(8) is the base. The copper(II)⋯silver(I) distance is 3.302 Å, while the Cu(2)–O(7)–Ag(I) and Cu(2)–O(8)–Ag(I) bridge angles are 101.45(9) and 99.65(9)°, respectively.
In the CuII3MII compounds 1 (M = Zn) and 2 (M = Cd) the molecules lie on a crystallographic 2-fold axis. The zinc(II) ion, Zn(1), in the diphenoxo-bridged [CuIILZnII(H2O)2]2+ dication of [{CuIILZnII(H2O)2}{CuIIL}2](ClO4)2 (1) is tetracoordinated by the two bridging phenoxo oxygen atoms, O(7) and O(7A), and two water oxygen atoms, O(5) and O(5A). In this case also, both the ethoxy oxygen atoms, O(6) and O(6A), of the O4 compartment remain uncoordinated. The zinc(II)–water oxygen atom distance [1.960(2) Å] is shorter by ca. 0.07 Å than the zinc(II)–phenoxo oxygen atom bond length [2.0333(18) Å]. While the O–Zn–O bond angles range between 74.67(10) and 131.16(12)°, indicating that the ZnO4 coordination environment is distorted tetrahedral. On the other hand, the cadmium(II) centre, Cd(1), in the diphenoxo-bridged [CuIILCdII(H2O)2(CH3CN)]2+ dication of [{CuIILCdII(H2O)2(CH3CN)}{CuIIL}2](ClO4)2 (2) is heptacoordinated. In this case, all four oxygen atoms of the O4 compartment coordinate to the metal centre. The remaining three coordination positions are occupied by two water oxygen atoms and one acetonitrile nitrogen atom. The cadmium–phenoxo bond distance [Cd(1)–O(7) = 2.2576(14) Å] is slightly shorter than the cadmium–acetonitrile bond length [Cd(1)–N(4) = 2.285(3) Å]. However, the cadmium–ethoxy bond distance is significantly greater [Cd(1)–O(6) = 2.6049(14) Å]. The coordination environment of cadmium(II) can be best approximated as a distorted pentagonal bipyramidal; two phenoxo oxygen atoms, O(7) and O(7C), two ethoxy oxygen atoms, O(6) and O(6C), and the acetonitrile nitrogen atom, N(4), define the pentagonal plane, whereas the two water oxygen atoms, O(5) and O(5C), occupy the axial positions. The average deviation of the constituent atoms from the least-squares O4N plane is very small, 0.047 Å, and the metal centre is exactly pocketed in this pentagonal plane. Again, in comparison to the ideal value of 90°, deviation of the bond angles [83.85(6)–95.57(4)°] involving an axial atom and an atom in the pentagonal plane are not significant. These structural parameters apparently indicate that the coordination environment is not significantly distorted. However, the transoid angle [O(5)–Cd(1)–O(5C) = 168.86(8)°] involving the two axial atoms deviate further from the ideal value of 180°. Moreover, instead of being 72° and 144° for the ideal case, the bond angles involving the donor centres in the pentagonal plane vary between 64.04(5)° to 82.02(3)° and 131.90(5)° to 164.04(7)°. In addition, as already mentioned, the metal–ligand bond distances vary in the wide range of 2.2576(14)–2.6049(14) Å. Clearly, the coordination environment of the cadmium(II) centre in 2 is highly distorted pentagonal bipyramidal.
As shown in Fig. 4, each of the two hydrogen atoms of the water molecule, coordinated to silver(I) in 4 forms bifurcated hydrogen bonds with one phenoxo and one ethoxy oxygen atoms of the O4 compartment of the mononuclear [CuIIL] moiety. Similarly, each of the two coordinated water molecules of the dinuclear units in the CuII3ZnII (1) and CuII3CdII (2) interacts with the O4 compartments of two different mononuclear [CuIIL] moieties due to water⋯phenoxo and water⋯ethoxy bifurcated hydrogen bonds (Fig. 1 and 2). The geometries of the bifurcated hydrogen bonds in these three molecules are summarized in Table 4. The D⋯A contacts lie in the range 2.661(3)–3.217(2) Å, indicating that the hydrogen bonds vary between being weak and moderately strong. Each of the coordinated water molecules in 1, 2 and 4, therefore, may be considered as encapsulated in the O4 compartment of one mononuclear [CuIIL] moiety. The extent of encapsulation is not, however, strong, as evidenced by the displacement (dO; 1.39 Å in 1, 1.18 Å in 2, 1.24 Å in 4) of water oxygen atom from the corresponding least-squares O(phenoxo)2O(ethoxy)2 planes. Clearly, in [{CuIILAgI(H2O)}{CuIIL}](ClO4) (4), the dinuclear [CuIILAgI(H2O)]+ unit is self-assembled and co-crystallized with one mononuclear [CuIIL] moiety and therefore compound 4 is a [2 × 1 + 1 × 1] co-crystal. Similarly, each of the dinuclear units [CuIILZnII(H2O)2]2+ in [{CuIILZnII(H2O)2}{CuIIL}2](ClO4)2 (1) and [{CuIILCdII(H2O)2(CH3CN)]2+ in [{CuIILCdII(H2O)2(CH3CN)}{CuIIL}2](ClO4)2 (2) are self-assembled and co-crystallized with two mononuclear [CuIIL] moieties and therefore these two compounds are [2 × 1 + 1 × 2] co-crystals. The reason for the co-crystallization of dinuclear and mononuclear units in 1, 2 and 4 is the tendency of a water molecule, to be encapsulated in the O4 compartment of the mononuclear [CuIIL] moiety.
| Compound | D–H⋯A | H⋯A | D⋯A | D–H⋯A |
|---|---|---|---|---|
| 1 | O(5)–H(5A)⋯O(1) | 2.51(2) | 3.158(3) | 140(3) |
| O(5)–H(5A)⋯O(2) | 2.11(3) | 2.776(3) | 142(3) | |
| O(5)–H(5B)⋯O(3) | 1.93(3) | 2.661(3) | 144(3) | |
| O(5)–H(5B)⋯O(4) | 2.31(2) | 3.024(3) | 142(3) | |
| 2 | O(5)–H(5A)⋯O(1) | 2.47(2) | 3.217(2) | 150(3) |
| O(5)–H(5A)⋯O(2) | 2.25(2) | 2.921(2) | 138(2) | |
| O(5)–H(5B)⋯O(3) | 2.12(2) | 2.840(2) | 147(2) | |
| O(5)–H(5B)⋯O(4) | 2.28(2) | 2.940(2) | 138(2) | |
| 4 | O(5)–H(5A)⋯O(1) | 2.11(2) | 2.929(3) | 161(3) |
| O(5)–H(5A)⋯O(2) | 2.43(3) | 2.859(3) | 112(3) | |
| O(5)–H(5B)⋯O(3) | 2.14(2) | 2.902(3) | 151(3) | |
| O(5)–H(5B)⋯O(4) | 2.29(2) | 2.968(3) | 138(3) |
In all four compounds π–π stacking interactions23 exist which result in π bonded dimers between pairs of adjacent molecules, this results in CuIIHgII dimers in 3 (Fig. 5) and one-dimensional self-assembled stacks in the CuII3ZnII (1; Fig. S1, ESI),†CuII3CdII (2; Fig. S2, ESI)† and CuII2AgI (4; Fig. 6) complexes. While both the aromatic rings of the CuIIHgII (3) complex and all the four aromatic rings of the mononuclear and dinuclear units of the CuII2AgI (4) complex participate in π–π stacking interactions, aromatic rings of only the mononuclear units of the CuII3ZnII (1) and CuII3CdII (2) complexes participate in the stacking interactions. The centroid to centroid distances and the offset distances between the interacting aromatic rings are listed in Table 5. It may also be mentioned that copper(II)⋯phenolate semi-coordination between the neighbouring molecules is also responsible for the generation of the dimeric self-assembly in the CuIIHgII (3; Fig. 5) compound and one-dimensional self-assembly in the CuII2AgI (4; Fig. 6) complex. The copper(II)⋯phenolate distances are 2.795 Å and 2.872 Å for 3 and 4, respectively.
 (3) demonstrating the dimeric self-assembly resulted due to π–π stacking interactions and copper(ii)⋯phenolate semi-coordination. Symmetry code: C, −x, −y, 1 − z.](/image/article/2011/CE/c0ce00158a/c0ce00158a-f5.gif) | ||
| Fig. 5 Perspective view of [CuIILHgII(CH2COCH3)](ClO4) (3) demonstrating the dimeric self-assembly resulted due to π–π stacking interactions and copper(II)⋯phenolate semi-coordination. Symmetry code: C, −x, −y, 1 − z. | ||
 (4) demonstrating the one-dimensional self-assembly resulted due to π–π stacking interactions and copper(ii)⋯phenolate semi-coordination. Symmetry code: A, −x, 2 − y, −z; B, −1 + x, y, z; C, 1 − x, 2 − y, −z.](/image/article/2011/CE/c0ce00158a/c0ce00158a-f6.gif) | ||
| Fig. 6 Perspective view of [{CuIILAgI(H2O)}{CuIIL}](ClO4) (4) demonstrating the one-dimensional self-assembly resulted due to π–π stacking interactions and copper(II)⋯phenolate semi-coordination. Symmetry code: A, −x, 2 − y, −z; B, −1 + x, y, z; C, 1 − x, 2 − y, −z. | ||
| Compound | Plane 1 | Plane 2 | Centroid–centroid distances/Å | Offset distance/Å |
|---|---|---|---|---|
| a Symmetry code = −x, −y, −z + 1. b Symmetry code = 0.5 − x, 1.5 − y, 2 − z. c Symmetry code = −x, −y, 1 − z. d Symmetry code = 1 − x, 2 − y, −z. e Symmetry code = −x, 2 − y, −z. | ||||
| 1 | C(3)–C(8) | C(13)–C(18)a | 3.660(2) | 1.396(4) |
| C(13)–C(18) | C(3)–C(8)a | 3.660(2) | 1.396(4) | |
| 2 | C(3)–C(8) | C(13)–C(18)b | 3.622(1) | 1.361(3) |
| C(13)–C(18) | C(3)–C(8)b | 3.622(1) | 1.361(3) | |
| 3 | C(3)–C(8) | C(13)–C(18)c | 3.572(2) | 1.194(4) |
| C(13)–C(18) | C(3)–C(8)c | 3.572(2) | 1.194(4) | |
| 4 | C(3)–C(8) | C(13)–C(18)d | 3.845(2) | 1.868(5) |
| C(13)–C(18) | C(3)–C(8)d | 3.845(2) | 1.868(5) | |
| C(23)–C(28) | C(33)–C(38)e | 3.774(2) | 1.767(5) | |
| C(33)–C(38) | C(23)–C(28)e | 3.774(2) | 1.767(5) |
Syntheses and characterization
The dinuclear compound [CuIILHgII(CH2COCH3)](ClO4) (3), trinuclear compound [{CuIILAgI(H2O)}{CuIIL}](ClO4) (4) and tetranuclear compounds [{CuIILZnII(H2O)2}{CuIIL}2](ClO4)2 (1) and [{CuIILCdII(H2O)2(CH3CN)}{CuIIL}2](ClO4)2 (2), are readily produced in crystalline form from the reactions of [CuIIL⊂(H2O)] with perchlorate salts of mercury(II), silver(I), zinc(II) and cadmium(II), respectively, in appropriate solvent, acetone for 1 and 3, acetonitrile for 2 and acetone-acetonitrile for 4. Although no base was used, metal assisted deprotonation of acetone takes place during the synthesis of the CuIIHgII compound 3, in which the mono-deprotonated acetone anion is coordinated to the HgII centre through the CH2 carbon atom (vide supra).The stretching of the water molecule (s) in 1, 2 and 4 are observed as a broad and medium or weak intensity band, centred at 3452, 3425 and 3434 cm−1, respectively. Four weak intensity bands in the range 2878–3057 cm−1 in the IR spectra of 1–4 can be assigned to C–H stretching. Appearance of a weak intensity band at 2279 cm−1 in the IR spectrum of 2 is indicative of the presence of acetonitrile. The characteristics C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching in 1–4 is observed as a strong signal at ca. 1630 cm−1. The presence of carbonyl moiety, [CH2COCH3]−, in 3 is evidenced from the appearance of a weak intensity shoulder at 1671 cm−1. The perchlorate vibrations are observed as a strong intensity band in the range 1080–1087 cm−1 and a weak intensity band in the range 619–627 cm−1.
N stretching in 1–4 is observed as a strong signal at ca. 1630 cm−1. The presence of carbonyl moiety, [CH2COCH3]−, in 3 is evidenced from the appearance of a weak intensity shoulder at 1671 cm−1. The perchlorate vibrations are observed as a strong intensity band in the range 1080–1087 cm−1 and a weak intensity band in the range 619–627 cm−1.
Conclusions
The objective of the present investigation has been to understand the composition of the heteronuclear products obtained on reacting mononuclear inclusion compound [CuIIL⊂(H2O)] with d10 metal ions, ZnII, CdII, HgII and AgI [H2L = N,N′-ethylenebis(3-ethoxysalicylaldimine)]. While a monophenoxo-bridged dinuclear CuIIHgII compound (3) is obtained in the case of HgII, the other three products are co-crystals of one diphenoxo-bridged CuIIM species [M = ZnII (1), CdII (2), AgI (4)] and one (for 4) or two (for 1 and 2) mononuclear [CuIIL] moieties. Therefore, 4 is a [2 × 1 + 1 × 1] co-crystal of dinuclear and mononuclear moieties, while 1 and 2 are [2 × 1 + 1 × 2] co-crystals of dinuclear and mononuclear moieties. The silver(I) ion in 4 is coordinated to one water molecule, the hydrogen atoms of which form bifurcated hydrogen bonds to the oxygen atoms of the O4 compartment of a [CuIIL] moiety, and are believed to account for the formation of the trinuclear CuII2AgI self-assembly. Similarly, the tetranuclear CuII3MII self-assemblies in 1 (M = Zn) and 2 (M = Cd) are thought to be formed through bifurcated hydrogen bonding of the hydrogen atoms of the two coordinated water molecules with the oxygen atoms of the O4 compartments of the two [CuIIL] moieties. Although [2 × 1 + 1 × 2] co-crystallization in this ligand system has been observed previously in the CuII3M (M = CuII, CoII, MnII, NaI) self-assemblies of one diphenoxo-bridged dinuclear CuIIM unit and two mononuclear [CuIIL] moieties, the [2 × 1 + 1 × 1] co-crystallization in the CuII2AgI compound (4) is a new type of structure. Again, the second metal centres in the dinuclear cores of the previously published CuII3M (M = CuII, CoII, MnII, NaI) co-crystals are either five- or six-coordinated. In contrast, the second metal centre (AgI, ZnII, CdII), in the dinuclear core of 4, 1 and 2 are, respectively, tri-, tetra- and hepta-coordinated. In all three species, co-crystallization appears to take place through hydrogen bonding due to the presence of coordinated water molecule(s). The environment of the second metal centre in the CuII3M (M = CuII, CoII, MnII, NaI) co-crystals are either square pyramidal or intermediate between square pyramidal and trigonal bipyramidal, while that in the CuII3NaI compound remains ill defined. On the other hand, the coordination environment of silver(I), zinc(II) and cadmium(II) in 4, 1 and 2 are, respectively, irregular pyramidal, distorted tetrahedral and distorted pentagonal bipyramidal. However, if the second metal centre is coordinated to water molecule (s), self-assembled co-crystallization tends to take place in the homo- or hetero-nuclear complexes derived from H2L, forming the self-assembled co-crystals of dinuclear and mononuclear units. Another interesting aspect of the present investigation and of our previous studies is that the O4 compartment of H2L can accommodate/coordinate varieties of metal ions, namely, 3d (CuII, CoII, MnII, ZnII), 4d (AgI, CdII), 5d (HgII), 4f (CeIII–YbIII), s-block (NaI, KI, BaII) and p-block (BiIII, PbII).Acknowledgements
Financial support from the Department of Science and Technology (SR/S1/IC–12/2008), the Government of India and University Grants Commission (fellowship to SS) and Council for Scientific and Industrial Research (fellowship to SH) is gratefully acknowledged. HAS would like to thank the Leverhulme Trust for financial assistance.References
- R. Bogue, Assem. Autom., 2008, 28, 211 CrossRef.
- J. A. Thomas, in Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, CRC Press, Boca Raton, FL, 2004, p. 1248 Search PubMed.
- M. Andruh, D. G. Branzea, R. Gheorghe and A. M. Madalan, CrystEngComm, 2009, 11, 2571 RSC.
- (a) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (b) D. Braga, L. Maini, M. Polito, L. Scaccianoce, G. Cojazzi and F. Grepioni, Coord. Chem. Rev., 2001, 216–217, 225 CrossRef; (c) A. J. Blake, N. R. Champness, P. Hubberstey, M. A. Withersby and M. Schröder, Coord. Chem. Rev., 1999, 183, 117 CrossRef CAS; (d) J.-P. Sauvage, Ed. Transition Metals in Supramolecular Chemistry; Perspectives in Supramolecular Chemistry 5; Wiley: London, 1999 Search PubMed; (e) G. R. Desiraju, ed. The Crystal as a Supramolecular Entity; Perspectives in Supramolecular Chemistry 2; Wiley: London, 1996 Search PubMed; (f) D. Braga, F. Grepioni and A. G. Orpen, Crystal Engineering: from Molecules and Crystals to Materials; Kluwer Academic: Dordrecht, The Netherlands, 1999 Search PubMed.
- (a) G. J. McManus, J. J. Perry Iv, M. Perry, B. D. Wagner and M. J. Zaworotko, J. Am. Chem. Soc., 2007, 129, 9094 CrossRef CAS; (b) M. R. Marvel, J. Lesage, J. Baek, P. S. Halasyamani, C. L. Stern and K. R. Poeppelmeier, J. Am. Chem. Soc., 2007, 129, 13963 CrossRef CAS; (c) F. Nouar, J. F. Eubank, T. Bousquet, L. Wojtas, M. J. Zaworotko and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 1833 CrossRef CAS; (d) K. Uemura, K. Saito, S. Kitagawa and H. Kita, J. Am. Chem. Soc., 2006, 128, 16122 CrossRef CAS.
- (a) A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998 CrossRef CAS; (b) J. L. C. Rowsell and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 1304 CrossRef CAS; (c) M. Nayak, R. Koner, H. Stoeckli-Evans and S. Mohanta, Cryst. Growth Des., 2005, 5, 1907 CrossRef CAS; (d) B. Dutta, B. Adhikary, P. Bag, U. Flörke and K. Nag, J. Chem. Soc., Dalton Trans., 2002, 2760 RSC; (e) P. Agnihotri, E. Eringathodi, P. Paul and P. K. Ghosh, Eur. J. Inorg. Chem., 2006, 3369 CrossRef CAS.
- (a) J. Hamblin, S. P. Argent, A. J. Blake, C. Wilson and N. R. Champness, CrystEngComm, 2008, 12, 1782 Search PubMed; (b) S. Bianketti, A. J. Blake, C. Wilson, P. Hubberstey, N. R. Champness and M. Schröder, CrystEngComm, 2009, 5, 763 Search PubMed; (c) M. L. Cheney, N. Shan, E. R. Healey, M. Hanna, L. Wojtas, M. J. Zaworotko, V. Sava, S. Song and J. R. Sanchez-Ramos, Cryst. Growth Des., 2010, 10, 394 CrossRef CAS; (d) N. Kundu, A. Audhya, Sk Md T. Abtab, S. Ghosh, E. R. T. Tiekink and M. Chaudhury, Cryst. Growth Des., 2010, 10, 1269 CrossRef CAS.
- (a) J. F. Remenar, S. L. Morissette, M. L. Peterson, B. Moulton, J. M. MacPhee, H. R. Guzman and O. Almarsson, J. Am. Chem. Soc., 2003, 125, 8456 CrossRef CAS; (b) T. E. Keyes, R. J. Forster, A. M. Bond and W. Miao, J. Am. Chem. Soc., 2001, 123, 2877 CrossRef CAS; (c) H. Koshima, H. Miyamoto, I. Yagi and K. Uosaki, Cryst. Growth Des., 2004, 4, 807 CrossRef CAS; (d) P. L. Magueres, S. M. Hubig, S. V. Lindeman, P. Veya and J. K. Kochi, J. Am. Chem. Soc., 2000, 122, 10073 CrossRef CAS.
- (a) J. A. Bis, O. L. McLaughlin, P. Vishweshwar and M. J. Zaworotko, Cryst. Growth Des., 2006, 6, 2648 CrossRef CAS; (b) G. P. Stahly, Cryst. Growth Des., 2007, 7, 1007 CrossRef CAS; (c) S. Karki, L. Fábián, T. Friščić and W. Jones, Org. Lett., 2007, 9, 3133 CrossRef CAS; (d) F. G. Vogt, J. S. Clawson, M. Strohmeier, A. J. Edwards, T. N. Pham and S. A. Watson, Cryst. Growth Des., 2009, 9, 921 CrossRef CAS; (e) W.-H. Wang, P.-H. Xi, X.-Y. Su, J.-B. Lan, Z.-H. Mao, J.-S. You and R.-G. Xie, Cryst. Growth Des., 2007, 7, 741 CrossRef CAS; (f) L. R. MacGillivray, G. S. Papaefstathiou, T. Friščicć, T. D. Hamilton, D.-K. Bučar, Q. Chu, D. B. Varshney and I. G. Georgiev, Acc. Chem. Res., 2008, 41, 280 CrossRef CAS.
- (a) H. Koshima, M. Nagano and T. Asahi, J. Am. Chem. Soc., 2005, 127, 2455 CrossRef CAS; (b) S. L. Childs, L. J. Chyall, J. T. Dunlap, V. N. Smolenskaya, B. C. Stahly and G. P. Stahly, J. Am. Chem. Soc., 2004, 126, 13335 CrossRef CAS; (c) B. Olenik, R. Boese and R. Sustmann, Cryst. Growth Des., 2003, 3, 175 CrossRef CAS; (d) F. Pan, M. S. Wong, V. Gramlich, C. Bosshard and P. Gunter, J. Am. Chem. Soc., 1996, 118, 6315 CrossRef CAS.
- M. Nayak, R. Koner, H.-H. Lin, U. Flörke, H.-H. Wei and S. Mohanta, Inorg. Chem., 2006, 45, 10764 CrossRef CAS.
- S. Sarkar, M. Nayak, M. Fleck, S. Dutta, U. Flörke, R. Koner and S. Mohanta, Eur. J. Inorg. Chem., 2010, 735 CrossRef CAS.
- M. Nayak, S. Hazra, P. Lemoine, R. Koner, C. R. Lucas and S. Mohanta, Polyhedron, 2008, 27, 1201 CrossRef CAS.
- S. Hazra, R. Koner, M. Nayak, H. A. Sparkes, J. A. K. Howard and S. Mohanta, Cryst. Growth Des., 2009, 9, 3603 CrossRef CAS.
- S. Hazra, S. Sasmal, M. Nayak, H. A. Sparkes, J. A. K. Howard and S. Mohanta, CrystEngComm, 2010, 12, 470 RSC.
- M. Nayak, A. Jana, M. Fleck, S. Hazra and S. Mohanta, CrystEngComm, 2010, 12, 1416 RSC.
- M. Fleck, S. Hazra, S. Majumder and S. Mohanta, Cryst. Res. Technol., 2008, 43, 1220 CrossRef CAS.
- M. Nayak, S. Sarkar, P. Lemoine, S. Sasmal, R. Koner, H. A. Sparkes, J. A. K. Howard and S. Mohanta, Eur. J. Inorg. Chem., 2010, 744 CrossRef CAS.
- (a) C.-C. Chou, C.-C. Su, H.-L. Tsai and K.-H. Lii, Inorg. Chem., 2005, 44, 628 CrossRef CAS; (b) M. Palaniandavar, R. J. Butcher and A. W. Addison, Inorg. Chem., 1996, 35, 467 CrossRef CAS; (c) R. C. Holz and L. C. Thompson, Inorg. Chem., 1993, 32, 5251 CrossRef CAS; (d) P. Jones, R. S. Vagg and P. A. Williams, Inorg. Chem., 1984, 23, 4110 CrossRef CAS; (e) W. J. Evans, T. J. Boyle and J. W. Ziller, Inorg. Chem., 1992, 31, 1120 CrossRef CAS.
- (a) R. Koner, H.-H. Lin, H.-H. Wei and S. Mohanta, Inorg. Chem., 2005, 44, 3524 CrossRef CAS; (b) R. Koner, G.-H. Lee, Y. Wang, H.-H. Wei and S. Mohanta, Eur. J. Inorg. Chem., 2005, 1500 CrossRef CAS.
- (a) Bruker–Nonius 2004, APEX-II, SAINT- Plus and TWINABS. Bruker–Nonius AXS Inc., Madison, Wisconsin, USA Search PubMed; (b) Bruker 2003, SAINT- Plus. Version 6.45. Bruker AXS Inc., Madison, Wisconsin, USA Search PubMed; (c) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112; (d) G. M. Sheldrick, SHELXL-97, Crystal Structure Refinement Program, University of Göttingen, 1997 Search PubMed.
- (a) D. C. Bebout, J. F. Bush II, K. K. Crahan, E. V. Bowers and R. J. Butcher, Inorg. Chem., 2002, 41, 2529 CrossRef CAS; (b) J. Vicente, A. Arcas, J. M. Fernandez-Hernandez and D. Bautista, Organometallics, 2001, 20, 2767 CrossRef CAS.
- (a) C. Janiak, J. Chem. Soc., Dalton Trans., 2000, 3885 RSC; (b) J. Liu, E. M. Murray and V. G. Young, Jr., Chem. Commun., 2003, 1904 RSC; (c) J. R. Price, N. G. White, A. Perez-Velasco, G. B. Jameson, C. A. Hunter and S. Brooker, Inorg. Chem., 2008, 47, 10729 CrossRef CAS; (d) A. N. Sokolov, T. Friščicć, S. Blais, J. A. Ripmeester and L. R. MacGillivray, Cryst. Growth Des., 2006, 6, 2427 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional structural details (Fig. S1 and S2). CCDC reference numbers 761685–761688 for 1–4. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ce00158a |
| This journal is © The Royal Society of Chemistry 2011 |
