Fabrication of hollow cubic Ag microboxes with net-like nanofiber structures and their surface plasmon resonance†
Bo
Chen
,
Xiuling
Jiao
and
Dairong
Chen
*
Key Laboratory for Special Functional Aggregate Materials of Education Ministry, School of Chemistry & Chemical Engineering, Shandong University, Jinan, 250100, P. R. China. E-mail: cdr@sdu.edu.cn; Fax: +86-0531-88364281; Tel: +86-0531-88364280
First published on 31st August 2010
Abstract
We designed a new templating synthesis to produce hollow cubic Ag microboxes with net-like nanofiber structures in an ethanol system under ambient conditions. The Ag boxes are characterized by TEM, HR-TEM, FE-SEM, and XRD, which illustrates their novel hierarchical structure. The boxes are composed of metallic Ag crystallites which connect together into a net-like structure to form the walls of the boxes, and the six walls close to form the final hollow cubic Ag microbox. The fabrication mechanism of the Ag boxes is demonstrated by tracking the intermediates in the synthesis procedures. Structural analysis and comparative tests are employed to shed light on the formation mechanism of the net-like nanofiber structure. The structure-dependent surface plasma resonance (SPR) properties of the as-prepared Ag microboxes and nanofibers are described based on the UV/Vis/NIR absorption spectra.
1. Introduction
Metal nanostructured materials with functional properties have been extensively studied for many decades, because of the importance of these materials to catalysis, photography, electronics, photonics, information storage, optoelectronics, biological labelling, imaging, and sensing.1 In particular, noble metal nanostructured materials such as Ag and Au have attracted considerable attention scientifically and industrially, owing to their numerous applications that include surface plasmon resonance (SPR), surface-enhanced Raman scattering (SERS), as well as chemical and biological sensing.2 The intrinsic properties of a metal nanocrystal are determined by a set of physical parameters that may include its size, shape, composition, and structure (e.g., solid or hollow).1 For example, in the case of a Au or Ag nanocrystal, both computational and experimental studies have demonstrated that their shape and structure play important roles in determining the number, position, and intensity of localized surface plasmon resonance (LSPR) modes, as well as the spectral region or polarization dependence for effective molecular detection by SERS.3 Therefore, preparation of metal nanostructures with controllable morphologies is essential in order to fully exploit their peculiar properties and unique applications.A wealth of chemical methods have been developed for the synthesis of Ag and Au nanostructures that have well-controlled morphologies, including nanoplates,4 cubes,5 belts,6 wires,7 rods,8 and branched multipods.9 Recently, many research efforts have been focused on developing hollow (nanoshell) Au nanostructures due to their special optical properties and promising applications in biomedicine.10 The strong surface plasmon absorption band for Au nanoshells can be tuned from the visible to the near-infrared (NIR) region, where optical transmission through tissue is optimal. Thus, hollow Au nanoshells have become an ideal candidate for cancer cell imaging and photothermal therapy in the NIR region.10 Although Ag nanoparticles are frequently used as sacrificial templates to synthesize hollow gold nanostructuresvia galvanic replacement,11 the preparation of hollow Ag nanostructures have been less successful. However, the surface plasmon resonance of hollow (nanoshell) Ag nanostructures can also be tuned across the visible and the near-infrared regions of the electromagnetic spectrum.12 This tunability of optical response has promising applications in areas from photonics and electronics to biology and medicine.12 In this sense, we believe that it is significant to explore simple and efficient strategies for synthesizing hollow Ag nanostructures to fully exploit these applications.
The synthetic approaches for hollow nanostructures can be broadly divided into four categories: (1) conventional hard templating synthesis,13 (2) sacrificial templating synthesis,14 (3) soft templating synthesis,15 and (4) template-free methods.16 There are a few precedents for hollow Ag nanostructures. For example, Qi and co-workers have presented a route for the synthesis of submicrometer-sized hollow Ag spheres using PEO-b-PMAA-SDS complex micelles as soft templates.17 Wang et al. have demonstrated the fabrication of two- and three-dimensional ordered structures of hollow Ag spheres by colloidal crystal hard templates.18 Also, monodisperse hollow Ag spheres have been prepared with phase-transformable emulsions composed of natural beeswax as hard templates.19 Each method has several intrinsic advantages and disadvantages. Templating against hard (solid) templates is arguably the most effective, and certainly the most common, method for synthesizing hollow micro-/nanostructures, however, the template-removal step not only significantly complicates the process but also diminishes the quality of the product particles.20 It remains a challenge to develop novel hollow nanostructures with easy-to-control synthesis conditions. Preparation of non-spherical hollow structures introduces additional challenges, because the synthetic approaches for spherical hollow structures does not generally apply to the synthesis of non-spherical hollow structures.20 Furthermore, the fabrication of Ag nanoshells has proven more difficult than other types, such as gold nanoshells.12 As a result, reports on the synthesis of non-spherical Ag hollow structures are relatively few. Previously, Qi's laboratory prepared rhombododecahedral Ag cages by self-assembly coupled with a template of the precursor crystals, which is analogous to the sacrificial templates based on the Kirkendall effect.21 This process seemingly provides a general route to the synthesis of superstructures with unique morphologies and complex hierarchies. However, there are still difficulties in fabricating non-spherical hollow structures of Ag, such as the lack of non-spherical templates, the difficulty in forming a uniform coating around template surfaces with large variation in curvature, as well as the complexity of the template-removal step.
Herein, we report a facile fabrication of hollow cubic Ag microboxes, whose walls are constructed of netlike Ag nanofibers. This net-like nanofiber and hollow structure evidently influences the optical properties of the Ag microboxes, engendering a surface plasma resonance (SPR) across the visible and the near-infrared spectral regions. Our synthesis strategy is firstly to prepare NaCl/AgCl core/shell structures in ethanol, and then fabricate hollow Ag microboxes by chemically reducing these core/shell structures with ascorbic acid, finally removing the sodium chloride cores by simple water washing. The whole synthesis process is under normal temperature and pressure, wet-chemistry conditions and is easy to control. Cubic NaCl crystals serve as unconventional non-spherical hard templates, easily coated by AgCl particles around the cube surfaces probably because of the similar crystalline structures of AgCl and NaCl. Ascorbic acid is employed because it can provide mild reducing conditions, which favour the morphology-preserved transformation of the precursor templates.21 The net-like nanofiber structure of the as-prepared Ag microboxes is important to preserve the hollow structure from destruction when we remove the NaCl core. We discuss the formation mechanism for this nanofiber net-like structure, as well as the structure-dependent surface plasma resonance (SPR) properties of the as-prepared Ag microboxes and nanofibers.
2. Experiment
2.1 Synthesis
All reagents were of analytical grade and used without further purification. In a typical synthesis of the hollow cubic Ag boxes, 200 μL NaCl solution (5 M) was added into 25 mL anhydrous ethanol with vigorous stirring, immediately giving a cloudy solution indicating the formation of NaCl crystals. This cloudy solution was then poured into a solution containing 25.0 mL anhydrous ethanol, 5.0 mL AgNO3 (0.05 M ethanolic solution), and 0.3 g PVP. After stirring the mixture for 2 h, the precipitate was collected by centrifugation at 5000 rpm, washed with and then dispersed in 20 mL NaCl-saturated anhydrous ethanol,22 followed by addition of 10.0 mL ascorbic acid (1.0 g 100 mL−1NaCl-saturated anhydrous ethanol) and 5 mL NaOH (0.25 M ethanolic solution). After gently stirring to produce a homogeneous mixture and then equilibrating without stirring for 12 h, the resultant gray precipitate was collected by centrifugation at 9000 rpm and repeatedly washed with aqueous ethanol (VH2O/VEthanol = 1/1).2.2 Characterization
The morphology and microstructure of the products were characterized by a transmission electron microscope (TEM, JEM 100-CXII) with an accelerating voltage of 80 kV, a high resolution transmission electron microscope (HR-TEM, JEOL-2010) with an accelerating voltage of 200 kV, and a field emission-scanning electron microscope (FE-SEM JSM-6700F). X-Ray diffraction (XRD) patterns were collected on a Rigaku D/Max 2200PC diffractometer with a graphite monochrometer and Cu-Kα radiation (λ = 0.15418 nm). UV-vis absorption spectra of the products dispersed in water were collected on a UV-vis spectrophotometer (Lambda-35, Perkin-Elmer). Thermogravimetric analysis (TGA) was carried out on a thermal analyzer (Mettler Toledo, TGA/SDTA851) at a heating rate of 10 °C min−1 from room temperature to 700 °C under N2 flow. Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 5DX FT-IR spectrometer using KBr pellet technique.3. Results and discussion
The colour contrast in the TEM images (Fig. 1a–c) clearly shows the hollow structure of the boxes with side lengths of ca. 1 μm and wall thicknesses of ca. 100 nm. Fig. 1c shows a distorted cubic box, and shows that the box face is composed of a net-like nanofiber structure. The selected area electron diffraction (SAED) pattern of this face exhibits bright diffraction rings or discontinuous spots, indicating the box is composed of polycrystalline nanofibers. The left side in Fig. 1d is the TEM images of two cubic Ag boxes, the right part outlines the corresponding sketch, which clearly distinguishes the cubic morphology, and shows that there is some distortion. FE-SEM images of the broken boxes (Fig. 1f) further demonstrate their hollow structure. The image of the ensemble (Fig. 1e) also shows the boxes facilely distorted morphology and polydisperse sizes, which may be the reason that the ensemble seems disordered. Considering the distorted hollow morphology, we speculate that the flexible polycrystalline nanofibers are easy to bend (marked by arrows in Fig. 1a). The walls of the box are thin compared to their lengths, and are hardly strong enough to support the framework of the cubic boxes. However, the boxes are composed of nanofibers which link into a net-like structure. We believe that this net-like structure ensures the flexibility of these Ag boxes, and is beneficial to maintain the hollow structure. | ||
| Fig. 1 TEM (a, b, c, d) and FE-SEM (e, f) images of the hollow cubic Ag boxes, the inset in (c) is an SAED pattern of one face of a box; the right side in (d) is the structural outline of two cubic boxes. | ||
The XRD pattern of the Ag boxes (Fig. 2) shows four obvious diffraction peaks corresponding to the (111), (200), (220) and (311) planes of metallic Ag with face-centered cubic (fcc) structure (JCPDS no. 04-0783), and no other peaks, indicating a single phase is present. The XRD pattern also shows a relatively broad diffraction, indicating that the Ag crystallites constructing the boxes are relatively small. Based on the diffraction peaks of the (111) planes, we calculated by the Scherrer formula that the average size of the Ag crystallites in the Ag boxes is about 13.5 nm. According to the above results, we speculate that the box is composed of metallic Ag crystallites, which connect together into a net-like structure to form the walls of the box, so that the six walls close to form the final hollow cubic Ag microbox.
 | ||
| Fig. 2 XRD pattern of as-prepared hollow cubic Ag boxes. | ||
HR-TEM image (Fig. 3a) shows the loose texture of a wall in a hollow cubic Ag box, which indicates the net-like nanofiber structure. The fibers are of diameter about 10–20 nm and intercross into a net. HR-TEM images (Fig. 3b–d) show the lattice patterns of the fiber's top, middle, and a branch, respectively. The lattice fringes appear in different directions. Most correspond to (111) plane spacing while some correspond to the (200) plane spacing of the face-centered cubic (fcc) metallic Ag, indicating the net-like nanofibers are polycrystalline. Considering the reaction conditions for preparation of the cubic Ag boxes with this net-like nanofiber structure, we assume that the ascorbic acid provides a kinetically controlled chemical environment for the generation of Ag nanosheets or nanowires due to its slow redox reaction rate.1f However, these net-like nanofibers with polycrystalline structure are significantly different from Ag nanosheets with oriented growth parallel to the [111] direction,23 or from long, straight Ag nanowires with a twinned crystalline structure,24 so their formation mechanism is sure to be associated with the specific experimental conditions. Therefore, we must first comprehend the experimental process for fabricating the hollow cubic Ag microboxes.
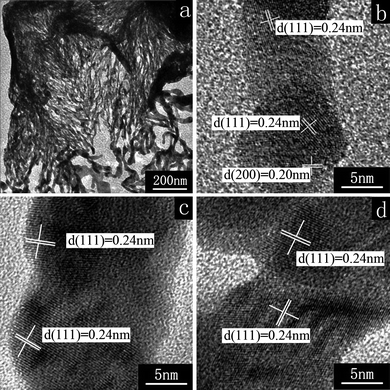 | ||
| Fig. 3 HR-TEM images of a loose wall (a), the top of a fiber (b), the middle of a fiber (c), and a fiber branch (d) in the as-prepared hollow cubic Ag boxes. | ||
Fig. 4 schematically illustrates the fabrication process for the hollow cubic Ag boxes. Firstly, the cubic NaCl crystals are formed in ethanol and then react with AgNO3 to generate AgCl. The AgCl particles heterogeneously nucleate on the surface of NaCl crystals to form NaCl/AgCl core-shell structures. These core-shell structures are collected by centrifugation and then reduced by ascorbic acid to generate metallic Ag. Finally we gain the hollow cubic Ag boxes by removing the NaCl core. This process design is tracked experimentally to demonstrate the formation mechanism of the hollow cubic Ag boxes.
 | ||
| Fig. 4 Schematic illustration of the fabrication process for the hollow cubic Ag boxes. | ||
The TEM image (Fig. 5a) of the NaCl crystals formed by addition of aqueous NaCl to ethanol shows they are cubic and have smooth surfaces with side lengths of ca.1.0 μm. Actually, these crystals do not have a highly uniform size-distribution, which is likely the primary reason that the final hollow cubic Ag boxes do not have a uniform size-distribution. The XRD pattern of these crystals (curve a of Fig. 6) confirms that they are NaCl crystals with face-centered cubic structure (JCPDS no. 05-0628). Fig. 5b shows that after they react with AgNO3, the surface of the NaCl crystals is no longer smooth and some small particles appear, indicating the formation of NaCl/AgCl core-shell structures by heterogeneous nucleation of AgCl on the NaCl surface. Small particles can also be observed in the background of this image which we consider are from the homogeneous nucleation of AgCl particles. The small particles can be removed from the product by centrifugation at an appropriate speed because of their tiny size compared with the NaCl/AgCl core-shell structures. As shown in Fig. 5c, after centrifugal separation, there are exclusively cubic NaCl/AgCl core-shell structures. The XRD pattern (curve b of Fig. 6) confirms that at this time the product is a mixture of NaCl (JCPDS no. 05-0628) and AgCl (JCPDS no. 31-1238). Both salts are of face-centered cubic structure and have very close lattice parameters, 5.640 and 5.549 Å, respectively. Though their XRD patterns almost coincide, we can distinguish them by the split diffraction peaks at the (200) and (220) planes. The former corresponds to NaCl and the latter to AgCl.
 | ||
| Fig. 5 TEM images of the intermediates in the formation process of the hollow cubic Ag boxes. (a) NaCl crystals, (b) NaCl/AgCl core-shell structures before centrifugation, (c) NaCl/AgCl core-shell structures after centrifugation, (d) hollow cubic AgCl boxes (e) an AgCl frame (f) some fragments of the AgCl boxes. | ||
 | ||
| Fig. 6 XRD patterns of the intermediates in the formation process of the hollow cubic Ag boxes. | ||
The products characteristic core-shell structure in Fig. 5c can be confirmed by removing the NaCl core to form a hollow cubic AgCl box. Fig. 5d is a TEM image of the product in Fig. 5c after washing with aqueous ethanol (VH2O/Vethanol = 1/1). The colour contrast indicates a hollow cubic structure. The corresponding XRD pattern (curve c of Fig. 6) confirms it is a single phase of AgCl with a face-centered cubic structure. All these results illuminate the formation of the designed intermediate product, the cubic NaCl/AgCl core-shell structures, which can form hollow cubic AgCl boxes by washing to remove the NaCl core. However, these AgCl boxes are fragile, so they may collapse when ultrasound is employed in the washing process (shown as Fig. 5e and f). When we use such AgCl boxes as a precursor to reduce AgCl to metallic Ag, we cannot obtain the morphology-preserved product, which proves that the NaCl core is indispensable in fabrication of the Ag boxes (ESI, Fig. S1).† We speculate that the NaCl cores provide a support for the freshly generated Ag nanofibers winding along their surfaces. Therefore, experimentally we select the NaCl/AgCl core-shell structures as precursors to fabricate hollow cubic Ag boxes.
There are two issues yet to be noticed. One is promoting the heterogeneous nucleation of AgCl particles at the NaCl crystal surfaces, which is beneficial to the formation of the desirable core/shell structures. Actually, the NaCl/AgCl core/shell structures cannot be obtained when we simply mix NaCl crystals with AgNO3 ethanolic solution. Under these conditions, the homogeneous nucleation of the AgCl particles will be overwhelming, resulting in successive consumption of the NaCl templates and formation of disordered agglomerates of AgCl precipitates. Therefore, we add PVP to the AgNO3 ethanolic solution before reacting with NaCl templates. PVP donates lone pair electrons from its oxygen and nitrogen atoms to the sp orbitals of the silver ions, and thus a coordination complex of Ag ions and PVP forms in solution,25 which proves to be a simple and effective means to eliminate homogeneous nucleation of the AgCl particles. Thus, the heterogeneous nucleation of the AgCl particles on the surface of the NaCl templates will dominate and we obtain the designed intermediate product, the cubic NaCl/AgCl core-shell structures.
Another issue is how to obtain the morphology-preserved product when these cubic NaCl/AgCl core-shell structures participate in the redox reaction. We have demonstrated that the NaCl core is indispensable in this reaction process. Therefore, experimentally we select NaCl-saturated ethanol as solvent to disperse the NaCl/AgCl core-shell structure precursors, which insures the NaCl cores are not dissolved in the whole redox reaction process. Moreover, a moderate reduction rate is critical to achieve this morphology-preserved synthesis. Qiet al. have demonstrated that a minimum reductant concentration is necessary for the morphology-preserved transformation from Ag3PO4 to metallic Ag.21 Analogously, our preliminary experiments show that a slow reduction rate favours the morphology-preserved transformation. We selected undisturbed reaction conditions and ascorbic acid as reductant, which is a widely used mild reducing agent. Its reduction of AgCl can be tuned by controlling the pH of the reaction system.26 Actually, the formation of morphology-preserved hollow cubic Ag boxes is so sensitive to the reaction environment that acute stirring influences the integrity of the boxes (ESI, Fig. S2).† When a stronger reductant such as NaBH4 is employed, we only see bulk particle agglomeration (ESI, Fig. S3).†
Though the hollow cubic Ag boxes are fabricated with a designed synthesis process, there is yet a puzzling issue as to how the net-like Ag nanofibers are generated. Considering the reaction conditions, using ascorbic acid as a mild reducing agent, the AgCl keeps the concentration of free Ag+ to a very low level in ethanolic solution. The normal temperature and static reaction process are also associated with a slow reaction rate. All these likely provide an opportunity for kinetically controlled syntheses, which, in the case of Ag, is generally favoured in the formation of plate-like products.1f Evidently, the net-like Ag nanofibers deviate from these plate-like products morphologically and structurally. In foregoing section, we speculated that the NaCl cores provide support for winding freshly generated Ag nanofibers along their surfaces. Would the net-like Ag nanofibers be generated when the NaCl cores are not present? Demonstrating this should be helpful for us in comprehending the formation mechanism of the Ag nanofibers. Therefore, the following comparative test was conducted.
We conducted the comparative test with a similar procedure to the fabrication of the hollow cubic Ag boxes, excepting not providing NaCl crystal templates. With other reaction conditions unchanged, we employ dispersive AgCl nano-particles as a substitute for the NaCl/AgCl core/shell structures (ESI, Fig. S4).† Net-like Ag nanofibers were prepared as predicted, but did not have the hollow cubic box morphology. As shown in Fig. 7a, the as-prepared Ag nanofibers agglomerate and disorder to some extent, though the SAED pattern is similar to the pattern of the nanofibers, which compose the hollow cubic Ag boxes. It should be noted that both SAED patterns display discontinuous concentric rings characteristic of fcc Ag, indicating that although the Ag nanofibers and the whole Ag boxes are not perfect single crystals, they have a certain amount of preferential crystal orientation.27 The SEM image (Fig. 7b) and the HR-TEM image (Fig. 7c) confirm that the as-prepared Ag nanofibers also have net-like morphology. The HR-TEM images of Fig. 7d–f show the lattice patterns of the nanofibers' middle, branch, and junction, respectively. Similar to the structures of the nanofibers which compose the hollow cubic Ag boxes, the as-prepared Ag nanofibers also show several lattice fringes with different directions; most correspond to the (111) plane spacing and some correspond to the (200) plane spacing of face-centered cubic (fcc) metallic Ag. Moreover, both fibers have similar X-ray powder diffraction patterns, thermogravimetric results and infrared spectra (ESI, Fig. S5–7).† Considering the architectural differences between the dispersed AgCl nanoparticles and NaCl/AgCl core/shell structures, all these results imply that both fibers have the same formation mechanism which may be independent of the form of the AgCl precursor, but must be closely associated with the special redox conditions where ascorbic acid reduces AgCl in ethanolic solution.
 | ||
| Fig. 7 TEM (a), SEM (b) and HR-TEM (c–f) images of Ag nanofibers, the inset in (a) is the SAED pattern. | ||
By closely investigating the HR-TEM images of the as-prepared Ag nanofibers (Fig. 7d–f), it can be observed that the nanofibers are composed of nanoparticles with grain boundaries (marked with straight lines in Fig. 7d–f) and defects (marked with arrows in Fig. 7d–f). These fibers have similar structural characteristics to the attachment structures of SnO2 ultra-thin nanowires,28 so we speculate on an attachment mechanism to form the net-like Ag nanofibers. This attachment could be considered as an attachment based on preferential crystal orientation, which is similar to the Ag nanodendrites.27 At the primary stage, portions of the AgCl are reduced to fine Ag nanocrystals. These Ag nanocrystals coexist with the AgCl particles which have not been reduced. Thus, at the intermediate reaction stage, there are two competitive pathways for the newly generated Ag atoms. One is to grow at the surfaces of the pre-existing Ag nanocrystals, and then to form large particles such as rods, wires or plates. Another is to nucleate and form new nanocrystals, and then Ag particles are attached due to the copious supply of Ag nanocrystals.27 At an appropriate reaction rate, the nanocrystals attach in series to form the nanofibers. Occasionally they attach side by side to form a branch or junction, which eventually forms the net-like Ag nanofiber structures.
Therefore, there are two factors which favour the formation of these net-like Ag nanofiber structures. One is continuously generating new nuclei which form new nanocrystals for subsequent attachment growth. Another is an appropriate reaction rate for the linear attachment to form the net-like nanofiber structures. In the anhydrous ethanol system that we use to fabricate the hollow cubic Ag boxes, we believe the above two factors to be set appropriately.
Firstly, Ag+ is reduced to metallic Ag by ascorbic acid (H2Asc),29 according to the following reaction.
| 2Ag+ + H2Asc + 2OH− → 2Ag0 + 2H2O + Asc | (1) |
It should be noted that H2O is generated in the reaction system, which would favour generating new nuclei continuously. The reason may be demonstrated as follows: sodium ascorbate is insoluble in ethanol, but soluble in water. In the anhydrous ethanol system coupled with NaOH, ascorbic acid would be precipitated in advance, and then be dissolved gradually as H2O is generated. That is to say, the concentration of the reducing agent is increased gradually, and then the redox reaction rate is increased gradually. Therefore, continuous nucleation should be reasonable and the new nanocrystals are in adequate supply for subsequent attachment growth.
Secondly, the redox reaction is accelerated by the gradually generated H2O, whereas the reaction rate is essentially slow because of the low concentration of free Ag+ and the mild reducing power of ascorbic acid, which provides an opportunity for particle attachment growth in a somewhat ordered mode. At an appropriate reaction rate, the particles attach in series to form the nanofiber net-like structures. This attachment mode is highly sensitive to the reaction conditions, as testified by the comparative test of preparing Ag nanofibers. Even if stirring is employed to change the reaction rate, the final product deviates from the net-like nanofibers and a disordered collection of aggregated Ag nanoparticles is obtained instead (ESI, Fig. S8).†
Ag nanostructured materials exhibit a strong ultraviolet/visible/near infrared (UV/vis/NIR) absorption band arising from a localized surface plasmon resonance (LSPR).3 The numbers and positions of the LSPR are closely related to the Ag nanostructures' physical parameters such as shape, size and composition, and structure (e.g. solid or hollow).30 Computational studies have also demonstrated the LSPR's dependency on these physical parameters.3,31 We anticipate that the as-prepared Ag nanofibers and boxes may have plasmon resonance across the visible and the near-infrared regions of the electromagnetic spectrum. However, we have not established corresponding simulation modes for theoretical calculation, due to the complex hierarchical structures of these Ag nanofibers and boxes. Here, we describe the qualitative features of the plasmon resonances for the as-prepared Ag nanofibers and boxes, compared to the aggregated Ag nanoparticles.
In Fig. 8, the curves describe the SPR patterns of as-prepared Ag aggregated particles, fibers, and boxes. A broad plasmon peak at ∼410 nm appears in the LSPR patterns of the aggregated Ag particles, indicating the presence of silver particles with nanometre-sized dimensions.32 This peak exhibits a broad full-width at half-maximum (FWHM) and a tailing effect, indicating a broad distribution in size and morphology for these silver nanoparticles.33 The broadening of the peak may also be attributed to random interparticle electromagnetic coupling owing to aggregation.34 With regard to the Ag nanofiber absorption band in Fig. 8, the most intense resonance peak blue-shifts to ∼380 nm. This blue shift is usually attributed to transverse modes in wire-shaped Ag nanostructures.7,25 It should also be noted in the fibers' SPR patterns that an obvious tailing effect appears across the visible and the near-infrared regions of the electromagnetic spectrum, which may be attributed to the longitudinal modes of the Ag nanofibers. There is no centralized absorption to form a peak for the longitudinal modes, which implies a highly polydisperse sample containing nanofibers with a wide range of aspect ratios.35 These results are consistent with the as-prepared Ag nanofibers' netlike structure- an ensemble of wire-shaped Ag nanostructures with polydisperse aspect ratios. In contrast, the plasmon peak for the Ag boxes further blue-shifts to ∼350 nm, which can be attributed to the plasmon response of the long silver nanowires (nanofibers), and also may be due to the optical signatures of bulk silver.7,25 Notably, the increasing intensity of the Ag boxes' absorption around the visible and near infrared regions indicates that the optical response of the hollow Ag nanostructures can be tuned and red-shifted significantly, however, the as-prepared Ag boxes do not exhibit a centralized plasmon peak in the visible and near infrared spectrum region, which is probably due to their polydispersity and facile distortion.
 | ||
| Fig. 8 UV/vis/NIR absorption spectra of the Ag nanostructures. | ||
4. Conclusions
In summary, hollow cubic Ag microboxes with net-like nanofiber structures have been fabricated in an ethanol system under ambient conditions, by chemically reducing NaCl/AgCl core/shell structured precursors with ascorbic acid. The net-like nanofiber and hollow structure bring the Ag microboxes a surface plasma resonance (SPR) across the visible and the near-infrared regions, which are promising for applications to various areas, including catalysis, surface-enhanced Raman scattering (SERS) detection, biology and medicine. The synthetic approach based on templating methods provides a useful model for the synthesis of Ag hollow structures. Furthermore, this approach may be extended to other reaction systems and could possibly be used for device fabrication.Acknowledgements
This work is supported by the Major State Basic Research Development Program of China (973 Program) (no. 2010CB933504) and the National Natural Science Foundation of China (no. 20671057). The authors thank Dr Pamela Holt for help with editing the manuscript.References
- A. Henglein, Chem. Rev., 1989, 89, 1861 CrossRef CAS; G. Schmid and L. F. Chi, Adv. Mater., 1998, 10, 515 CrossRef CAS; P. V. Kamat, J. Phys. Chem. B, 2002, 106, 7729 CrossRef CAS; R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2005, 109, 12663 CrossRef CAS; A. Tao, S. Habas and P. Yang, Small, 2008, 4, 310 CrossRef CAS; Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60 CrossRef CAS.
- S. Nie and S. R. Emery, Science, 1997, 275, 1102 CrossRef CAS; K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667 CrossRef CAS; A. J. Haes and R. P. Van Duyne, J. Am. Chem. Soc., 2002, 124, 10596 CrossRef CAS; K. Kneipp, H. Kneipp and J. Kneipp, Acc. Chem. Res., 2006, 39, 443 CrossRef CAS; M. L. Tran, S. P. Centeno, J. A. Hutchison, H. Engelkamp, D. Liang, G. Van Tendeloo, B. F. Sels, J. Hofkens and H. Uji-i, J. Am. Chem. Soc., 2008, 130, 17240 CrossRef CAS; T. Dadosh, J. Sperling, G. W. Bryant, R. Breslow, T. Shegai, M. Dyshel, G. Haran and I. Bar-Joseph, ACS Nano, 2009, 3, 1988 CrossRef CAS; H. Peng, C. M. Strohsahl, K. E. Leach, T. D. Krauss and B. L. Miller, ACS Nano, 2009, 3, 2265 CrossRef CAS.
- T. Jensen, L. Kelly, A. Lazarides and G. C. Schatz, J. Cluster Sci., 1999, 10, 295 CrossRef CAS; J. P. Kottmann, O. J. F. Martin, D. R. Smith and S. Schultz, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 235402 CrossRef; M. A. El-Sayed, Acc. Chem. Res., 2001, 34, 257 CrossRef CAS; C. J. Murphy and N. R. Jana, Adv. Mater., 2002, 14, 80 CrossRef CAS; F. Kim, J. Song and P. Yang, J. Am. Chem. Soc., 2002, 124, 14316 CrossRef CAS; P. Jain, K. Lee, I. H. El-Sayed and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 7238 CrossRef CAS; B. J. Wiley, S. Im, Z. Li, J. McLellan, A. Siekkinen and Y. Xia, J. Phys. Chem. B, 2006, 110, 15666 CrossRef CAS.
- R. Jin, C. Cao, A. Mirkin, K. L. Kelly, G. G. Schatz and J. Zheng, Science, 2001, 294, 1901 CrossRef CAS; R. Jin, C. Cao, E. Hao, G. S. Metraux, G. C. Schatz and C. A. Mirkin, Nature, 2003, 425, 487 CrossRef CAS; I. Washio, Y. Xiong, Y. Yin and Y. Xia, Adv. Mater., 2006, 18, 1745 CrossRef CAS.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176 CrossRef CAS; T. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648 CrossRef CAS; R. Jin, S. Egusa and N. F. J. Scherer, J. Am. Chem. Soc., 2004, 126, 9900 CrossRef CAS.
- Y. Sun, B. Mayers and Y. Xia, Nano Lett., 2003, 3, 675 CrossRef CAS; N. Zhao, Y. Wei, N. Sun, Q. Chen, J. Bai, L. Zhou, Y. Qin, M. Li and L. Qi, Langmuir, 2008, 24, 991 CrossRef CAS.
- Y. Sun, Y. Yin, B. T. Mayers, T. Herricks and Y. Xia, Chem. Mater., 2002, 14, 4736 CrossRef CAS; Y. Sun, B. Mayers, T. Herricks and Y. Xia, Nano Lett., 2003, 3, 955 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065 CrossRef CAS; B. Pietrobon, M. McEachran and V. Kitaev, ACS Nano, 2009, 3, 21 CrossRef CAS.
- S. Chen, Z. Wang, J. Ballato, S. H. Foulger and D. L. Carroll, J. Am. Chem. Soc., 2003, 125, 16186 CrossRef CAS; E. Hao, R. C. Bailey, G. C. Schatz, J. T. Hupp and S. Li, Nano Lett., 2004, 4, 327 CrossRef CAS; Y. Han, S. Liu, M. Han, J. Bao and Z. Dai, Cryst. Growth Des., 2009, 9, 3941 CrossRef CAS.
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549 CrossRef CAS; C. Loo, A. Lowery, N. Halas, J. West and R. Drezek, Nano Lett., 2005, 5, 709 CrossRef CAS; J. Chen, D. Wang, J. Xi, L. Au, A. Siekkinen, A. Warsen, Z. Li, H. Zhang, Y. Xia and X. Li, Nano Lett., 2007, 7, 1318 CrossRef CAS; G. Wu, A. Milkhailovsky, H. A. Khant, C. Fu, W. Chiu and J. A. Zasadzinski, J. Am. Chem. Soc., 2008, 130, 8175 CrossRef CAS; J. Zhu, J. Nanopart. Res., 2009, 11, 785 CrossRef CAS; G. B. Braun, A. Pallaoro, G. Wu, D. Missirlis, J. A. Zasadzinski, M. Tirrell and N. O. Reich, ACS Nano, 2009, 3, 2007 CrossRef CAS; Y. Hu, Q. Chen, Y. Ding, R. Li, X. Jiang and B. Liu, Adv. Mater., 2009, 21, 3639 CrossRef CAS.
- Y. Sun and Y. Xia, J. Am. Chem. Soc., 2004, 126, 3892 CrossRef CAS; Y. Yin, C. Erdonmez, S. Aloni and A. P. Alivisatos, J. Am. Chem. Soc., 2006, 128, 12671 CrossRef CAS; X. Lu, H. Tuan, J. Chen, Z. Li, B. A. Korgel and Y. Xia, J. Am. Chem. Soc., 2007, 129, 1733 CrossRef CAS.
- J. B. Jackson and N. J. Halas, J. Phys. Chem. B, 2001, 105, 2743 CrossRef CAS; Z. Jiang and C. Liu, J. Phys. Chem. B, 2003, 107, 12411 CrossRef CAS; K. Yong, Y. Sahoo, M. T. Swihart and P. N. Prasad, Colloids Surf., A, 2006, 290, 89 CrossRef CAS; C. Noguez and J. Zhang, J. Phys. Chem. A, 2009, 113, 4068 CrossRef.
- F. Caruso, R. A. Caruso and H. Mohwald, Science, 1998, 282, 1111 CrossRef CAS; X. W. Lou, C. Yuan and L. A. Archer, Small, 2007, 3, 261 CrossRef CAS.
- Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS; H. Zeng, W. Cai, P. Liu, X. Xu, H. Zhou, C. Klingshirn and H. Kalt, ACS Nano, 2008, 2, 1661 CrossRef CAS.
- V. S. Marthy, J. N. Cha, G. D. Stucky and M. S. Wong, J. Am. Chem. Soc., 2004, 126, 5292 CrossRef; Y. Wan and S. H. Yu, J. Phys. Chem. C, 2008, 112, 3641 CrossRef CAS.
- J. Li and H. C. Zeng, J. Am. Chem. Soc., 2007, 129, 15839 CrossRef CAS; J. G. Yu, H. G. Yu, H. T. Guo, M. Li and S. Mann, Small, 2008, 4, 87 CrossRef CAS.
- D. Zhang, L. Qi, J. Ma and H. Cheng, Adv. Mater., 2002, 14, 1499 CrossRef CAS.
- Z. Chen, P. Zhan, Z. Wang, J. Zhang, W. Zhang, N. Ming, C. Chan and P. Sheng, Adv. Mater., 2004, 16, 417 CrossRef CAS.
- Z. Wang, M. Chen and L. Wu, Chem. Mater., 2008, 20, 3251 CrossRef CAS.
- X. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- J. Yang, L. Qi, C. Lu, J. Ma and H. Cheng, Angew. Chem., Int. Ed., 2005, 44, 598 CrossRef CAS.
- The NaCl-saturated anhydrous ethanol is prepared as follows: ∼2 g NaCl is added into 500 mL anhydrous ethanol, then equilibrated for more than 1 day. After centrifugation, we obtain the clear NaCl-saturated anhydrous ethanol for subsequent use as solvent.
- X. Sun, S. Dong and E. Wang, Angew. Chem., Int. Ed., 2004, 43, 6360 CrossRef CAS; J. Du, B. Han, Z. Liu, Y. Liu and D. Kang, Cryst. Growth Des., 2007, 7, 900 CrossRef CAS.
- J. Hu, Q. Chen, Z. Xie, G. Han, R. Wang, B. Ren, Y. Zhang, Z. Yang and Z. Tian, Adv. Funct. Mater., 2004, 14, 183 CrossRef CAS; Z. Wang, J. Liu, X. Chen, J. Wan and Y. Qian, Chem.–Eur. J., 2005, 11, 160 CrossRef; M. Tsuji, K. Matsumoto, N. Miyamae, T. Tsuji and X. Zhang, Cryst. Growth Des., 2007, 7, 311 CrossRef CAS.
- Z. Zhang, B. Zhao and L. Hu, J. Solid State Chem., 1996, 121, 105 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957 CrossRef CAS; M. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2004, 108, 5882 CrossRef CAS.
- X. Wen, Y. Xie, W. Mak, K. Cheung, X. Li, R. Renneberg and S. Yang, Langmuir, 2006, 22, 4836 CrossRef CAS.
- X. Xu, J. Zhuang and X. Wang, J. Am. Chem. Soc., 2008, 130, 12527 CrossRef CAS.
- K. P. Velikov, G. E. Zegers and A. van Blaaderen, Langmuir, 2003, 19, 1384 CrossRef CAS.
- K. L. Kelly, E. Coronado, L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668 CrossRef CAS; D. D. Evanoff and G. Chumanov, J. Phys. Chem. B, 2004, 108, 13957 CrossRef CAS.
- I. O. Sosa, C. Noguez and R. G. Barrera, J. Phys. Chem. B, 2003, 107, 6269 CrossRef CAS.
- D. D. Evanoff and G. Chumanov, J. Phys. Chem. B, 2004, 108, 13948 CrossRef.
- M. N'Gom, J. Ringnalda, J. F. Mansfield, A. Agarwal, N. Kotov, N. J. Zaluzec and T. B. Norris, Nano Lett., 2008, 8, 3200 CrossRef CAS.
- W. Rechberger, A. Hohenau, A. Leitner, J. R. Krenn, B. Lamprecht and F. R. Aussenegg, Opt. Commun., 2003, 220, 137 CrossRef CAS; K. Su, Q. Wei, X. Zhang, J. J. Mock, D. R. Smith and S. Schultz, Nano Lett., 2003, 3, 1087 CrossRef CAS.
- C. Ah, S. Hong and D. Jang, J. Phys. Chem. B, 2001, 105, 7871 CrossRef CAS; R. Erni and N. D. Browning, Ultramicroscopy, 2007, 107, 267 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images of the products obtained by comparative experiments, which include using AgCl hollow boxes as precursors, reaction under acute stirring, using NaBH4 as reductant, dispersive AgCl nanoparticles and using them as precursors under acutely stirring, and XRD patterns, TG curves, IR curves of the Ag boxes and Ag fibers. See DOI: 10.1039/c0ce00132e |
| This journal is © The Royal Society of Chemistry 2011 |
