A facile approach to PbS nanoflowers and their shape-tunable single crystal hollow nanostructures: Morphology evolution †
Yingnan
Wang‡
a,
Quanqin
Dai‡
ab,
Xinyi
Yang
a,
Bo
Zou
*a,
Dongmei
Li
a,
Bingbing
Liu
a,
Michael Z.
Hu
b and
Guangtian
Zou
a
aState Key Laboratory of Superhard Materials, Jilin University, Changchun, 130012, China. E-mail: zoubo@jlu.edu.cn; Fax: (+86) 431-85168883
bOak Ridge National Laboratory, Oak Ridge, Tennessee, 37830, USA
First published on 27th August 2010
Abstract
In this work, we presented a facile approach for the preparation of three-dimensional PbS nanoflowers, which was attributed to the coexistence of two types of amines with different-length alkyl chains and different steric hindrance. These monodisperse PbS nanoflowers showed small particle sizes (∼35 nm) and narrow size distribution (δ ≈ 9%). On the basis of these nanoflowers, we obtained a series of single-crystal hollow PbS nanostructures with tunable morphologies (including sphere, cuboctahedron, cube, and tube/rod) through elevating reaction temperature and prolonging growth time. It was further followed by a detailed discussion of the mechanism of morphology evolution, where the recrystallization and intraparticle ripening made contributions.
1. Introduction
Semiconductor nanocrystals have attracted a great deal of attention because of their unique physical and chemical properties. It is well-known that these properties are greatly influenced by the nanocrystal shape and size.1 Significant progress in the synthesis of different-shaped and sized nanostructures has been achieved in the past two decades. Among these numerous nanostructures, complex three-dimensional (3D) colloidal nanocrystals, such as crystalline nanoflowers, are very interesting because of their unique technical potential, which is inaccessible with the 0D and 1D counterparts.2–6 For the synthesis of 3D nanoflowers, the previous mainstream was based on high-temperature approaches,3–8 while we inhere present a room-temperature preparation of PbS nanoflowers that have been rarely reported. Interestingly, these as-prepared PbS nanoflowers could evolve into hollow nanostructures with different shapes, dependent on the reaction temperature and time.Because of their high surface area and low material density, hollow nanostructures have many novel physical and chemical features that differentiate them from other 3D nanostructures. These features make hollow structures ideal building blocks for a range of applications, including catalysis, optical sensing, drug delivery, biomedical imaging and photothermal cancer treatment.9–14 For instance, it has been demonstrated that hollow nanocrystals could serve as resonant nanocavities for holding and probing small particles (e.g. biomolecules or quantum dots) in sensing applications.15 Although much effort was devoted to developing synthetic methods for hollow nanostructures, the related syntheses were mainly limited to template techniques,16–21 Kirkendall effect22–24 and Ostwald ripening process.25–27 These template-assisted methods usually require many complex procedures, including template modification, precursor attachment, and core removal. Comparatively, our reported preparation of different-shaped hollow PbS nanostructures is template-free, appearing facile and reproducible.
Lead sulfide (PbS), an important member of the IV-VI semiconductors, has a narrow band gap (0.41 eV) and a large exciton Bohr radius (18 nm). Its nanoscaled counterpart exhibits strong quantum confinement in the near-infrared wavelength, and shows potential applications in optical switching,28 field effect transistors (FET),29 solar-cell,30,31 telecommunication,32 and biotechnology applications.33 As a result, PbS nanomaterials with various morphologies (0D-3D) have been synthesized for such studies and applications.34–38 For example, PbS hollow nanostructures were synthesized through the surfactant-assisted sonochemical approach39 and the cation exchange method,40 respectively. However, it still remains a challenge to develop a facile approach for the synthesis of 3D PbS nanostructures, because the previously reported PbS and other IV-VI nanocrystals with 3D structures usually show large sizes (>100 nm) and wide size distributions. And these hollow structures mainly appear spherical and polycrystalline.
In this paper, we described a facile method to synthesize 3D PbS nanoflowers with relatively small and uniform sizes (∼35 nm). Based on these nanoflowers, a series of single-crystal hollow PbS nanostructures with tunable morphologies (including sphere, cuboctahedron, cube, and tube/rod) were obtained through elevating reaction temperature and prolonging growth time. Afterward, the formation mechanism of PbS nanoflowers and their shape transformation were systematically discussed.
2. Experimental section
2.1 Materials
Lead oxide (99.999%), sulfur powder (99.98%), oleic acid (technical grade 90%), phenyl ether (99%), oleylamine (OLA, 70%), dodecylamine (DDA, 98%) and octylamine (OA, 99%) were all purchased from Aldrich. Methanol, toluene, tetrachloroethylene (TCE) and acetone were obtained from commercial sources. All chemicals were used without further purification.2.2 Preparation of PbS nanoflowers
In a typical synthesis, a mixture of PbO powder (0.223 g, 1.0 mmol), oleic acid (1.0 ml) and OA (5.0 ml) was added to a 50-ml three-neck flask. This mixture was heated to 120 °C under the nitrogen protection, where yellow PbO powder was completely dissolved under stirring. After the mixture was naturally cooled to room temperature (RT, ∼25 °C), 3.5 ml of sulfur solution (including 0.016 g of sulfur dissolved in both OLA and phenyl ether with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 volume ratio of OLA to phenyl ether) was added into the flask. Upon stirring, the initial pale-yellow clear solution gradually turned black, indicating the formation of the PbS nanoflowers at RT. At different reaction intervals, aliquots were taken from the flask, and immediately purified with methanol and excess acetone. Subsequently, the residual samples were re-dispersed in TCE for characterization.
4 volume ratio of OLA to phenyl ether) was added into the flask. Upon stirring, the initial pale-yellow clear solution gradually turned black, indicating the formation of the PbS nanoflowers at RT. At different reaction intervals, aliquots were taken from the flask, and immediately purified with methanol and excess acetone. Subsequently, the residual samples were re-dispersed in TCE for characterization.
2.3 Preparation of hollow PbS nanostructures
The above homogeneous black solution of PbS nanoflowers was either directly heated to a certain reaction temperature, or transferred to a 10-ml Teflon-lined autoclave. The autoclave was kept in a furnace at a certain temperature for 30 min and then naturally cooled to RT. The same purification process shown above was carried out before the characterization of the resulting hollow nanostructures.2.4 Characterization
All measurements were performed at RT. The phase purity of the obtained samples was characterized by X-ray powder diffraction (XRD, Bruker D8 diffractometer working with a Cu-Kα target). Samples for TEM characterization were prepared by adding several drops of PbS solution in TCE onto the 300-mesh copper grids with carbon support film. Transmission electron microscopy (TEM) images and selected area electron diffraction (SAED) pattern were obtained on a Hitachi H-8100 microscope operated at 200 kV. High-resolution transmission electron microscopy (HRTEM) images were obtained on a JEM-2100F operated at 200 kV, where the corresponding fast Fourier transform (FFT) algorithms were analyzed.3. Results and discussion
Although there are some publications reporting the synthesis of PbS nanocrystals,34–36 these reported syntheses generally employed individual fatty acids and amines, and generated dot-shaped nanocrystals. Comparatively, we here utilized two different types of amines to produce 3D PbS nanoflowers. The clear sulfur solution (prepared with sulfur, phenyl ether and OLA) was mixed with the stirred colorless lead solution (prepared with lead oxide, oleic acid and OA). Shortly, the initial clear mixture gradually turned black, indicative of the formation of PbS nanoflowers, as illustrated in Fig. 1. Fig. 2a presents the TEM image of flower-like PbS aggregates. These highly monodisperse nanoflowers have an average diameter of ∼35 nm. Their standard deviation (δ) was calculated to be ∼9%. The PbS nanoflowers were further investigated by the HRTEM analysis (Fig. 2b). It can be observed that the resulting nanoflowers are composed of small-sized PbS nanocrystals with average sizes of 3∼8 nm. Clear and continuous lattice fringe can be observed. The distance between neighboring fringes was measured to be 0.30 nm, close to the (200) lattice spacing of PbS. To determine the accurate structure of these nanoflowers, we characterized the SAED pattern of more than 80 nanoflowers, as shown in Fig. 2c. All the dot-rings in this SAED pattern could be perfectly indexed to the same positions as those of the bulk cubic rock-salt PbS. | ||
| Fig. 1 Synthesis of PbS nanoflowers at room temperature (RT). | ||
 | ||
| Fig. 2 (a) TEM image of PbS nanoflowers synthesized with OLA and OA included. (b) HRTEM image of PbS nanoflowers. (c) SAED pattern of PbS nanoflowers. | ||
As shown above, our presented synthesis of PbS nanoflowers are facile, via simply mixing the sulfur and lead solutions together at room temperature. Also, this synthesis is easily reproducible. One can readily reproduce PbS nanoflowers by strictly following the recipe shown in the Experimental Section or even reasonably changing the dosage of starting materials. For example, even though the amount of the fatty acid and/or amine was quadrupled, PbS nanoflowers could be prepared (Fig. S1†). This indicates that the chemical dosage related to the change of concentrations and ratios is not a major factor in the formation of PbS nanoflowers. Instead, we found that the coexistence of two types of fatty amines (such as OA and OLA) with different chain lengths were the key to the formation of nanoflowers. PbS nanoflowers could be prepared only when two fatty amines with different chain lengths were involved in the reaction (Fig. 2 with the coexistence of OA and OLA, and Fig. 3 with the coexistence of DDA and OLA). If individual amines (either of OA, OLA, and DDA) were used, no nanoflowers could be observed, where only monodisperse PbS nanoparticles were formed at both low and high temperatures (Fig. S2†). This provides a simple and effective approach to synthesize monodisperse PbS nanoparticles. Generally, between two types of bulky alkyl chains in fatty amines/acids, the shorter alkyl chains have smaller steric hindrance, which drastically increases the rate of oriented attachments of individual nanocrystals to form 3D nanostructures, such as flower-like aggregates.41 It should be noted that although it is not novel to use two types of fatty amines/acids with different chain lengths to produce nanoflowers, the previous syntheses needed to be performed at high temperatures. Houtepen et al. reported that the combination of acetic acid and oleic acid could result in a dramatic change in the nanocrystal shape, forming flower-like and star-like PbSe nanocrystals at ∼130 °C.41 Peng and co-workers combined acetic acid and myristic acid to synthesize a series of nanoflowers (In2O3, CoO, MnO, ZnO, and ZnSe) at >250 °C.3,42
 | ||
| Fig. 3 (a) TEM image of flower-like PbS aggregates synthesized with OLA and DDA included. (b) HRTEM image of corresponding PbS nanoflowers. | ||
Interestingly, the room-temperature formed PbS nanoflowers could transformed into hollow nanostructures through simply regulating the reaction temperature and reaction time. Fig. 4a presents the TEM image of spherical hollow PbS nanoparticles obtained at 150 °C for 30 min. The average diameter of these hollow nanospheres is ∼38 nm and the diameter ratio of sphere to cavity is close to 2.0. Compared with the original monodisperse PbS nanoflowers, these spherical hollow PbS nanoparticles also keep highly monodisperse and uniform size (δ ≈ 9%). In fact, the hollow nanospheres could be transformed from the as-prepared nanoflowers at a reaction temperature as low as 100 °C (Fig. S3†), but the obtained nanospheres had rough surfaces, indicative of poor crystallinity. When the reaction with PbS nanoflowers involved was conducted at 200 °C, monodisperse hollow PbS nanocuboctahedrons (Fig. 4b) and nanocubes (Fig. 4c) could be subsequently observed at different reaction intervals. This indicates that at higher temperatures the initial nanoflowers could transformed into hollow cuboctahedral morphologies; with the prolongation of reaction time these nanocuboctahedrons would further become cubic. Comparing with hollow nanospheres, the hollow cuboctahedrons and cubes had smooth surfaces and more regular geometrical morphologies.
 | ||
| Fig. 4 TEM images of the different-shaped hollow PbS nanostructures prepared at different reaction temperatures and times. (a) spherical hollow nanostructures, (b) cuboctahedral hollow nanostructures, (c) cubic-like hollow nanostructures. The scale bars represent 100 nm (top) and 50 nm (bottom) respectively. | ||
The morphology of these transformed PbS nanostructures are further investigated by HRTEM and XRD. Fig. 5a and b display HRTEM images of the hollow nanospheres. The fringe spacing was measured to be 0.34 nm, which is close to the (111) lattice spacing of PbS. The fast Fourier transform (FFT, inset in Fig. 5b) is consistent with the (111) projection, showing the well-crystalline feature. Similarly, the good crystallinity of hollow nanocubes was also confirmed by the HRTEM images (Fig. 5c and d). Additionally, from Fig. 5a–d, we can have a good view of the cavity by a clear contrast difference between their edges (dark color) and centers (light color). Both of the FFT patterns and clear lattice fringes indicate that these hollow structures are single crystals. Fig. 6 presents the XRD patterns of the initial and transformed PbS nanoparticles with different morphologies. All the diffraction peaks were labeled and could be indexed to the cubic rock salt PbS (JCPDS 05-0592). Compared with the original nanoflowers, PbS hollow nanocubes show better crystallinity, which is consistent with the corresponding TEM images.
 | ||
| Fig. 5 (a and b) HRTEM images of spherical hollow PbS particles. (c and d) HRTEM images of cubic-like hollow PbS particles. Insets in Figures b and d are the corresponding FFT patterns. | ||
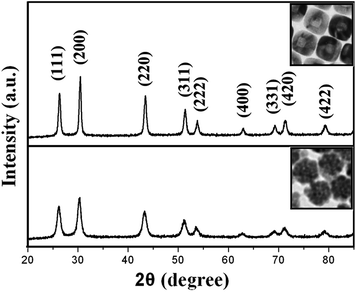 | ||
| Fig. 6 XRD spectra of the PbS nanoflowers (bottom) and cubic-like hollow PbS particles (top). | ||
As mentioned above, the morphologies of PbS hollow nanoparticles are strongly dependent on the reaction temperature and time. This dependence could be systematically observed in Fig. 7. With the reaction temperature (T) ranging from 100 °C to 200 °C and reaction time (t) lasting within 30 min, hollow PbS nanoparticles with different shapes can be obtained. However, further prolongation of reaction time (i.e. 60 min at 150 °C) could lead the resulting hollow PbS particles to gradually transform into solid and polydisperse PbS nanoparticles (Fig. S4†). The average size of these solid nanocubes is smaller (10–30 nm at 150 °C for 60 min) than that of their corresponding hollow nanostructures. As mentioned above, if the as-prepared PbS nanoflowers were heated to a reaction temperature below 100 °C, they were stable enough to resist the morphology transformation. In contrast, if they were heated to an extremely high reaction temperature (e.g. 250 °C), the original PbS nanoflowers would be transformed into highly crystalline nanotubes/nanorods (Fig. 7 and Fig. 8). It was found that the length of these nanotubes/nanorods can reach 5 μm (Fig. 8a); their diameter is ∼40 nm, similar to that of the hollow nanocubes (Fig. 8b and c). In Fig. 8c, some remaining nanocubes were observed to coexist with these nanotubes/nanorods. This implies that the formed nanotubes/nanorods were most possibly derived from the evolution of hollow nanocubes. At the extremely high reaction temperature, the adjacent hollow nanocubes would be able to link with each other leading to the formation of long nanotubes/rods.43
 | ||
| Fig. 7 (a) Shape evolutions of PbS nanoparticles synthesized under different reaction conditions. All scale bars represent 50 nm. (b) Schematic diagram of shape evolutions of PbS nanoparticles. | ||
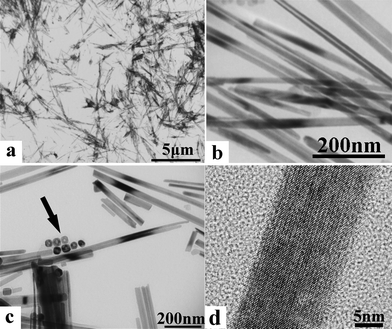 | ||
| Fig. 8 TEM images of PbS nanotubes/nanorods under different magnifications. The arrow in Panel c shows the coexisting hollow particles. | ||
After carefully calculated the effective volumes of the hollow nanostructures, we found that there almost was no change in these volumes. This indicates that there exists a typical intraparticle ripening process, in which the materials in a nanocrystal are redistributed in the same nanocrystal during the evolution of crystal shapes. This process is the most consistent with the experimental observation of nanoflower-based morphology transformations. With the increase of reaction temperatures, the structural defects (interspaces) of flower-like aggregates acted as active points to form the interior cavity gradually. Therefore, these loose flower-like PbS aggregates would crystallize gradually through a selective recrystallization process, evolving into hollow nanospheres with the relatively low interfacial energy.44 When the reaction temperature continued to increase, the hollow nanospheres could further evolve into more advanced morphologies, such as hollow nanocuboctahedrons and nanocubes. That is because, for rock salt structures of IV–VI nanocrystals, the growth of the higher surface energy (111) face in the <111> direction was faster than that of the lower surface energy (100) face in the <100> direction. This would favor the growth of the (111) facets, resulting in the formation of cubic-like nanostructures with the lowest total surface energy.37,45,46Annealing these hollow nanostructures at elevated temperatures for a prolonged period of time would improve their crystallinity and thus eliminate their defects. With the least defects in crystallinity, solid nanoparticles would finally replace their hollow morphology to terminate the shape evolution, indicating the termination of intraparticle ripening. As already discussed above, it is a tendency for rock salt IV–VI nanocrystals to transform form spheres to cubes in the high-temperature regime. This explains the formation of solid nanocubes, instead of solid nanospheres, when the reaction was kept long enough at high temperatures (Figure S4). It should be noted that a reaction running too long (e.g. 60 min, Fig. S4†) would trigger the occurrence of Ostwald ripening, causing the nanocrystal ensemble to appear polydisperse.
4. Conclusion
In summary, we report a facile and reproducible method to synthesize monodisperse PbS nanoflowers, which is attributed to the coexistence of two types of amines with different-length alkyl chains and different steric hindrance. Furthermore, during the increase of reaction temperature and time, the intraparticle ripening drives these prepared nanoflowers to evolve into single-crystal hollow nanostructures with different morphologies (sphere, cuboctahedron, cube, and tube/rod).Acknowledgements
This work is supported by NSFC (nos. 20773043 and 10979001), the National Basic Research Program of China (nos. 2005CB724400 and 2007CB808000), the LDRD program at the Oak Ridge National Laboratory and the Nanomanufacturing project, Industrial Technology Program of the US Department of Energy.References
- X. G. Peng, L. Manna, W. D. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Alivisators, Nature, 2000, 404, 59 CrossRef CAS.
- S. H. Sun, D. Q. Yang, D. Villers, G. X. Zhang, E. Sacher and J. Dodelet, Adv. Mater., 2008, 20, 571 CrossRef CAS.
- A. Narayanaswamy, H. F. Xu, N. Pradhan and X. G. Peng, Angew. Chem., Int. Ed., 2006, 45, 5361 CrossRef CAS.
- J. J. Ning, Q. Q. Dai, T. Jiang, K. K. Men, D. H. Liu, N. R. Xiao, C. Y. Li, D. M. Li, B. B. Liu, B. Zou, G. T. Zou and W. W. Yu, Langmuir, 2009, 25, 1818 CrossRef CAS.
- F. L. Jia, L. Z. Zhang, X. Y. Shang and Y. Yang, Adv. Mater., 2008, 20, 1050 CrossRef CAS.
- Y. S. Luo, S. Q. Li, Q. F. Ren, J. P. Liu, L. L. Xing, Y. Wang, Y. Yu, Z. J. Jia and J. L. Li, Cryst. Growth Des., 2007, 7, 87 CrossRef CAS.
- F. Cao, W. D. Shi, L. J. Zhao, S. Y. Song, J. H. Yang, Y. Q. Lei and H. J. Zhang, J. Phys. Chem. C, 2008, 112, 17095 CrossRef CAS.
- Q. Q. Dai, N. R. Xiao, J. J. Ning, C. Y. Li, D. M. Li, B. Zou, W. W. Yu, S. H. Kan, H. Y. Chen, B. B. Liu and G. T. Zou, J. Phys. Chem. C, 2008, 112, 7567 CrossRef CAS.
- S. Y. Chang, L. Liu and S. A. Asher, J. Am. Chem. Soc., 1994, 116, 6745 CrossRef CAS.
- F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111 CrossRef CAS.
- D. E. Bergbreiter, Angew. Chem., Int. Ed., 1999, 38, 2870 CrossRef CAS.
- H. Huang, E. E. Remsen, T. Kowalewski and K. L. Wooley, J. Am. Chem. Soc., 1999, 121, 3850.
- Y. Sun and Y. N. Xia, Science, 2002, 298, 2176 CrossRef CAS.
- Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS.
- J. Aizpurua, P. Hanarp, D. S. Sutherland, M. Kall, G. W. Bryant and F. J. G. de Abajo, Phys. Rev. Lett., 2003, 90, 057401 CrossRef CAS 401.
- T. He, D. R. Chen, X. L. Jiao and Y. L. Wang, Adv. Mater., 2006, 18, 1078 CrossRef CAS.
- X. M. Lu, L. Au, J. McLellan, Z. Y. Li, M. Marquez and Y. N. Xia, Nano Lett., 2007, 7, 1764 CrossRef CAS.
- Y. D. Yin, C. Erdonmez, S. Aloni and A. P. Alivisatos, J. Am. Chem. Soc., 2006, 128, 12671 CrossRef CAS.
- K. An, S. G. Kwon, M. Park, H. B. Na, S. Baik, J. H. Yu, D. Kim, J. S. Son, Y. W. Kim, I. C. Song, W. K. Moon, H. M. Park and T. Hyeon, Nano Lett., 2008, 8, 4252 CrossRef CAS.
- S. H. Jiao, L. F. Xu, K. Jiang and D. S. Xu, Adv. Mater., 2006, 18, 1174 CrossRef CAS.
- J. C. Park, J. Kim, H. Kwon and H. Song, Adv. Mater., 2008, 20, A1 CrossRef.
- X. Liang, X. Wang, Y. Zhuang, B. Xu, S. M. Kuang and Y. D. Li, J. Am. Chem. Soc., 2008, 130, 2736 CrossRef CAS.
- G. Q. Zhang, W. Wang, Q. X. Yu and X. G. Li, Chem. Mater., 2009, 21, 969 CrossRef CAS.
- A. Cabot, R. K. Smith, Y. D. Yin, H. M. Zheng, B. M. Reinhard, H. T. Liu and A. P. Alivisatos, ACS Nano, 2008, 2, 1452 CrossRef CAS.
- P. Hu, L. J. Yu, A. H. Zuo, C. Y. Guo and F. L. Yuan, J. Phys. Chem. C, 2009, 113, 900 CrossRef CAS.
- X. Wang, H. B. Fu, A. Peng, T. Y. Zhai, Y. Ma, F. L. Yuan and J. N. Yao, Adv. Mater., 2009, 21, 1.
- Z. M. Chen, Z. R. Geng, M. L. Shi, Z. H. Liu and Z. L. Wang, CrystEngComm, 2009, 11, 1591 RSC.
- F. W. Wise, Acc. Chem. Res., 2000, 33, 773 CrossRef CAS.
- D. V. Talapin and C. B. Murray, Science, 2005, 310, 86 CrossRef CAS.
- S. A. McDonald, G. Konstantatos, S. Zhang, P. W. Cyr, E. J. D. Klem, L. Levina and E. H. Sargent, Nat. Mater., 2005, 4, 138 CrossRef CAS.
- G. I. Koleilat, L. Levina, H. Shukla, S. H. Myrskog, S. Hinds, A. G. Pattantyus-Abraham and E. H. Sargent, ACS Nano, 2008, 2, 833 CrossRef CAS.
- L. Bekueva, S. Musikhin, M. A. Hines, T.-W. F. Chang, M. Tzolov, G. D. Scholes and E. H. Sargent, Appl. Phys. Lett., 2003, 82, 2895 CrossRef.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435 CrossRef CAS.
- K. A. Abel, J. N. Shan, J. C. Boyer, F. Harris and F. J. M. van Veggel, Chem. Mater., 2008, 20, 3794 CrossRef CAS.
- L. Cademartiri, J. Bertolotti, R. Sapienza, D. S. Wiersma, G. V. Freymann and G. A. Ozin, J. Phys. Chem. B, 2006, 110, 671 CrossRef CAS.
- J. Liu, H. Yu, Z. Wu, W. Wang, J. Peng and Y. Cao, Nanotechnology, 2008, 19, 345602 CrossRef.
- Z. P. Peng, Y. S. Jiang, Y. H. Song, C. Wang and H. J. Zhang, Chem. Mater., 2008, 20, 3153 CrossRef CAS.
- H. B. Li, D. Chen, L. L. Li, F. Q. Tang, L. Zhang and J. Ren, CrystEngComm, 2010, 12, 1127 RSC.
- S. F. Wang, F. Gu and M. K. Lü, Langmuir, 2006, 22, 398 CrossRef CAS.
- Y. F. Zhu, D. H. Fan and W. Z. Shen, Langmuir, 2008, 24, 11131 CrossRef CAS.
- A. J. Houtepen, R. Koole, D. Vanmaekelbergh, J. Meeldijk and S. G. Hickey, J. Am. Chem. Soc., 2006, 128, 6792 CrossRef CAS.
- A. Narayanaswamy, H. F. Xu, N. Pradhan, M. Kim and X. G. Peng, J. Am. Chem. Soc., 2006, 128, 10310 CrossRef CAS.
- C. Zhang, Z. H. Kang, E. Shen, E. Wang, L. Gao, F. Luo, C. G. Tian, C. L. Wang, Y. Lan, J. X. Li and X. J. Cao, J. Phys. Chem. B, 2006, 110, 184 CrossRef CAS.
- Y. X. Gao, S. H. Yu, H. P. Cong, J. Jiang, A. W. Xu, W. F. Dong and H. Cölfen, J. Phys. Chem. B, 2006, 110, 6432 CrossRef CAS.
- W. G. Lu, J. Y. Fang, K. L. Stokes and J. Lin, J. Am. Chem. Soc., 2004, 126, 11798 CrossRef CAS.
- J. E. Murphy, M. C. Beard, A. G. Norman, S. P. Ahrenkiel, J. C. Johnson, P. R. Yu, O. I. Mićić, R. J. Ellingson and A. J. Nozik, J. Am. Chem. Soc., 2006, 128, 3241 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional TEM images. See DOI: 10.1039/c004459h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2011 |
