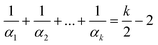On water nets L4(x)6(y)
Alexandru
Banaru
*
Moscow State University, Chemistry Faculty, Leninskie Gory, 1, Moscow, 119991, Russia. E-mail: banaru@phys.chem.msu.ru; Tel: +7 495 9392258
First published on 3rd September 2010
Abstract
On the basis of the Infantes–Motherwell classification of water clusters in organic hydrates and graph theory, topologies of 2-homeohedral 2-isogonal nets with tetra- and hexagonal cycles were derived. The implication on the structure of water layers in a crystal is discussed.
Introduction
In the early 2000's the attention of some researchers specialized in the field of crystal engineering was attracted to crystalline hydrates. By means of statistical analysis of their structures deposited to CSD1 it was shown2 that water molecules frequently form planar (i.e. capable of being projected onto a plane surface without intersecting edges) nets. Such nets are denoted2 by the symbol Lm1(n1)m2(n2)…mk(nk), where mi is the size of disjoint cycles, ni is the number of cycles, which share water molecules with the i-th one. The most widespread water layers are L4(6)5(7)26(8) and L5(7) (Fig. 1(a) and (b)) having average size of cycles m = 5. Subsequently it was found3 that this fact is not occasional. The protic excess p of a layer defined as the number of protons not binding the vertices of a net (excess protons) is generally divisible by 1/n (Table 1), where n is the number of water molecules per formula unit M·nH2O. | ||
| Fig. 1 Water layers in hydrates retrieved from CSD:1 (a) L4(6)5(7)26(8) (AMHPYR), (b) L5(7) (PINOLH01), (c) L4(6)6(8) (HEKXAW). | ||
| p | Layer | CSD refcode | p ∑ | Composition | Space group | Layer symmetry group |
|---|---|---|---|---|---|---|
| a There are branches of water molecules on the layer. b The are water molecules isolated from the layer. | ||||||
| 1/3 | L5(7) | BIVMUO | 1/3 | C7H12N2O2S·3H2O | P212121 | P l (YZ)21 |
| CEYVEH | 1/3 | C10H18N2·6H2O | P21/c | P l (XY)21 | ||
| PINOLH01 | 1/3 | C6H14O2·6H2O | Pnnm | P l (XY)21212 | ||
| PIPERH | 1/3 | C4H10N2·6H2O | P21/n | P l (XY)21 | ||
| L4(6)6(8) | HEKXAW | 1/3 | C5H7N3OS·3H2O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P
l
(XY)![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
|
| RABCEY | 1/2a | C2HFClO2·4H2O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P
l
(XY)![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
||
| L4(6)5(7)26(8) | AMHPYR | 1/3 | C4H8N2O2·3H2O | P21/c | P l (YZ)21/c | |
| PYRTHA10 | 1/3 | C5H5N·3H2O | Pbca | P l (XZ)21/a | ||
| XIHSEM | 1/3 | C8H8N4O·3H2O | P21/c | P l (YZ)21/c | ||
| YINFAC | 1/3 | C6H7N·3H2O | P21/c | P l (YZ)21/c | ||
| YINDUU | 1/3 | C6H15N·3H2O | P21/c | P l (YZ)21/c | ||
| PUHPOX | 1/3 | C12H24N4·6H2O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P
l
(XY)![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
||
| HOMPRO10 | 3/4b | C6H4NO2·4H2O | P21/c | P l (YZ)21/c | ||
| 2/5 | L4(6)5(6)7(8) | GIXDUM | 2/5 | C4H12N2·5H2O | C2/c | P l(YZ)2/c |
| 3/8 | L4(6)5(6)26(8)2 | HASPIA | 3/8 | C10H13N5O2·4H2O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P
l
(XY)![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| 1/2 | L6(6) | BEWYAE | 1/2 | C6H8B2O4·4H2O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P
l
(XZ)![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| HOSHIG | 1/2 | C8H12N2O2·4H2O | Pbcn | P l(YZ) bc21 | ||
| SAJXEG | 2/3a | C16H25NO2·3H2O | Pna21 | P l(YZ) n | ||
| IMIPID | 1b | C4H6N2O2·3H2O | P21ab | P l (XY)21ab | ||
| L4(4)8(8) | GIKZOP | 1/2 | C16H30N2O2·8H2O | C2/m | C l (XY)2/m | |
| 3/5 | L4(6)8(6)8(8)2 | GIXDOG | 2/3a | C7H18N2·3H2O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P
l
(XY)![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| L4(2)4(4)4(6)16(12) | EBUNIZ | 2/3a | C16H18N2O2·6H2O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P
l
(011)![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
|
| 2/3 | L4(4)12(8) | SIFFAO | 2/3 | C14H13NO·3H2O | P21/c | P l (YZ)21/c |
| L6(4)10(8) | REGREA | 3/4a | C12H24O6·8H2O | I2/a | P l(XY)2/a | |
| 3/4 | L4(4)16(8) | DUDPIB | 3/4 | C20H38N4O6P2·8H2O | P1 | P l(YZ)1 |
| L8(8)12(8) | JAMNER | 3/4 | C22H20N2O6·8H2O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P
l
(XY)![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
|
| 4/5 | L12(6) | ITEWOT | 4/5 | C12H10N2O2·5H2O | Pbcn | P l(XY) b2n |
| 5/6 | L14(6) | CPMIAL10 | 6/7a | C21H20N6O3·7H2O | P21/c | P l(XZ) c |
| 7/8 | L18(6) | CATXIE | 7/8 | C17H19N3O3·4H2O | P21/c | C l (XY)2 |
| EHULAU | 7/8 | C12H24N3O6·4H2O | P63 | P l(XY)3 |
Water layers in crystalline hydrates are usually formed when n ≥ 3. At the same time the amount of structural data in CSD dramatically decreases with increase of n. Therefore the most widespread layered hydrates are trihydrates.3 If a net contains merely 3- and 4-coordinated vertices (O atoms), then 0 ≤ p ≤ 1/2, specifically, in trihydrates p = 1/3 corresponding to m = 5.
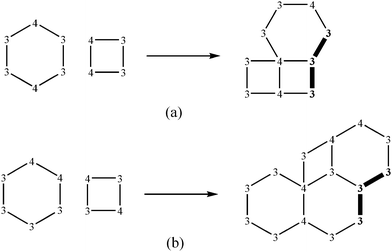
The third most popular water layer with p = 1/3 is L4(6)6(8). In contrast to homeohedral tilings L5(7) of a plane, for which merely two combinatorially distinct types are known,4 the number of respective 2-homeohedral tilings (with two transitivity classes of faces with respect to automorphism group of a net) is apparently much greater, however, in concrete hydrates the only variant occurred to date (Fig. 1(c)). In order to understand the causes of this, one should investigate all theoretically feasible nets formed by equivalent tetra- and hexagons. This work is aimed on derivation of such nets in the most ordinary case, when a tiling is 2-isogonal, i.e. each vertex (of degree 3 or 4) generates the sole transitivity class.
Remark that even cycles tend to occupy inversion centres causing centrosymmetric layer as well as the crystal (Table 1). A few exceptions correspond to L6(6) (HOSHIG, SAJXEG, IMIPID) and layers with large 12–18-membered rings (DUDPIB, ITEWOT, CPMIAL10, CATXIE, EHULAU).
Euler’s formula for 2-periodic nets
Homeohedral 2-periodic nets made of k-gons satisfy the following modification of Euler’s formula:4where αi is the degree of i-th vertex. Let modify this formula for a 2-homeohedral tiling L4(x)6(y) (k = 10) with vertices of degrees 3 and 4:
(n = number of vertices, lower index = number of polygon sides, upper index = vertex degree, nIII4 + nIV4 = 4, nIII6 + nIV6 = 6). Several cases arise (Table 2). Remark the net L4(8)6(6) is impossible since tetra- and hexagon have no vertex shared in this case. Let consider the other cases.
| n III 4 | n IV 4 | n III 6 | n IV 6 | L4(x)6(y) |
|---|---|---|---|---|
| 4 | 0 | 2 | 4 | L4(4)6(10) |
| 3 | 1 | 3 | 3 | L4(5)6(9) |
| 2 | 2 | 4 | 2 | L4(6)6(8) |
| 1 | 3 | 5 | 1 | L4(7)6(7) |
| 0 | 4 | 6 | 0 | L4(8)6(6) |
2-Isogonal nets L4(x)6(y)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Therefore, no L4(4)6(10) exists.
1. Therefore, no L4(4)6(10) exists.
 | ||
| Scheme 1 Requisite environment of monohedron 4(4). Numbers denote vertex degrees. | ||
 | ||
| Scheme 2 Monohedra 4(5) and 6(9). | ||
 | ||
| Scheme 3 Contradictory environments of 4(6) and 6(8). | ||
From ortho-arrangement of 4-coordinated vertices in a hexagon the tape follows, its margin being formed by 4-coordinated vertices, and interior—by 3-coordinated (Scheme 4(a)). Such tapes can be imposed in two ways generating two layers. At para- arrangement of 4-coordinated vertices in both polygons a tape from hexagons is made, which tetragons univocally adjoin to. Such tapes can be imposed in the only fashion (Scheme 4(b)). In final case (Scheme 3(b)) one finds the same contradiction as for (a).
 | ||
| Scheme 4 Requisite generation of L4(6)6(8). | ||
 | ||
| Scheme 5 Requisite generation of L4(7)6(7). | ||
Conclusion
There are four 2-homeohedral 2-isogonal tilings of a plane surface formed by tetra- and hexagonal tiles (![[m with combining macron]](https://www.rsc.org/images/entities/i_char_006d_0304.gif) = 5). Why are not all of these variants realized in crystalline hydrates? The answer can prove to be the following. Since water cycles tend to be localized in inversion centers of a crystal, they are to have even number of adjacent cycles that fits L4(6)6(8), the latter existing of the only type, as shown above.
= 5). Why are not all of these variants realized in crystalline hydrates? The answer can prove to be the following. Since water cycles tend to be localized in inversion centers of a crystal, they are to have even number of adjacent cycles that fits L4(6)6(8), the latter existing of the only type, as shown above.
Remark there can exist (and do exist5) 3-isogonal nets L4(5)6(9) and L4(6)6(8). For exhaustive derivation one may utilize the method discussed in current work (instead of “truncating” and “splitting” 1-isohedral tilings of a plane which are less comprehensive). On the other hand, 2-isogonal nets look the most feasible in crystals since water net, in general, tends to be produced at least number of non-equal intermolecular contacts6H2O⋯OH2.
References
- F. H. Allen and W. D. S. Motherwell, Acta Cryst., 2002, B 58, 407 CAS.
- L. Infantes and S. Motherwell, CrystEngComm, 2002, 4(75), 454 RSC.
- A. Banaru and Yu. L. Slovokhotov, CrystEngComm, 2010, 12, 1054, 10.1039/b918793f.
- B. N. Delone, Izv. Akad. Nauk SSSR Ser. Mat., 1959, 23, 365 Search PubMed.
- B. Grünbaum, H.-D. Löckenhoff, G. C. Shepard and Á. H. Temesvári, Geom. Dedicata, 1985, 19, 109 Search PubMed.
- A. M. Banaru, Moscow University Chemistry Bulletin, 2009, 64, 80 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |