Pendant orientation and its influence on the formation of hydrogen-bonded thiacalixarene nanotubes†
Yan
Li
,
Weiping
Yang
,
Yuanyin
Chen
and
Shuling
Gong
*
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072, PR China. E-mail: gongsl@whu.edu.cn; Fax: + 86 27 68754067; Tel: + 86 27 68752701
First published on 7th September 2010
Abstract
Five 1,3-alternatethiacalix[4]arene derivatives bearing carboxylic acid and/or urea hydrogen-bonding groups were prepared and their crystal structures were determined by single-crystal X-ray diffraction methods. In compound 1, where the pendant arms are all adorned with carboxylic acid groups, the pendants all orientate along the base of the molecular axis. An interesting three-dimensional network of “endo-inclusion” aquatubes is formed by stacking the water contained cavities of 1 up and down. While concerning to the other four compounds to which the urea groups are introduced, their pendant arms either orientate towards the inner side of the cavities, or orientate towards the outside, depending on the types of hydrogen-bonding groups and the position of these groups. When the urea groups are in the same side (compounds 2, 4 and 5), the opposite chains in the molecule will locate away from each other which may be due to the steric repulsions. But when the urea group and carboxylic acid group are in the same side (compounds 3 and 4), the opposite chains all orientate inwards because of the intramolecular, inter-chain hydrogen bonds between the opposite chains. Although these four compounds can also self-assemble through the cavity stacking motif, the inwardly orientated pendant arms which protrude into the thiacalixarene cavity obstruct the channels.
Introduction
Organic nanotubes formed by molecular self-assembly have drawn considerable attention because of their unique encapsulation, release, and transport properties.1 A variety of molecular building blocks have been rationally designed and synthesized as tube-forming compounds.2 The rigid skeleton of calixarenes has proved fruitful in the synthesis of tubular systems.3–5 Most of these nanotubes are formed by arranging calixarenes along the surface of a cylinder4 and only a few are formed by stacking the calixarene cavities up and down with a shared central axis.5 Among these architectures, we are attracted by the latter self-assembly motif which involves not only the functional groups on the lower or upper rims, but also the native concave cavities of calixarenes whose importance can be clearly seen from the words “The ability of calixarenes to act as baskets is one of their most intriguing properties, accounting for much of the interest that they have received since their reincarnation in the 1970s ” said by C. David Gutsche.6Our early work reported such a one-dimensional channel formed by the self-assembly of 1,2-alternate p-tert-butylcalix[4]arene tetra-acetic acid.5d In the present contribution we have expanded our studies with the 1,3-alternate p-tert-butylthiacalix[4]arene framework mainly due to the following reasons: (1) the tubular shape of 1,3-alternate skeleton7 usually invokes the idea of forming the channels in the solid state by the cavity stacking motif;5a,5b (2) the presence of sulfur atoms in place of the usual CH2 bridges makes thiacalixarenes behaving many unique features compared with “classical” calixarenes, such as the larger cavity dimensions and metal complexation through the sulfur atoms which make these compounds good candidates for exploring the guest inclusion properties of cavity; and (3) the 1,3-alternate derivatives of p-tert-butylthiacalix[4]arene are easily accessible in multi-gram amounts without chromatographic purification, while the 1,3-alternate derivatives of p-tert-butylcalix[4]arene can only be obtained rarely by the trivial procedure.8
A variety of reversible intermolecular interactions such as coordination bonding, electrostatic, van der Waals forces or hydrogen and halogen bonding have been used, sometimes in combination, for the generation of solid state supramolecular architectures.9 For the formation of purely organic materials multiple hydrogen bonds are particularly attractive, since they are relatively strong, directional, and many of them can act simultaneously.9,10
Based upon the above conception, five 1,3-alternate thiacalix[4]arene derivatives bearing carboxylic acid and/or urea hydrogen-bonding groups at the lower rim have been synthesized (Scheme 1). Their molecular structures and self-assembly behaviour in the solid state are studied. Mainly, the orientation of the pendant arms and its influence on the formation of nanotubes formed by the cavity sacking motif are emphasized.
 | ||
| Scheme 1 Syntheses of the compounds. | ||
Results and discussion
Molecular structures in the solid state
The crystal structures of the five compounds are shown in Fig. 1. The torsion angles of the five compounds at the sulfur bridges are all in the sequence of ++, −−, ++, −− that is consistent with the 1,3-alternate conformation found in calix[4]arene.11 The values of the dihedral angles between the phenolic rings and the reference molecular plane R, defined as least squares plane containing the sulfur atoms of the bridges indicate the tilting of the rings which leads to an enlargement of the cavities on both sides of the molecules (Table 1). But the enlargement degree of the cavities of the five compounds is different, which can be seen from the interplanar angles between the pairs AC and BD (Table 2). This could be mainly ascribed to the intramolecular, inter-chain hydrogen bond between the opposite pendant arms which connect to the lower rim of the molecules. The four pendant arms connected to aromatic rings A, B, C and D are called chain A, chain B, chain C and chain D, respectively.| Structure no. | Plane AR | Plane BR | Plane CR | Plane DR |
|---|---|---|---|---|
| 1 | 103.94 | 102.43 | 102.58 | 100.25 |
| 2 | 107.87 | 121.56 | 104.38 | 112.54 |
| 3 | 116.47 | 104.17 | ||
| 4 | 102.96 | 108.17 | 116.38 | 115.49 |
| 5 | 112.90 | 109.28 | 110.58 | 119.51 |
| Structure no. | Plane AC | Plane BD |
|---|---|---|
| 1 | 26.53 | 22.69 |
| 2 | 32.25 | 54.13 |
| 3 | 40.68 | 40.68 |
| 4 | 39.44 | 43.72 |
| 5 | 43.49 | 48.86 |
 | ||
| Fig. 1 Molecular structures of the five compounds. The hydrogen atoms connected to carbon atom and disordered atoms are all omitted for clarity. | ||
However, compared with the cavity shape, the most significant differences among these thiacalix[4]arenes are the spatial orientation of the pendant arms with respect to the calixarene cavity which can be established by examining the torsion angles related to the four chains reported in Table 3.
| Chain A | Chain B | Chain C | Chain D | |
|---|---|---|---|---|
| 1 | C6–O1–C11–C12 | C38–O4–C47–C48 | C30–O7–C35–C36 | C14–O10–C23–C24 |
| −175.5(3) | −175.9(4) | −173.7(4) | −175.1(4) | |
| O1–C11–C12–O2 | O4–C47–C48–O5 | O7–C35–C36–O9 | O10–C23–C24–O11 | |
| −2.0(7) | −1.4(8) | −7.3(6) | −4.8(8) | |
| O1–C11–C12–O3 | O4–C47–C48–O6 | O7–C35–C36–O8 | O10–C23–C24–O12 | |
| 178.3(4) | 178.5(5) | 174.4(4) | 177.0(5) | |
| 2 | C1–O1–C11–C12 | C15–O4–C24–C25 | C27–O7–C36–C37 | C40–O10–C49–C50 |
| 177.4(2) | 175.9(2) | 116.9(3) | 60.3(3) | |
| O1–C11–C12–N2 | O4–C24–C25–O5 | O7–C36–C37–N1 | O10–C49–C50–O11 | |
| 6.7(4) | 8.5(4) | −4.7(3) | 93.3(3) | |
| O1–C11–C12–O2 | O4–C24–C25–O6 | O7–C36–C37–O8 | O10–C49–C50–O12 | |
| −172.2(3) | −171.2(3) | 176.4(2) | −88.1(4) | |
| C11–C12–N2–C13 | C36–C37–N1–C38 | |||
| −176.3(3) | −176.5(3) | |||
| C12–N2–C13–O3 | C37–N1–C38–O9 | |||
| 171.7(3) | −176.7(3) | |||
| C12–N2–C13–N4 | C37–N1–C38–N3 | |||
| −7.8(5) | 5.1(4) | |||
| 3 | C1–O1–C11–C12 | C14–O4–C23–C24 | ||
| 171.9(4) | −167.1(5) | |||
| O1–C11–C12–O3 | O4–C23–C24–N1 | |||
| 15.1(7) | −12.7(9) | |||
| O1–C11–C12–O2 | O4–C23–C24–O5 | |||
| −164.9(6) | 170.3(6) | |||
| C23–C24–N1–C25 | ||||
| −177.9(6) | ||||
| C24–N1–C25–O6 | ||||
| 180.0(6) | ||||
| C24–N1–C25–N2 | ||||
| −0.1(9) | ||||
| 4 | C6–O1–C11–C12 | C19–O4–C24–C25 | C32–O7–C37–C38 | C45–O10–C50–C51 |
| 114.4(9) | 171.1(4) | 175.4(8) | 178.6(6) | |
| O1–C11–C12–N1 | O4–C24–C25–N3 | O7–C37–C38–N5 | O10–C50–C51–O11 | |
| −6.3(3) | 7.0(6) | −0.2(7) | 166.0(1) | |
| O1–C11–C12–O2 | O4–C24–C25–O5 | O7–C37–C38–O8 | O10–C50–C51–O12 | |
| 170.1(9) | 171.0(5) | 179.9(0) | −18.2(4) | |
| C11–C12–N1–C13 | C24–C25–N3–C26 | C37–C38–N5–C39 | ||
| 172.8(8) | 175.3(9) | 178.4(2) | ||
| C12–N1–C13–O3 | C25–N3–C26–O6 | C38–N5–C39–O9 | ||
| 176.1(6) | 174.5(3) | 177.1(4) | ||
| C12–N1–C13–N2 | C25–N3–C26–N4 | C38–N5–C39–N6 | ||
| 4.7(0) | −10.1(4) | 1.0(8) | ||
| 5 | C1–O1–C11–C12 | C19–O4–C24–C25 | C28–O7–C37–C38 | C45–O10–C50–C51 |
| −177.4(3) | −79.0(3) | 81.0(3) | −167.0(2) | |
| O1–C11–C12–N1 | O4–C24–C25–N3 | O7–C37–C38–N5 | O10–C50–C51–N7 | |
| −9.5(4) | −76.7(3) | 79.9(3) | −7.6(4) | |
| O1–C11–C12–O2 | O4–C24–C25–O5 | O7–C37–C38–O8 | O10–C50–C51–O11 | |
| 172.6(3) | 101.1(3) | −99.2(3) | 172.8(3) | |
| C11–C12–N1–C13 | C24–C25–N3–C26 | C37–C38–N5–C39 | C50–C51–N7–C52 | |
| −179.4(3) | 164.7(3) | −179.2(3) | −178.9(3) | |
| C12–N1–C13–O3 | C25–N3–C26–O6 | C38–N5–C39–O9 | C51–N7–C52–O12 | |
| 172.8(3) | −172.8(3) | −179.3(3) | 170.5(3) | |
| C12–N1–C13–N2 | C25–N3–C26–N4 | C38–N5–C39–N6 | C51–N7–C52–N8 | |
| −5.6(5) | 6.7(5) | 0.3(5) | −11.4(4) |
The torsion angles of the four pendant arms of compound 1 show that the pendants' orientations are very similar. As illustrated in Fig. 1, the four pendant arms are all oriented along the base of the molecular axis. There is no intramolecular hydrogen bond between the two opposing pendant arms. But there are two water molecules, which occupy the cavity of compound 1. The intermolecular hydrogen bond between water and the methyleneoxycarboxylic acid group will be discussed in detail in the following.
In compound 2, two carboxyl groups are located on one side; two urea groups are located on the other side. The values of torsion angle in Table 3 show that the four pendant arms in compound 2 are oriented in pairs, one with chain B and chain A pointing towards the inner side of the calixarene cavity and the second with chain C and chain D pointing towards the outside, as illustrated in Fig. 1. The carboxyl group on chain B formed one intramolecular hydrogen bond O5⋯O10 (2.923 Å) with the opposing chain D, which leads to the burying of chain B in the cavity of the thiacalixarene and also the unusual large interplanar angles between the opposing aromatic rings B and D. There is no intramolecular, inter-chain hydrogen bond between the opposing chains A and C. But four intra-chain N–H⋯O hydrogen bonds exist in these two urea chains. Two are between NH2 group and CH2CO group, N3⋯O8 (2.705 Å) and N4⋯O2 (2.705 Å). The other two are between phenolic oxygen atom and NH group, N1⋯O7 (2.712 Å) and N2⋯O1 (2.627 Å).
Compound 3 also has two carboxyl groups and two urea groups as compound 2, the only difference is that the same groups are no longer located on the same side, but averagely on the two sides separated by the S1–S2–S3–S4 plane. And one half of the molecule is deduced from the other half by a twofold screw axis which located at (1/2, y, 1/4). The four pendant arms all orientate inwardly with respect to the calixarene cavity which may be due to the two intramolecular hydrogen bonds between the opposing pendant arms: O3⋯O6 (2.557 Å) and N1⋯O3 (3.170 Å) (Fig. 1). Besides, there are also four intra-chain N–H⋯O hydrogen bonds which is similar to compound 2.
There are three urea groups and one carboxyl group in compound 4 whose structure is comprised of two parts: (I) half part of compound 2 (the urea part) and (II) half part of compound 3. In part (I), one urea chain orientates outwardly (chain A) and one urea chain orientates inwardly (chain C). There is no intramolecular hydrogen bond between these two chains. In part (II), both chains (chain B and chain D) orientate inwardly because of the hydrogen bonds O12⋯O6 (2.504 Å) and N3⋯O12 (3.109 Å) (Fig. 1). The pendant orientation of the two parts is also consistent with the same parts in compound 2 and compound 3. There are six intra-chain N–H⋯O hydrogen bonds, three between NH2 group and CH2CO group and three between phenolic oxygen atom and NH group.
The spatial orientation of the four pendant arms of compound 5 is similar to compound 2 (Fig. 1). Two orientate inwardly (chain A and chain D) and two orientate outwardly (chain B and chain C). There is one intramolecular hydrogen bond N3⋯O12 (2.862 Å) between the opposing chains B and D and one N5⋯O3 (2.951 Å) between the opposing chains A and C. And like compounds 2, 3 and 4, the intra-chain N–H⋯O hydrogen bonds are also present in the four urea chains. Four are between NH2 group and CH2CO group, N2⋯O2 (2.691 Å), N4⋯O5 (2.737 Å), N6⋯O8 (2.675 Å) and N8⋯O11 (2.663 Å). Two are between phenolic oxygen atom and NH group, N1⋯O1 (2.607 Å), N7⋯O10 (2.654 Å).
From the above analysis we can see that in compound 1 where the pendant arms are all adorned with carboxylic acid groups, the pendants all orientate along the base of the molecular axis. Water molecules may also play a role in stabilizing the structure. While concerning to the other four compounds to which the urea groups are introduced, their pendant arms either orientate towards the inner side of the cavity, or orientate towards the outside. When the same substituted groups are in the same side, the opposite chains in the molecule will locate away from each other. That is, if one chain orientates towards the cavity, the relative opposite chain will orientate outwards, which is obvious in compounds 2, 4 and 5. This may be due to the steric repulsions between the substituted opposite chains. But when the carboxylic acid group and urea group are in the same side (compounds 3 and 4), i.e. the hydrogen-bond recognition sites are arranged in a complementary manner, the situation is different. The opposite chains all orientate inwards because of the intramolecular, inter-chain hydrogen bonds between the opposite chains. Besides, there is a common characteristic in the inwardly orientated urea chains, all the atoms on these chains are almost in the same plane, as can be seen from the torsion angles in Table 3. There are always two types of intra-chain N–H⋯O hydrogen bonds in these chains, the hydrogen bond between NH2 group and CH2CO group, and the hydrogen bond between phenolic oxygen atom and NH group.
The self-assembly behaviour of the compounds
In the crystal structure of compound 1, the asymmetric unit contains one thiacalix[4]arene molecule, two water molecules and four ethanol molecules. The two water molecules locate on the two sides of molecule 1 and both situate almost in the middle of the cavity (O13–C17 = 3.273 Å, O13–C41 = 3.258 Å, angle C17–O13–C41 = 170.87°; O14–C3 = 3.363 Å, O14–C27 = 3.362 Å, angle C3–O14–C27 = 172.22°). They act as both a hydrogen donor (O13⋯O7 (2.799 Å), O13⋯O1 (2.948 Å), O13⋯O2 (2.751 Å), O14⋯O5 (2.641 Å), O14⋯O4 (2.909 Å)) and acceptor (O9⋯O13 (2.554 Å), O11⋯O14 (2.641 Å)) bridging the opposite pendant arms. The intermolecular hydrogen bond between water and methyleneoxycarboxylic group makes the structure of 1 rigid, which can be seen from the similarity of the torsion angles of the four pendant arms (−175.58°, −175.82°, −173.81° and −175.08°) (Fig. 1). In the extended structure, each thiacalix[4]arene molecule hydrogen bonds to four ethanol molecules using the four carboxylic groups on the pendant arms, i.e. O6⋯O17 (2.563 Å), O16⋯O12 (2.728 Å), O3⋯O18 (2.552 Å) and O15⋯O8 (2.745 Å) (Fig. 2)). Due to the pseudo-tetrahedronal distribution of the pendant arms, the four ethanol molecules thus occupy the apexes of a pseudo-tetrahedron. Then each ethanol molecule binds to another ethanol molecule which connects with the neighbouring thiacalix[4]arenes. Along the [101] direction, this hydrogen-bonding pattern affords a wave-ladder type of one-dimensional hydrogen-bonded network of the compound (tape structure) so that deep grooves are formed. Neighbouring tapes stack along the b axis without any molecular translation along the a and c axis, only with alternating thiacalix[4]arenes in each tape inserting into the grooves of the neighbouring tapes, which give rise to an infinite 2D array of ethanol-bridged thiacalix[4]arenes (Fig. 2). In other words, this stacking generates infinite nanotubes along the b axis, which are filled with water molecules and these tubes are lined up along the [101] direction by the bridged ethanol molecules. In the formation of these aquatubes, the interspaces between the neighbouring thiacalix[4]arene molecules are filled with the pseudo-tetrahedronally distributed ethanol molecules, which only locate on the wall of the nanotube without obstructing the channel. Along the a axis, the aligned nanotubes in the same sheet are layered via the connection of ethanol (O18⋯O15, 2.713 Å) to form a three-dimensional network of aquatubes (Fig. 3). | ||
| Fig. 2 Stacking diagrams of tapes of 1. Up: side view perpendicular to b axis; down: top view along the b axis. Hydrogen-bonded interactions are shown as broken lines. The hydrogen atoms connected to carbon atom, disordered atoms and water molecules are all omitted for clarity. | ||
![The three-dimensional network of aquatubes of 1. The thiacalix[4]arene molecules in the same sheet are coloured with the same colour.](/image/article/2011/CE/c0ce00129e/c0ce00129e-f3.gif) | ||
| Fig. 3 The three-dimensional network of aquatubes of 1. The thiacalix[4]arene molecules in the same sheet are coloured with the same colour. | ||
In the crystal structure of compound 2, two molecules form a dimer through the cavity stacking motif viahydrogen bonds N4⋯O3 (3.091 Å) and N4⋯O8 (2.961 Å) (Fig. 4). Then neighbouring dimers connect each other side-by-side by the outwardly orientated chain C and chain D via a cyclic motif R22(8) which is formed by hydrogen bond N3⋯O12 (3.013 Å) and O11⋯O9 (2.622 Å). The buried chain B doesn't take part in the formation of the intermolecular hydrogen bond. The overall packing arrangement of 2 takes on a 1D double-stranded ladder-like chain form.
 | ||
| Fig. 4 The 1D double-stranded ladder-like chain structures of 2. Hydrogen-bonded interactions are shown as broken lines. The hydrogen atoms connected to carbon atom and disordered atoms are all omitted for clarity. | ||
The self-assembly behaviour of compound 3 is the simplest among the five compounds. Neighbouring compounds connect to each other viahydrogen bond N2⋯O2 (3.154 Å) resulting in a 1D single-stranded linear chain parallel to the (101) plane (Fig. 5). In the 1D network, all the molecules are connected to each other through the cavity stacking motif.
 | ||
| Fig. 5 The 1D single-stranded linear chain structures of 3. Hydrogen-bonded interactions are shown as broken lines. The hydrogen atoms connected to carbon atom are omitted for clarity. | ||
In the extended structure of compound 4 (Fig. 6), each molecule binds to three neighbouring molecules viahydrogen bond N4⋯O11 (3.053 Å), N2⋯O9 (2.928 Å) and N6⋯O3 (2.887 Å). The hydrogen bond motif in N4⋯O11 is the same as that in compound 3. The hydrogen bond between the urea groups, i.e. N2⋯O9 and N6⋯O3 is in a cyclic R22(8) motif.
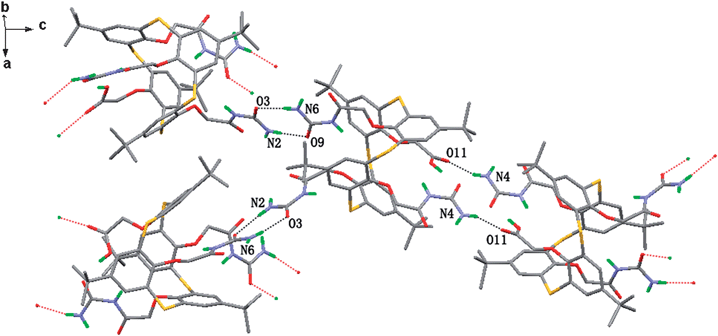 | ||
| Fig. 6 The crystal structure of 4. Hydrogen-bonded interactions are shown as broken lines. The hydrogen atoms connected to carbon atom and disordered atoms are all omitted for clarity. | ||
In the crystal structure of compound 5, neighbouring molecules are linked by the inwardly orientated chains A and D viahydrogen bond N2⋯O6 (2.974 Å) and cyclic R22(8) motif hydrogen bond N2⋯O12 (2.978 Å), N8⋯O3 (3.012 Å) in a cavity stacking motif. A 1D polymeric chain is formed (Fig. 7). The overall 3D crystal architecture of 5 is characterized by the propagating of this chain system along two different directions directed by two outwardly orientated chains B and C. As shown in Fig. S1 (ESI),† neighbouring 1-D chains hydrogen-bond to each other via a cyclic R22(8) motif N4⋯O6 (2.942 Å), i.e. via the outwardly orientated chain B resulting the propagation of the infinite chain along [101] direction. Another outwardly orientated chain C leads to the propagation of the chain system perpendicular to (101) plane viahydrogen bond N6⋯O5 (2.976 Å) (Fig. S2 (ESI)).†
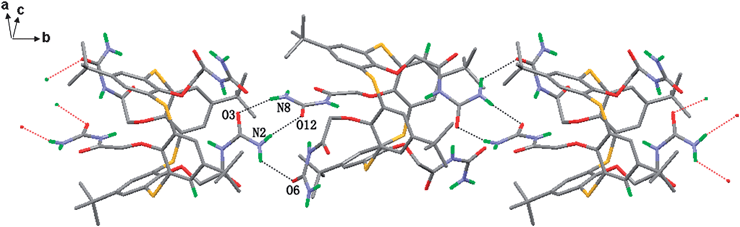 | ||
| Fig. 7 The 1D chain structure of 5 along b axis. Hydrogen-bonded interactions are shown as broken lines. The hydrogen atoms connected to carbon atom are omitted for clarity. | ||
Although various types of intermolecular organizations are formed by varying the type of hydrogen-bonding functionalities and the position of these interaction sites, there are some recurring hydrogen bond synthons in these supramolecular architectures. The hydrogen bond synthons that appear in compounds 2, 3, 4 and 5 are shown in Scheme 2. The participating carboxylic acid preferentially engages in heteromeric acid to amide hydrogen bond, either in a acid⋯amide discrete pattern II or in a acid⋯amide ring pattern I. This is consistent with the conclusion made by Seaton.12 The remaining urea groups tend to form amide⋯amide ring pattern III, which exists in most cases. These supramolecular synthons derived from the intermolecular hydrogen bond that are between the pendant arms of neighboring thiacalix[4]arene molecules play an important role in controlling the final crystalline form of each compound.
 | ||
| Scheme 2 The hydrogen bond synthons that appear in compounds 2, 3, 4 and 5. | ||
In the solid state, all the compounds can self-assemble through the cavity stacking motif by the axis-orientated or the inwardly orientated pendant arms, the outwardly orientated pendant arms are not favorable for this packing motif. And only compound 1 forms the cavity stacking motif nanotubes. Although the assembly is formed with the assistance of solvent molecules, the formation of this kind of nanotubes involves the fully usage of the framework of 1,3-alternatethiacalix[4]arene, both the four pendant arms and the tubular cavity. For the other four compounds, although they can connect with the neighbouring molecules through the cavity stacking motif, the inwardly orientated pendant arms which protrude into the thiacalixarene cavity obstruct the passages in these assemblies. So they couldn't be deemed as nanotubes any more. In order to form the nanotubes that we expected, further efforts are being made to prevent the inward orientation of the pendant arms.
Conclusion
In summary, we have structurally characterized the crystalline forms of five 1,3-alternatethiacalix[4]arene derivatives. The differences in their molecular structures and self-assembly behaviour in the solid state are illustrated. The most significant difference between these thiacalix[4]arenes is the spatial orientation of their pendant arms. There are many factors that may influence their orientation, such as the types of hydrogen-bonding groups, the position of these groups. In particular, we have found that the axis-orientated or the inwardly orientated pendant arms are favorable for the cavity stacking packing motif. All the compounds can self-assemble through this packing motif. But only compound 1 forms the nanotubes which is consistent with our initial assumption. For the other four compounds, the inwardly orientated pendant arms which protrude into the thiacalixarene cavity obstruct the channels. These findings will provide further insights in understanding and designing new hydrogen-bonded nanotubes formed by the cavity stacking motif.Experimental
Syntheses of the compounds
Compounds 2, 3, 4 and 5 were all derived from 1,3-alternate p-tert-butylthiacalix[4]arene tetra-methyleneoxycarboxylic acid 113via two steps. Firstly, a mixture of compound 1 (0.40 g, 0.42 mmol) and thionyl chloride (0.8 mL, 11.2 mmol) in dichloromethane (20 mL) was reflux for 24 h. Removal of the solvent and residual thionyl chloride under reduced pressure furnished the middle product acid chloride as an off-white solid. Second, after the crude middle product was cooled to room temperature and dissolved in solvent (20 mL), urea was added. The stirred mixture was refluxed for 72 h, then approximate half of the solvent was distilled off, and the residue was treated with 10 mL of distilled water; after filtration, the precipitate was washed with distilled water (3 × 10 mL) to give a white to yellow precipitate and the mixture of products was then separated by column chromatography (silica gel) to give thiacalix[4]arene urea derivatives.For compounds 2, 3 and 4, in the second step the solvent used was THF and a catalyzed amount of trifluoroacetic acid was also added. For compounds 5, in the second step the solvent used was acetonitrile.
Compound 2. Obtained as a white solid. Conversion: 11.5%, mp > 260 °C. 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.17 (s, 18 H, t-Bu), 1.21 (s, 18 H, t-Bu), 4.39 (s, 4 H, ArOCH2), 4.63 (s, 4 H, ArOCH2), 7.42 (s, 4 H, ArH), 7.46 (s, 4 H, ArH), 7.69 (s, 4 H, NH2), 8.79 (s, 2 H, NH), 11.02 (br, 2 H, COOH). ESI-MS: m/z 1037 [M + H] +, 1059 [M + Na]+, 1075 [M + K]+. Anal. calcd for C50H60N4O12S4: C 57.89, H 5.83, N 5.40, S 12.36. Found: C 57.59, H 5.66, N 5.15, S 12.03.
Compound 3. Obtained as a white solid. Conversion: 11.5%, mp > 300 °C. 1H NMR (300 MHz, CDCl3): δ (ppm) 1.20 (s, 18 H, t-Bu), 1.22 (s, 18 H, t-Bu), 4.36 (d, 2 H, J = 15.6 Hz, ArOCH2), 4.51 (s, 2 H, ArOCH2), 4.56 (s, 2 H, ArOCH2), 4.89 (d, 2 H, J = 15.9 Hz, ArOCH2), 5.39 (s, 2 H, NH), 7.15 (s, 2 H, ArH), 7.23 (s, 2 H, ArH), 7.44 (s, 2 H, ArH), 7.50 (s, 2 H, ArH), 8.54 (s, 2 H, NH2), 8.76 (s, 2 H, NH2), 11.20 (br, 2 H, COOH). ESI-MS: m/z 1037 [M + H] +, 1059 [M + Na]+, 1075 [M + K]+. Anal. calcd for C50H60N4O12S4: C 57.89, H 5.83, N 5.40, S 12.36. Found: C 57.63, H 5.72, N 5.11, S 12.09.
Compound 4. Obtained as a white solid. Conversion: 24.3%, mp: 219–222 °C. 1H NMR (300 MHz, CDCl3): δ (ppm) 1.20 (s, 18 H, t-Bu), 1.22 (s, 9 H, t-Bu), 1.25 (s, 9 H, t-Bu), 4.31 (s, 2 H, ArOCH2), 4.62 (d, 2 H, J = 14.7 Hz, ArOCH2), 4.71 (s, 2 H, ArOCH2), 4.75 (d, 2 H, J = 14.4 Hz, ArOCH2), 5.16 (s, 2 H, NH2), 5.39 (s, 1 H, NH), 7.29 (s, 2 H, ArH), 7.33 (s, 2 H, ArH), 7.35 (s, 2 H, ArH), 7.63 (s, 2 H, ArH), 8.12 (s, 2 H, NH2), 8.25 (s, 2 H, NH2), 8.59 (s, 1 H, NH), 9.57 (s, 1 H, NH), 11.24 (s, 1 H, COOH). ESI-MS: m/z 1079 [M + H] +, 1101 [M + Na]+, 1117 [M + K]+. Anal. calcd for C51H62N6O12S4: C 56.75, H 5.79, N 7.79, S 11.88. Found: C 56.38, H 5.68, N 7.52, S 11.53.
Compound 5. Obtained as a white solid. Conversion: 43%, mp > 300 °C. 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.19 (s, 36H, t-Bu), 4.53 (s, 8 H, CH2), 7.48 (s, 8 H, ArH), 7.64 (s, 4 H, NH2), 7.68 (s, 4 H, NH2), 7.99 (s, 4 H, NH). ESI-MS: m/z 1143 [M + Na]+; 1159 [M + K]+. Anal. calcd For C52H64N8O12S4: C 55.70, H 5.75, N 9.99, S 11.44. Found: C 55.47, H 5.51, N 9.61, S 11.06.
Crystallization
A solution of 1 in ethanol is prepared in a small open container, then placed this receptacle inside a larger one that contains water and seal the outer vessel well. Colourless crystals of 1 were obtained by vapour diffusion of the solvents at room temperature after several days. Colourless crystals of 2, 3 and 4 were obtained by slow evaporation of their methanol–dichloromethane mixed solution. Colourless crystals of 5 were obtained by slow evaporation of the solvent from THF.X-Ray crystallography
X-Ray data of the crystals were collected on a Bruker SMART CCD single-crystal diffractometer with graphite filtered Mo-Kα (λ = 0.71073 Å) radiation. Data collections for crystals of 1 and 2 were carried out at 150 K. For crystals of 3, 4 and 5 were carried out at room temperature. The structure was solved by direct methods using the SHELXS-97 program14 and refined by Full-matrix least-squares on F2 using SHELXL97.15 All the non-hydrogen atoms were located directly by successive Fourier calculations and were refined anisotropically. Hydrogen atoms except for that of the disordered lattice solvent molecule and water molecule were placed in geometrically calculated positions by using a riding model. Referring to compound 1, two ethanol molecules were found to be disordered. C(50), C(55), C(56) and O(16) were refined with a split atom model. The occupancy factors are 0.51 for C(50), 0.49 for C(50′), 0.52 for C(55), C(56) and O(16), 0.48 for C(55′), C(56′) and O(16′). In compound 2, one tert-butyl group and one CH2Cl2 were found to be disordered. Split atoms were used for C(8)–C(10), C(52), Cl(3) and Cl(4). The occupancy factors are 0.578 for C(8)–C(10), 0.422 for C(8′)–C(10′), 0.573 for C(52), Cl(3) and Cl(4), 0.427 for C(52′), Cl(3′) and Cl(4′). In compound 4, three tert-butyl groups were found to be disordered. Split atoms were used for C(8)–C(10), C(34)–C(36) and C(47)–C(49). The occupancy factors are 0.73 for C(8)–C(10), 0.27 for C(8′)–C(10′), 0.57 for C(34)–C(36), 0.43 for C(34′)–C(36′), 0.62 for C(47)–C(49) and 0.38 for C(47′)–C(49′). Commands ‘DFIX’, ‘SADI’ and ‘ISOR’ were used in the above refinement. In compounds 4 and 5, the non-coordinated solvent molecules are severely disordered which could not be modelled by discrete atoms in the complexes. Correspondingly, the contribution of the solvent to the diffraction pattern was subtracted using SQUEEZE procedure of the PLATON.16 The total number of electrons accounted for in this way led to the proposed stoichiometry of the solvents we have used. That is each host molecule 4 was distributed 1.17 methanol and 0.67 CH2Cl2 molecules and each host molecule 5 was distributed one THF. Crystallographic data and structural correction parameters are listed in Table 4.| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| CCDC no. | 760511 | 760512 | 760513 | 773844 | 760514 |
| Formula | C48H56O12S4·4(C2H5OH)·2(H2O) | C50H60N4O12S4·2(CH2Cl2) | C50H60N4O12S4 | C51H62N6O12S4·1.17(CH3OH)·0.67(CH2Cl2) | 2(C52H64N8O12S4)·H2O·2(C4H8O) |
| M r/g mol−1 | 1173.47 | 1207.11 | 1037.26 | 1173.63 | 2404.92 |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Trigonal | Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
C2/c | C2/c |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif)
|
P21/n |
| a/Å | 10.5110(1) | 42.4396(7) | 24.5640(7) | 20.3779(1) | 14.9385(5) |
| b/Å | 16.749(2) | 14.2832(3) | 20.0520(5) | 20.3779(1) | 25.0431(8) |
| c/Å | 18.137(2) | 21.6031(5) | 10.7714(3) | 76.563(9) | 15.9995(5) |
| α/° | 88.538(2) | 90.00 | 90.00 | 90 | 90.00 |
| β/° | 77.585(2) | 117.638(4) | 93.394(2) | 90 | 91.281(2) |
| γ/° | 87.949(2) | 90.00 | 90.00 | 120 | 90.00 |
| V/Å3 | 3115.8(7) | 11601.0(6) | 5296.2(2) | 27534(4) | 5984.0(3) |
| Z | 2 | 8 | 4 | 18 | 2 |
| ρ c/g cm−3 | 1.251 | 1.382 | 1.301 | 1.172 | 1.255 |
| μ/mm−1 | 0.219 | 0.410 | 0.242 | 0.213 | 0.223 |
| F(000) | 1256 | 5056 | 2192 | 11131 | 2540 |
| Crystal size/mm | 0.20 × 0.10 × 0.10 | 0.23 × 0.20 × 0.10 | 0.16 × 0.12 × 0.08 | 0.30 × 0.20 × 0.05 | 0.20 × 0.10 × 0.10 |
| θ range/° | 1.15–25.00 | 1.53–25.10 | 2.34–25.04 | 1.76–25.00 | 1.59–25.00 |
| Reflections collected/unique | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971/10 971/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 (Rint = 0.1217) 863 (Rint = 0.1217) |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 715/10 715/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 (Rint = 0.2234) 327 (Rint = 0.2234) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 406/4648 (Rint = 0.0719) 406/4648 (Rint = 0.0719) |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 006/10 006/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 797 (Rint = 0.1200) 797 (Rint = 0.1200) |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926/10 926/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 509 (Rint = 0.0966) 509 (Rint = 0.0966) |
| GOF | 1.037 | 1.015 | 1.102 | 0.809 | 0.981 |
| Final R indices [I > 2σ(I)] | R 1 = 0.0831, wR2 = 0.2157 | R 1 = 0.0691, wR2 = 0.1764 | R 1 = 0.0920, wR2 = 0.2161 | R 1 = 0.0638, wR2 = 0.1450 | R 1 = 0.0600, wR2 = 0.1389 |
| R indices (all data) | R 1 = 0.1092, wR2 = 0.2330 | R 1 = 0.0811, wR2 = 0.1832 | R 1 = 0.1201, wR2 = 0.2342 | R 1 = 0.1431, wR2 = 0.1634 | R 1 = 0.0821, wR2 = 0.1494 |
Acknowledgements
We are grateful to the National Natural Science Foundation of China (20772092) and the Hubei Province Natural Science Fund for Distinguished Young Scholars (2007ABB021) for financial support.References
- (a) T. Shimizu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2601 CrossRef CAS; (b) T. Shimizu, Bull. Chem. Soc. Jpn., 2008, 81, 1554 CrossRef CAS.
- (a) D. T. Bong, T. D. Clark, J. R. Granja and M. R. Ghadiri, Angew. Chem., Int. Ed., 2001, 40, 988 CrossRef CAS; (b) H. Fenniri, P. Mathivanan, K. L. Vidale, D. Sherman, K. Hallenga, K. V. Wood and J. G. Stowell, J. Am. Chem. Soc., 2001, 123, 3854 CrossRef CAS; (c) T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401 CrossRef CAS; (d) K. Ono, K. Tsukamoto, R. Hosokawa, M. Kato, M. Suganuma, M. Tomura, K. Sako, K. Taga and K. Saito, Nano Lett., 2009, 9, 122 CrossRef CAS.
- C. Gaeta, C. Tedesco, and P. Neri, in Calixarenes in the Nanoworld, ed. J. Vicens and J. Harrowfield, Springer, The Netherlands, 2007, ch. 16, pp. 335–354 Search PubMed.
- For nanotubes formed by arranging calixarenes along the surface of a cylinder, see: (a) G. W. Orr, L. J. Barbour and J. L. Atwood, Science, 1999, 285, 1049 CrossRef; (b) B. H. Hong, J. Y. Lee, C.-W. Lee, J. C. Kim, S. C. Bae and K. S. Kim, J. Am. Chem. Soc., 2001, 123, 10748 CrossRef; (c) S. Stumpf, G. Goretzki, K. Gloe, K. Gloe, W. Seichter, E. Weber and J. W. Bats, J. Inclusion Phenom. Macrocyclic Chem., 2003, 45, 225 CrossRef CAS; (d) H. Mansikkamäki, M. Nissinen and K. Rissanen, Angew. Chem., Int. Ed., 2004, 43, 1243 CrossRef; (e) A. N. Lazar, N. Dupont, A. Navaza and A. W. Coleman, Chem. Commun., 2006, 1076 RSC; (f) F. Perret, A. N. Lazar, O. Shkurenko, K. Suwinska, N. Dupont, A. Navaza and A. W. Coleman, CrystEngComm, 2006, 8, 890 RSC; (g) S. J. Dalgarno, G. W. V. Cave and J. L. Atwood, Angew. Chem., Int. Ed., 2006, 45, 570 CrossRef CAS; (h) S. J. Dalgarno, J. E. Warren, J. Antesberger, T. E. Glass and J. L. Atwood, New J. Chem., 2007, 31, 1891 RSC; (i) R. M. McKinlay and J. L. Atwood, Angew. Chem., Int. Ed., 2007, 46, 2394 CrossRef CAS; (j) S. Kennedy and S. J. Dalgarno, Chem. Commun., 2009, 5275 RSC.
- For nanotubes formed by stacking the calixarene cavities, see: (a) P. Lhoták, M. Himl, S. Pakhomova and I. Stibor, Tetrahedron Lett., 1998, 39, 8915 CrossRef CAS; (b) J. Sýkora, J. Budka, P. Lhoták, I. Stibor and I. Císařová, Org. Biomol. Chem., 2005, 3, 2572 RSC; (c) L. Baldini, F. Sansone, C. Massera, A. Casnati, F. Ugozzoli and R. Ungaro, Inorg. Chim. Acta, 2007, 360, 970 CrossRef CAS; (d) W. Wang, S. L. Gong, Y. Y. Chen and J. P. Ma, New J. Chem., 2005, 29, 1390 RSC.
- C. D. Gutsche, Calixarenes: An Introduction, 2nd Edition, ed. J. F. Stoddard, The Royal Society of Chemistry, Cambridge, 2008 Search PubMed.
- B. Pulpoka, L. Baklouti, J. S. Kim and J. Vicens, in Calixarenes in the Nanoworld, ed. J. Vicens and J. Harrowfield, Springer, The Netherlands, 2007, ch. 7, pp. 135–149 Search PubMed.
- N. Morohashi, F. Narumi, N. Iki, T. Hattori and S. Miyano, Chem. Rev., 2006, 106, 5291 CrossRef CAS.
- (a) A. D. Burrows, Struct. Bond., 2004, 108, 55 CAS; (b) M. W. Hosseini, Acc. Chem. Res., 2005, 38, 313 CrossRef CAS; (c) P. Metrangolo and G. Resnati, Chem.–Eur. J., 2001, 7, 2511 CrossRef; (d) A. Casnati, G. Cavallo, P. Metrangolo, G. Resnati, F. Ugozzoli and R. Ungaro, Chem.–Eur. J., 2009, 15, 7903 CrossRef CAS.
- L. J. Prins, D. N. Reinhoudt and P. Timmerman, Angew. Chem., Int. Ed., 2001, 40, 2382 CrossRef CAS.
- F. Ugozzoli and G. D. Andreetti, J. Inclusion Phenom. Mol. Recognit. Chem., 1992, 13, 337 CAS.
- C. C. Seaton, A. Parkin, C. C. Wilson and N. Blagden, Cryst. Growth Des., 2009, 9, 47 CrossRef CAS.
- X. Li, S. L. Gong, W. P. Yang, Y. Y. Chen and X. G. Meng, Tetrahedron, 2008, 64, 6230 CrossRef CAS.
- G. M. Sheldrick, SHELXS-97, A Program for Crystal Structure Solution, University of Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, SHELXL-97, A Program for Crystal Structure Refinement; University of Göttingen, Germany, 1997 Search PubMed.
- A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 and S2: the propagated chain system of compound 5; Tables S3–S7: hydrogen bonds of compounds 1–5 [Å and °]; X-ray crystallographic information files (CIF) for compounds 1–5. CCDC reference numbers [CCDC NUMBER(S)]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ce00129e |
| This journal is © The Royal Society of Chemistry 2011 |
