DOI:
10.1039/C0CE00013B
(Paper)
CrystEngComm, 2011,
13, 251-258
Lanthanide-based 0D and 2D molecular assemblies with the pyridazine-3,6-dicarboxylate linker†
Received
22nd March 2010
, Accepted 13th June 2010
First published on 6th September 2010
Abstract
Two distinct series of lanthanides-based coordination polymers involving the pyridazine-3,6-dicarboxylate (pzdc) ligand have been structurally characterized by means of single-crystal XRD analysis. The structure of the compounds Ln2(H2O)4(pzdc)3·(3/3.5)H2O with Ln = Pr (1), Nd (2), Sm (3), Eu (4) is built up from dinuclear bricks [Ln2O8N5(H2O)4] containing two μ3-oxo bridged nine-fold coordinated lanthanide cations, connected via the pzdc ligand, which acts as tetratendate, tridentate or monodentate linkers. It results in the generation of layers of mixed pzdc-Ln2O8N5(H2O)4 networks, intercalated by free water molecules. The second series with chemical formula Ln2(H2O)6(pzdc)3·3H2O (Ln = Gd (5), Tb (6), Dy (7), Er (8), Lu (9)) is a molecular assembly of discrete dinuclear bricks similar to those of the previous compounds. They only differ by the number of terminal water molecules within the dimer [Ln2O6N5(H2O)6]. Interactions viahydrogen bond occur between terminal water species attached the lanthanide and free intercalated water molecules. Europium- and terbium-based compounds 4 and 6 exhibit fluorescence emission in the visible region at room temperature.
Introduction
Since the last decade, a large number of studies has been devoted to the synthesis of crystalline hybrid materials exhibiting extended open structures with porosity properties. In this context, the so-called metal–organic frameworks (MOF) or coordination polymers1–6 associate inorganic building bricks connected to each other through organic spacers in order to generate multidimensional 1–3D architectures delimiting cavities or/and tunnels. Depending on the nature of the inorganic center and organic ligand, many potential industrial applications could be developed in the fields of gas adsorbers, molecular separation, catalysis, drug delivery, etc.4,7–10 Among the different metal candidates, the hybrid lanthanide-organic compounds have paid a special attention since specific properties have been observed in the areas of magnetism,11 molecular recognition,12 luminescence,13–15 sensors,16 catalysis17–20etc. Moreover, due to the wide variety of coordination environments of f-block elements, numerous infinite networks have been described with diverse structural topologies.15,21,22 In the other hand, investigations have been also focused on the functionalities and geometries of the organic ligands. For instance, the family of rigid poly-carboxylates containing benzene ring has been intensively used for the construction of such structural frameworks. Particularly, the 1,4-benzenedicarboxylate (terephthalate) linker has attracted many interests for the generation of unusual flexible networks incorporating trivalent metals (Cr,23Fe,24,25Al,26,27 Ga,27–29 In30) in the MIL-53 series, for instance. This bifunctional ligand was also successfully incorporated in many coordination polymers structures with the trivalent lanthanides,31–39 some for them exhibiting both porous and luminescence properties. At the same time, mixed O- and N-donor molecules gave the opportunity to discover new polymeric structures with higher complexity due to the fact of possible bonding via both nitrogen or carboxyl oxygen species. They include the pyridine-2,5-dicarboxylate40–50 (one N-donor center) or pyrazine-2,5-dicarboxylate ligands,51,52 which are derived from the terephthalate motif. The latter has two N-donor centers in the 1,4 position within the benzene-like ring. Another closely related organic derivative called pyridazine-3,6-dicarboxylate is characterized by the occurrence of two bonded nitrogen atoms, forming an azine function within the aromatic cycle. The reactivity of this azo derivative (hereafter noted pzdc) towards metals was mainly investigated with divalent cations, giving rise to the isolation of discrete mononuclear (Mn2+,53Cu2+,54Pb2 +55) or dinuclear (Mn2+,56,57Co2+,57,58Ni2+,58Zn2 +57,59) complexes in molecular hydrogen-bonded assemblies.
In this contribution, we have explored the possibility of the reaction of the pyridazine-3,6-dicarboxylate molecules as a linker between trivalent lanthanide cations in order to build atomic arrangements with higher dimensionalities. Lanthanides cations are known displaying various coordination surroundings from VI to IX, establishing bonding with oxygen as well as nitrogen from the mixed functionalized organic ligands. The occurrence of high coordination state for lanthanide centers could favour the formation of multiple bonds for the generation of infinite extended polymeric networks. Here two series of phases have been synthesized in water solvent. The compounds Ln2(H2O)4(pzdc)3·(3/3.5)H2O with Ln = Pr (1), Nd (2), Sm (3), Eu (4) exhibit a two-dimensional structure whereas the compounds Ln2(H2O)6(pzdc)3·3H2O with Ln = Gd (5), Tb (6), Dy (7), Er (8), Lu (9) are characterized by the molecular arrangement of discrete dinuclear bricks. The paper deals with the synthesis, structure description of the different phases as well as the luminescence spectrum of the europium- and terbium-based solids.
Experimental
Synthesis
All the lanthanide nitrates or chlorides were purchased from Acros (Pr, Nd, Sm, Eu, Gd, Er) or Aldrich (Tb, Dy, Lu) and used as received without any further purification. Pyridazine-3,6-dicarboxylic acid (H2pzdc) was obtained from the synthesis procedure described by Sueur et al.60
Pr2(H2O)4(pzdc)3·3.5H2O (1).
0.25 g of praseodymium nitrate pentahydrate (99.9%, 0.6 mmol) was dissolved in 25 ml deionized hot water with 0.1 g pyridazine-3,6-dicarboxylic acid (0.6 mmol) and then left at room temperature. After one week, well formed pale yellow single crystals of 1 appeared in the mother liquid and dried in air.
Tb2(H2O)6(pzdc)3·3H2O (6).
Pale brown single-crystals of 6 were prepared by using the method similar to 1, with terbium chloride hexahydrate (99.9%, 0.26 g, 0.7 mmol) instead of praseodymium nitrate pentahydrate.
Crystals were selected under polarizing optical microscope and glued on a glass fiber for a single-crystal X-ray diffraction experiments. X-Ray intensity data were collected on a Bruker X8-APEX2 CCD area-detector diffractometer using Mo-Kα radiation (λ = 0.71073 Å) with an optical fiber as collimator. Several sets of narrow data frames (20 s per frame) were collected at different values of θ for two initial values of ϕ and ω, respectively, using 0.3° increments of ϕ or ω. Data reduction was accomplished using SAINT V7.53a.61 The substantial redundancy in data allowed a semi-empirical absorption correction (SADABS V2.1062) to be applied, on the basis of multiple measurements of equivalent reflections. The structure was solved by direct methods, developed by successive difference Fourier syntheses, and refined by full-matrix least-squares on all F2 data using SHELX63 program suite. Hydrogen atoms of the benzene ring were included in calculated positions and allowed to ride on their parent atoms. The final refinements include anisotropic thermal parameters of all non-hydrogen atoms, except the oxygen atoms of the water molecules. The crystal data are given in Table 1.
Table 1
Crystal data and structure refinement for lanthanides pyridazine-3,6-dicarboxylates
| |
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
| Formula |
C18H21N6O19.5Pr2 |
C18H21N6Nd2O19.5 |
C18H21N6O19.5Sm2 |
C18H20Eu2N6O19 |
C18H24Gd2N6O21 |
C18H24N6O21Tb2 |
C18H24Dy2N6O21 |
C18H24Er2N6O21 |
C18H24Lu2N6O21 |
| Formula weight |
915.2 |
921.86 |
934.08 |
928.32 |
974.91 |
978.13 |
985.41 |
994.93 |
1010.35 |
|
T/K |
292 (2) |
296(2) |
296(2) |
302(2) |
296(2) |
293(2) |
296(2) |
296(2) |
296(2) |
| Crystal type |
yellow platelet |
purple platelet |
yellow platelet |
Light brown platelet |
yellow platelet |
Light brown platelet |
yellow platelet |
pink platelet |
yellow platelet |
| Crystal size/mm |
0.45 × 0.20 × 0.16 |
0.25 × 0.12 × 0.03 |
0.27 × 0.14 × 0.03 |
0.08 × 0.05 × 0.01 |
0.16 × 0.14 × 0.04 |
0.38 × 0.23 × 0.09 |
0.48 × 0.19 × 0.10 |
0.37 × 0.10 × 0.07 |
0.37 × 0.10 × 0.07 |
| Crystal system |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
Monoclinic |
| Space group |
P21/c |
P21/c |
P21/c |
P21/c |
P21/n |
P21/n |
P21/n |
P21/n |
P21/n |
|
a/Å |
10.5283(8) |
10.4836(5) |
10.4555(19) |
10.4063(10) |
11.8519(2) |
11.8076(6) |
11.8365(3) |
11.8162(8) |
11.7980(7) |
|
b/Å |
13.4817(12) |
13.4043(8) |
13.333(3) |
13.1997(13) |
19.6425(3) |
19.6020(10) |
19.5914(5) |
19.4943(13) |
19.4168(12) |
|
c/Å |
20.5006(18) |
20.4238(13) |
20.372(3) |
20.244(2) |
14.9977(3) |
14.9743(7) |
15.0554(4) |
15.0763(10) |
14.8932(10) |
|
β/° |
102.016(4) |
102.163(3) |
102.245(10) |
102.695(5) |
102.5080(10) |
102.750(2) |
102.6320(10) |
102.854(3) |
103.041(3) |
|
V/Å3 |
2846.1(4) |
2805.6(3) |
2775.2(9) |
2712.8(5) |
3408.61(10) |
3380.4(3) |
3406.74(15) |
3385.8(4) |
3323.7(4) |
|
Z, ρcalcd/g cm−3 |
4, 2.101 |
4, 2.147 |
4, 2.199 |
4, 2.273 |
4, 1.865 |
4, 1.887 |
4, 1.889 |
4, 1.951 |
4, 2.018 |
|
μ/mm−1 |
3.480 |
3.758 |
4.290 |
4.682 |
3.944 |
4.237 |
4.960 |
5.010 |
5.996 |
|
θ range/° |
1.82–33.73 |
1.83–26.14 |
1.84–31.00 |
1.86–25.13 |
1.73–30.54 |
1.74–30.51 |
1.73–36.28 |
1.99–33.32 |
1.75 to 30.51 |
| Limiting indices |
−16 ≤ h ≤ 16 |
−12 ≤ h ≤ 12 |
−14 ≤ h ≤ 14 |
−12 ≤ h ≤ 12 |
−16 ≤ h ≤ 16 |
−16 ≤ h ≤ 16 |
−17 ≤ h ≤ 19 |
−18 ≤ h ≤ 18 |
−16 ≤ h ≤ 16 |
| −21 ≤ k ≤ 20 |
−16 ≤ k ≤ 16 |
−19 ≤ k ≤ 19 |
−15 ≤ k ≤ 15 |
−28 ≤ k ≤ 28 |
−28 ≤ k ≤ 28 |
−32 ≤ k ≤ 32 |
−29 ≤ k ≤ 30 |
−26 ≤ k ≤ 27 |
| −32 ≤ l ≤ 31 |
−25 ≤ l ≤ 25 |
−29 ≤ l ≤ 29 |
−24 ≤ l ≤ 2 4 |
−21 ≤ l ≤ 21 |
−21 ≤ l ≤ 20 |
−25 ≤ l ≤ 25 |
−23 ≤ l ≤ 23 |
−20 ≤ l ≤ 20 |
| Collected reflections |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 298 298 |
194![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 149 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 377 377 |
103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 337 337 |
115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552 552 |
138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 135 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 837 837 |
122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645 645 |
| Unique reflections |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 [Rint = 0.0473] 292 [Rint = 0.0473] |
5570 [Rint = 0.0752] |
8585 [Rint = 0.1317] |
4822 [Rint = 0.0480] |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 [Rint = 0.0628] 419 [Rint = 0.0628] |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305 [Rint = 0.0447] 305 [Rint = 0.0447] |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 [Rint = 0.0632] 270 [Rint = 0.0632] |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985 [Rint = 0.0530] 985 [Rint = 0.0530] |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 139 [Rint = 0.0410] 139 [Rint = 0.0410] |
| Parameters |
415 |
415 |
415 |
406 |
433 |
433 |
433 |
433 |
434 |
| Goodness-of-fit on F2 |
1.025 |
1.084 |
1.067 |
0.977 |
1.024 |
1.085 |
1.019 |
1.041 |
1.157 |
| Final R indices [I > 2σ(I)] |
R
1 = 0.0238 |
R
1 = 0.0318 |
R
1 = 0.0322 |
R
1 = 0.0224 |
R
1 = 0.0355 |
R
1 = 0.0353 |
R
1 = 0.0352 |
R
1 = 0.0433 |
R
1 = 0.0336 |
|
wR2 = 0.0680 |
wR2 = 0.0802 |
wR2 = 0.0778 |
wR2 = 0.0546 |
wR2 = 0.1147 |
wR2 = 0.1156 |
wR2 = 0.1176 |
wR2 = 0.1301 |
wR2 = 0.1041 |
|
R indices (all data) |
R
1 = 0.0316 |
R
1 = 0.0493 |
R
1 = 0.0637 |
R
1 = 0.0295 |
R
1 = 0.0520 |
R
1 = 0.0486 |
R
1 = 0.0564 |
R
1 = 0.0656 |
R
1 = 0.0428 |
|
wR2 = 0.0801 |
wR2 = 0.0965 |
wR2 = 0.1025 |
wR2 = 0.0582 |
wR2 = 0.1362 |
wR2 = 0.1359 |
wR2 = 0.1377 |
wR2 = 0.1451 |
wR2 = 0.1229 |
| Largest diffraction peak and hole/e Å−3 |
1.865 and −1.571 |
1.134 and −0.954 |
1.281 and −1.507 |
0.651 and −0.493 |
3.714 and −1.059 |
3.338 and −1.329 |
3.784 and −1.396 |
2.990 and −2.130 |
3.413 and −1.258 |
The thermogravimetric experiments have been carried out on a thermoanalyzer TGA 92 SETARAM under dry air atmosphere with a heating rate of 1 °C min−1 from room temperature up to 800 °C.
Luminescence (emission) spectra were measured on a SAFAS FLX-Xenius fluorescence spectrometer between 400 and 800 nm, equipped with a xenon lamp. Slid widths were 1 nm and scan rates of 125 nm s−1 were used.
Results
Crystal structures of 1–4
The compounds Ln2(H2O)4(pzdc)3·(3/3.5)H2O (Ln = Pr (1), Nd (2), Sm (3), Eu (4)) exhibit identical layered networks for the lightest lanthanides and are described in the monoclinic symmetry with the P21/c space group with a ≈ 10.46, b ≈ 13.34, c ≈ 20.36 Å, β ≈ 102.3°, V ≈ 2775 Å3 (Table 1). The structure contains two crystallographically independent sites for lanthanides with the same nine-fold coordination surroundings, described by distorted capped antiprism polyhedra (Fig. 1). Ln1 cation is bonded to six carboxyl oxygen atoms, two nitrogen atoms (N1B and N1C) from two distinct pyridazinedicarboxylate groups and one terminal water molecule (O1H). The Ln–O distances are ranging from 2.408(2) up to 2.741(2) Å and Ln–N distances from 2.723(2) up to 2.738(2) Å for Pr (1). Due to the lanthanide radius contraction, these bond distances decrease when the atomic number of lanthanide increases, with Ln–O distances in the range 2.346(3)–2.687(3) Å and Ln–N distances in the range 2.635(4)–2.674(4) Å in the Eu (4) compound. The second lanthanide cation Ln2 is coordinated to three carboxyl oxygen atoms, three nitrogen atoms from the three distinct pzdc linkers and three terminal aquo species. Typical distances Ln–O of 2.398(2)–2.564(2) Å and Ln–N of 2.699(2)–2.748(2) Å are observed for the Pr (1) compound and they decrease, as expected, for the heaviest lanthanides with Ln–O = 2.347(3)–2.491(3) Å and Ln–N = 2.618(4)–2.684(4) Å for the Eu (4) phase. For both cations, the existence of terminal aquo groups agrees well with the bond valence calculations64 with values in the range 0.44–0.31 (expected value for H2O: 0.4). The two lanthanide polyhedra Ln1O6N2(H2O) and Ln2O3N3(H2O)3 are linked through an oxo group O4A, which also belongs to one of the carboxylate arm of the pzdc ligand. It results in the occurrence of discrete lanthanide-centered dinuclear motifs [Ln2O8N5(H2O)4], bridged by μ3-oxo groups, with Ln⋯Ln distances ranging from 4.475(5) for Pr (1) down to 4.3897(4) Å for Eu (4) (Fig. 1). The inorganic dimers are connected to each other via the three crystallographically independent pyridazinedicarboxylate ligands [O2C–C4H2N2-CO2]2− (noted A, B and C), which adopt different connection modes (Fig. 1). Two organic molecules (B and C) act as bis(bidentate) linker towards the two lanthanide cations; the two nitrogen atoms are bound to two distinct lanthanide centers and one of the carboxyl oxygen is bound to one lanthanide center. Three of the remaining carboxyl oxygen atoms of the two pzdc species are non-bonded with relatively short C–O distance (1.20–1.22 Å) resulting in a monodentate bridging mode for the carboxylate groups (configuration μ2-η1:η1 for B). The fourth one is connected to another lanthanide cation (Ln1) from a distinct dimeric unit (syn–anti bidentate bridging mode for the carboxylate group: configuration μ3-η1:η1:η1 for C). This particular tetradentate coordination fashion was previously reported in other isolated molecular dinuclear complexes involving divalent transition metals such as Zn,57,59Mn,56,57Co,57,58Ni.57,58 In these compounds, the dimeric species is tetra-coordinated by three pzdc ligands, describing a trigonal prismatic molecular brick around a C3 axis (inducing an angle of 120° between the pzdc species). In our lanthanide-based series, the two pzdc molecules have an angle of nearly 90° and three other organic ligands are attached to the dimer. One of them (noted A) is tridendate and links one lanthanide cation via one of the nitrogen atom (Ln2) and the two lanthanide centers via the carboxyl μ3-oxo group O4A. The carboxylate arm acts as chelating bridge with Ln1 and monodentate bridge with Ln1 and Ln2. The two last other carboxylates are in a monodentate bridging mode with a lanthanide cation from an adjacent dimeric unit. The carboxylate groups of the pzdc molecule A thus adopt the following configuration μ3-η1:η2:η1. The two last pyridazine-based species (A and C) are linked to the inorganic dimer in a monodentate fashion. The monodentate bridging mode of the species A and C allows for the connection of the dimers and this generates the formation of neutral mixed organic-inorganic sheets, developing in the (a,b) plane (Fig. 2 and Fig. 3). The corrugated layers are stacked along the c axis and intercalated by free water molecules. The latter interact viahydrogen bond with the terminal water molecules attached to lanthanide cations. Other hydrogen bonds also exist between the bonded water molecules and remaining free carboxyl oxygen atoms of the pzdc ligand, ensuring the three-dimensional cohesion of the structure. One observes that the europium-based compound (4) incorporates less water with only three crystallographic sites instead of four sites (one of them has a 50% occupancy) in the other members of the 1–4 series.
 |
| | Fig. 1 Views of the nine-fold coordination environments of lanthanide cations in the series 1–4 (top). Polyhedral representation of the dinuclear unit LnO8N5(H2O)3 (bottom). Both lanthanide centers have a distorted capped square antiprism surrounding and are connected to each other via a carboxyl μ3-oxo group. The oxygen species OnH, (n = 1–4) are water molecules in terminal position. A, B and C labels indicate the three crystallographically independent pzdc linkers. Purple = lanthanide; red = oxygen; blue = nitrogen; grey = carbon. Hydrogen atoms have been omitted for clarity. | |
 |
| | Fig. 2 View of layer in the compounds Ln2(H2O)4(pzdc)3·(3/3.5)H2O, 1–4, along the c axis. | |
![View showing the stacking of two corrugated layers [Ln2(H2O)4(pzdc)3] intercalated by water molecules in 1–4, along the c axis.](/image/article/2011/CE/c0ce00013b/c0ce00013b-f3.gif) |
| | Fig. 3 View showing the stacking of two corrugated layers [Ln2(H2O)4(pzdc)3] intercalated by water molecules in 1–4, along the c axis. | |
Crystal structures of 5–9
The compounds Ln2(H2O)6(pzdc)3·3H2O (Ln = Gd (5), Tb (6), Dy (7), Er (8), Lu (9)) are closely related to the previous 1–4 series although they crystallize in a monoclinic cell (P21/n) with distinct parameters a ≈ 11.82, b ≈ 19.52, c ≈ 14.95 Å, β ≈ 102.8°, V ≈ 3365 Å3 (Table 1). The lanthanide cations lie on two crystallographically independent sites, coordinated to nine ligands, describing distorted capped antiprism polyhedra (Fig. 4). Ln1 is surrounded by three carboxyl atoms, three nitrogen atoms from the three distinct pzdc molecules and three water molecules in terminal position. For the Gd-based compound (5), the Gd–O distances are ranging from 2.322(3) up to 2.456(4) Å and Gd–N distances from 2.666(4) up to 2.707(4) Å in a GdO3N3(H2O)3 polyhedron. As expected with the heaviest lanthanides, the Ln–O and Ln–N distances decrease, with Lu–O = 2.240(3)–2.362(4) Å and Lu–N = 2.585(4)–2.668(4) Å in (9). The nine-fold coordinated Ln2 cation involves four carboxyl oxygen atoms, two nitrogen atoms and three terminal water molecules. The Gd–O distances are in the range 2.354(4)–2.595(4) Å and Gd–N equal to 2.662(4) Å for a GdO4N2(H2O)3 polyhedron in (5). With the radius contraction of lanthanides, shorter bond distances are observed: Lu–O = 2.265(4)–2.538(4) Å and Lu–N = 2.566(4)–2.565(4) Å in (9). The presence of terminal aquo species is well confirmed from bond valence64 considerations. The connection of the lanthanide cations by a μ3-oxo group (O1C) is identical to that found in compounds 1–4. That generates a dinuclear unit [Ln2O6N5(H2O)6], which is linked to three crystallographically independent pyridazinedicarboxylate ligands (Fig. 4). The same connection mode of the organic occurs in the compounds 5–9, except the absence of two additional monodentate pzdc ligands. Two of them (noted A and B) are tetra-coordinated to the lanthanides through the nitrogen and carboxyl atoms. The remaining non-bonded carboxyl oxygen atoms are free and short terminal C–O distances (1.20–1.25 Å) reflect their non-protonated character. The third pzdc molecule (noted C) is linked to Ln2 in chelating bridging mode and also connects Ln1via the carboxyl μ3-oxo species and one nitrogen atom as in compounds 1–4. The second carboxylate arm is not bonded and exists in its negatively charged form R–CO2−. The relatively short C–O bonding (1.22–1.25 Å) agree well with this configuration. A larger C–O distance (≈ 1.29 Å) would be expected in the case of protonated form of the carboxylate.65 The presence of this negative non-bonded carboxylate balances the charge of the dimer and ensures the electroneutrality of the dimeric brick. The structure is built up from the molecular assembly of isolated mixed organic-inorganic dinuclear units (Fig. 5) intercalated by free water molecules. The building blocks interact to each other through a hydrogen bond network involving terminal aquo species and free water (O7H⋯O1H, O8H⋯O2H, O9H⋯O6H, O9H⋯O2H, O10H⋯O5H), terminal carboxyl oxygen atoms (O8H⋯O4A, O9H⋯O3C, O9H⋯O2B, O10H⋯O1B) and free water molecules themselves (O7H⋯O10H, O8H⋯O10H). Beside the presence of free water, PLATON66 calculations also indicate the positions (around 0 ½ 0 and ½ 0 ½) of small unoccupied cavities (72 Å).
 |
| | Fig. 4 View of the nine-fold coordination environments of lanthanide cations in the series 5–9 (top). Polyhedral representation of the discrete dinuclear unit LnO6N5(H2O)6 (bottom). Both lanthanide centers have a distorted capped square antiprism surrounding and are connected to each other via a carboxyl μ3-oxo group. The oxygen species OnH, (n = 1–6) are water molecules in terminal position. A, B and C labels indicate the three crystallographically independent pzdc linkers. Purple = lanthanide; red = oxygen; blue = nitrogen; grey = carbon. Hydrogen atoms have been omitted for clarity. | |
![View of the molecular assembly of discrete dinuclear units [Ln2O6N5(H2O)6] along the b axis, in the compounds series 5–9, Ln2(H2O)6(pzdc)3·3H2O.](/image/article/2011/CE/c0ce00013b/c0ce00013b-f5.gif) |
| | Fig. 5 View of the molecular assembly of discrete dinuclear units [Ln2O6N5(H2O)6] along the b axis, in the compounds series 5–9, Ln2(H2O)6(pzdc)3·3H2O. | |
Thermal analysis
Only the two compounds 3 and 8 belonging to distinct series 1–4 or 5–9 have been selected for the thermogravimetric and differential thermogravimetric analyses. Data of the europium-based 4 compound are given in the ESI.†
The TG curves (Fig. 6) show two main events of weight losses. For the samarium-based compound (3), the first step is assigned to the departure of free water together with the terminal bonded water, with a total weight loss of 15.4% at 180 °C. It is also correlated to endothermic peaks at 80 °C (free water) and 110 & 165 °C (bonded water) respectively. This corresponds to a total water content of 8 H2O (calcd: 15.2%) per Sm2 unit, or 4 H2O (coordinated to Sm) and 4 H2O (free). The amount the intercalated water species is slightly higher than the value observed from the single-crystal analysis (3.5 H2O) and the difference may come from additional water adsorbed on the surface. It is also observed on the TG curve (ESI)† of the europium-based compound a smaller water weight loss with a value of 13.8%, corresponding to a total amount of water of 7.2 H2O, or 4 H2O (coordinated to Eu) and 3.2 H2O (free). This is in agreement with the X-ray diffraction analysis which indicates a lower water content. The second event occurs from 250 up to 600 °C and is attributed to the thermal decomposition the organic linker, with a series of large exothermic peaks at 335, 355, 405 and 495 °C (obsd: 48.3%; calcd: 48.1%). The final yellowish residue is samarium oxide (confirmed by powder X-ray diffraction) with a final weight of 36.3% (calcd: 36.7%).
 |
| | Fig. 6
TG and DTG curves of Sm2(H2O)4(pzdc)3·3.5H2O (3) (top) and Er2(H2O)6(pzdc)3·3H2O (8) (bottom) under air (heating rate: 1 °C min−1). | |
The TG curve of the erbium-based (8) phase is shown in Fig. 6. A first weight loss (16.1% or 8.9 H2O for one Er3 unit; expected 9 H2O) up to 150 °C is assigned to the departure of total content of water molecules (free and bonded) associated with an endothermic peaks at 85 °C. The second event is attributed to the thermal decomposition of the organic ligand with weight loss of 45.1% (calcd: 45.6%). Two exothermic peaks are visible at 390 and 420 °C. At 700 °C, the final residue is erbium oxide (Er2O3). It corresponds to a remaining weight of 38.8% (calcd: 38.2%).
Luminescence properties
The solid-state luminescent properties were investigated at room temperature for the europium- and terbium-based compounds (4) and (6).
Under excitation at 394 nm, the europium-based phase (4) exhibits a red luminescence with five groups of signals, which are typical for the Eu3+ cation (Fig. 7). They are located at 579, 592, 617, 649, 694 nm and are assigned to the five 5D0 → 7FJ transitions (J = 0, 1, 2, 3, 4, respectively).67 The 5D0 → 7F2 (as well as 5D0 → 7F4) transition is predominantly due to the electric dipole character, depending on the nature of bonded ligands around the Eu3+ cation whereas the 5D0 → 7F1 one is mainly sensitive to the magnetic dipole effect, reflecting crystal field environment. The strongest emissions are visible for the 5D0 → 7F2 and 5D0 → 7F1 transitions, with an intensity ratio of 2. This indicates that europium cations lie on low symmetry sites, involving the absence of inversion center. Moreover, the observation of the weak 5D0 → 7F0 transition, which is induced by crystal field Jmixing, is allowed on non-inversion sites. Two different signatures are consistent with the X-ray diffraction crystal structure of the compounds 1–4, which shows that the two crystallographically independent lanthanide cations occupy the general position 4e with no symmetry operation in the space groupP21/c. For this preliminary experience, it was also observed some splittings for different transitions (5D0 → 7F1,), which could be related to the distinct Stark components due to the crystal sites multiplicity observed for Eu3+ in the structure.
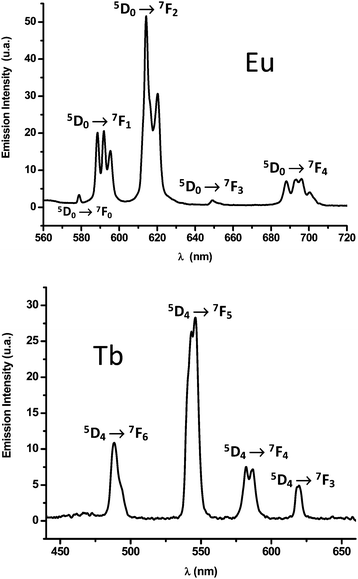 |
| | Fig. 7 solid-state emission spectra of Eu2(H2O)4(pzdc)3·3H2O (4) (excitation at 394 nm) Tb2(H2O)6(pzdc)3·3H2O (6) (excitation at 292 nm) at room temperature. | |
Under excitation at 292 nm, the terbium-based compound (6) emits a green luminescence with peaks located at 488, 545, 584 and 620 nm (Fig. 7). These signals typically correspond to the transitions 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4 and 5D4 → 7F3, respectively.67 As expected, the intensity of the 5D4 → 7F4 transition is the strongest one68 and is sensitive to the nature of the atoms surrounding the rare-earth cation.
Conclusion
To conclude, two types of coordination polymers based on pyridazine-3,6-dicarboxylate linkers have been successfully synthesized for the first time with trivalent metals such as lanthanides. Depending on the size of lanthanide cations, two series of compounds based on the similar dinuclear building brick of two μ3-oxo-bridged capped antiprisms have been isolated. The first ones (1–4), with the lightest lanthanides (Pr, Nd, Sm, Eu), consists of the connection of the dimeric units to each other through the pzdc ligands, generating a two-dimensional network. Free water molecules are intercalated between the hybrid organic-inorganic sheets. The second series 5–9 with the heaviest lanthanides (Gd, Tb, Dy, Er, Lu) contains the closely related dinuclear unit, coordinated by three pzdc species, which are isolated to each other by water molecules. The structural differences of the two molecular assemblies lie on the distinct number of water molecules in the coordination sphere of the metallic cations, correlated to the number of the pzdc species surrounding them. In series 1–4, five organic pzdc moieties are connected through a tetradentate, tridentate and monodentate connection scheme. In the series 5–9, two of the pzdc moieties are replaced by terminal water molecules, which prevent any further connection of the dimers to each other. It results in a 0D molecular arrangement of the isolated dinuclear units. From these observations, the lanthanides size seems to have a significant influence for the connection mode of the building blocks since for an identical nine-fold coordination, the number of water molecules attached to the cations varies drastically from the lightest to the heaviest lanthanides. It is also interesting to notice that with the multi-functionalized pzdc ligand, dimensionalities of frameworks higher than 0D can be generated in case of trivalent lanthanides. This structural feature has not been previously reported with the divalent metals.53–59
Acknowledgements
The authors would like to thank the GNR MATINEX of PACEN interdisciplinary program and the French ANR project no. ANR-08-BLAN-0216-01 for financial support. We also thank the technical help of Dr Pascal Roussel and Dr Frédéric Capet for the single-crystal X-ray diffraction intensities collection of 4 by means of a Bruker X8 apparatus equipped with an X-ray microsource.
References
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- A. K. Cheetham, C. N. R. Rao and R. K. Feller, Chem. Commun., 2006, 4780 RSC.
- C. N. R. Rao, S. Natarajan and R. Vaidhyanathan, Angew. Chem., Int. Ed., 2004, 43, 1466 CrossRef CAS.
- C. Janiak, Dalton Trans., 2003, 2781 RSC.
- A. U. Czaja, N. Trukhan and U. Müller, Chem. Soc. Rev., 2009, 38, 1284 RSC.
- J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC.
- L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC.
- C. Benelli and D. Gatteschi, Chem. Rev., 2002, 102, 2369 CrossRef CAS.
- H. Tsukube and S. Shinoda, Chem. Rev., 2002, 102, 2389 CrossRef CAS.
- J.-C. Bünzli and C. Piguet, Chem. Rev., 2002, 102, 1897 CrossRef.
- K. Binnemans, Chem. Rev., 2009, 109, 4283 CrossRef CAS.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC.
- B. V. Harbuzaru, A. Corma, F. Rey, P. Atienzar, J. L. Jorda, H. Garcia, D. Ananias, L. D. Carlos and J. Rocha, Angew. Chem., Int. Ed., 2008, 47, 1080 CrossRef CAS.
- M. Shibasaki and N. Yoshikawa, Chem. Rev., 2002, 102, 2187 CrossRef CAS.
- J. Inanaga, H. Furuno and T. Hayano, Chem. Rev., 2002, 102, 2211 CrossRef CAS.
- J. Gromada, J.-F. Carpentier and A. Mortreux, Coord. Chem. Rev., 2004, 248, 397 CrossRef.
- M. J. Vitorino, T. Devic, M. Tromp, G. Férey and M. Visseaux, Macromol. Chem. Phys., 2009, 210, 1923 CrossRef CAS.
- R. J. Hill, D.-L. Long, P. Hubberstey, M. Schröder and N. R. Champness, J. Solid State Chem., 2005, 178, 2414 CrossRef.
- C. L. Cahill, D. T. de Lill and M. Frisch, CrystEngComm, 2007, 9, 15 RSC.
- C. Serre, F. Millange, C. Thouvenot, M. Nogues, G. Marsolier, D. Louër and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519 CrossRef CAS.
- T. R. Whitfield, X. Wang, L. Liu and A. J. Jacobson, Solid State Sci., 2005, 7, 1096 CrossRef CAS.
- F. Millange, C. Serre, N. Guillou, G. Férey and R. I. Walton, Angew. Chem., Int. Ed., 2008, 47, 4100 CrossRef CAS.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Férey, Chem.–Eur. J., 2004, 10, 1373 CrossRef CAS.
- M. Vougo-Zanda, J. Huang, E. Anokhina, X. Wang and A. J. Jacobson, Inorg. Chem., 2008, 47, 11535 CrossRef CAS.
- C. Volkringer, T. Loiseau, N. Guillou, G. Férey, E. Elkaïm and A. Vimont, Dalton Trans., 2009, 2241 RSC.
- G. Chaplais, A. Simon-Masseron, F. Porcher, C. Lecomte, D. Bazer-Bachi, N. Bats and J. Patarin, Phys. Chem. Chem. Phys., 2009, 11, 5241 RSC.
- E. V. Anokhina, M. Vougo-Zanda, X. Wang and A. J. Jacobson, J. Am. Chem. Soc., 2005, 127, 15000 CrossRef CAS.
- T. M. Reineke, M. Eddaoudi, M. Fehr, D. Kelley and O. M. Yaghi, J. Am. Chem. Soc., 1999, 121, 1651 CrossRef CAS.
- T. M. Reineke, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 1999, 38, 2590 CrossRef CAS.
- L. Pan, N. Zheng, Y. Wu, S. Han, R. Yang, X. Huang and J. Li, Inorg. Chem., 2001, 40, 828 CrossRef CAS.
- C. Serre, F. Millange, J. Marrot and G. Férey, Chem. Mater., 2002, 14, 2409 CrossRef CAS.
- A. Deluzet, W. Maudez, C. Daiguebonne and N. Guillou, Cryst. Growth Des., 2003, 3, 475 CrossRef CAS.
- C. Daiguebonne, N. Kerbellec, K. Bernot, Y. Gérault, A. Deluzet and N. Guillou, Inorg. Chem., 2006, 45, 5399 CrossRef CAS.
- D. Weng, X.-J. Zheng and L. Jin, Eur. J. Inorg. Chem., 2006, 4184 CrossRef CAS.
- S.-L. Xie, B.-Q. Xie, X.-Y. Tang, N. Wang and S.-T. Yue, Z. Anorg. Allg. Chem., 2008, 634, 842 CrossRef CAS.
- S. Feng, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, m33 CrossRef.
- C. Qin, X.-L. Wang, E.-B. Wang and Z.-M. Su, Inorg. Chem., 2005, 44, 7122 CrossRef CAS.
- Y.-G. Huang, B.-L. Wu, D.-Q. Yuan, Y.-Q. Xu, F.-L. Jiang and M.-C. Hong, Inorg. Chem., 2007, 46, 1171 CrossRef CAS.
- C. Xie, B. Zhang, X. Wang, B. Yu, R. Wang, G. Shen and D. Shen, Z. Anorg. Allg. Chem., 2008, 634, 387 CrossRef CAS.
- Y. Huang, Y.-S. Song, B. Yan and M. Shao, J. Solid State Chem., 2008, 181, 1731 CrossRef CAS.
- P. C. R. Soares-Santos, L. Cunha-Silva, F. A. Almeida Paz, R. A. Sa Ferreira, J. Rocha, T. Trindade, L. D. Carlos and H. I. S. Nogueira, Cryst. Growth Des., 2008, 8, 2505 CrossRef CAS.
- Y.-G. Huang, F.-L. Jiang, D.-Q. Yuan, M.-Y. Wu, Q. Gao, W. Wei and M.-C. Hong, Cryst. Growth Des., 2008, 8, 166 CrossRef CAS.
- F. Luo, Y. Che and J. Zheng, Cryst. Growth Des., 2008, 8, 2006 CrossRef CAS.
- C.-M. Liu, D.-Q. Zhang and D. B. Zhu, Inorg. Chem. Commun., 2008, 11, 903 CrossRef CAS.
- Y.-G. Huang, F.-L. Jiang, D.-Q. Yuan, M.-Y. Wu, Q. Gao, W. Wei and M.-C. Hong, J. Solid State Chem., 2009, 182, 215 CrossRef CAS.
- F.-N. Shi, L. Cunha-Silva, T. Trindade, F. A. Almeida Paz and J. Rocha, Cryst. Growth Des., 2009, 9, 2098 CrossRef CAS.
- P. Mahata, K. V. Ramya and S. Natarajan, Inorg. Chem., 2009, 48, 4942 CrossRef CAS.
- X.-J. Zheng and L.-P. Jin, J. Chem. Crystallogr., 2005, 35, 865 CrossRef CAS.
- P. Yang, J.-Z. Wu and Y. Yu, Inorg. Chim. Acta, 2009, 362, 1907 CrossRef CAS.
- A. El Gueddi, S. Guesmi, B. Mernari, H. Stoeckli-Evans, J. Ribas and R. Vicente, Polyhedron, 1996, 15, 4283 CrossRef.
- S. Sobanska, M. Lagrenée, J.-P. Wignacourt and E. M. Holt, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 553 CrossRef.
- S. Sobanska, J.-P. Wignacourt, P. Conflant, M. Drache, M. Lagrenée and E. M. Holt, New J. Chem., 1999, 23, 393 RSC.
- W.-W. Sun, A.-L. Cheng, Q.-X. Jia and E.-Q. Gao, Inorg. Chem., 2007, 46, 5471 CrossRef CAS.
- W.-W. Sun, Q. Yue, A.-L. Cheng and E.-Q. Gao, CrystEngComm, 2008, 10, 1384 RSC.
- A. Escuer, R. Vicente, B. Mernari, A. El Gueddi and M. Pierrot, Inorg. Chem., 1997, 36, 2511 CrossRef CAS.
- M. Gryz, W. Starosta and J. Leciejewicz, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, m3470 CrossRef.
- S. Sueur, M. Lagrenée, F. Abraham and C. Bremard, J. Heterocycl. Chem., 1987, 24, 1285 CrossRef CAS.
-
SAINT Plus Version 7.53a, Bruker Analytical X-ray Systems, Madison, WI, 2008 Search PubMed.
-
G. M. Sheldrick, SADABS, Bruker-Siemens Area Detector Absorption and Other Correction, Version 2008/1, 2008 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112.
- N. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192 CrossRef.
- W. Starosta and J. Leciejewicz, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2004, 60, o2219 CrossRef.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef.
- F. S. Richardson, Chem. Rev., 1982, 82, 541 CrossRef CAS.
- R. Tripier, C. Platas-Iglesias, A. Boos, J.-F. Morfin and L. Charbonnière, Eur. J. Inorg. Chem., 2010, 2735 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040
040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 298
298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149
149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 377
377![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 337
337![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552
552![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135
135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 837
837![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645
645![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 [Rint = 0.0473]
292 [Rint = 0.0473]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 [Rint = 0.0628]
419 [Rint = 0.0628]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305 [Rint = 0.0447]
305 [Rint = 0.0447]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 [Rint = 0.0632]
270 [Rint = 0.0632]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985 [Rint = 0.0530]
985 [Rint = 0.0530]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 139 [Rint = 0.0410]
139 [Rint = 0.0410]

![View showing the stacking of two corrugated layers [Ln2(H2O)4(pzdc)3] intercalated by water molecules in 1–4, along the c axis.](/image/article/2011/CE/c0ce00013b/c0ce00013b-f3.gif)

![View of the molecular assembly of discrete dinuclear units [Ln2O6N5(H2O)6] along the b axis, in the compounds series 5–9, Ln2(H2O)6(pzdc)3·3H2O.](/image/article/2011/CE/c0ce00013b/c0ce00013b-f5.gif)


