Microwave-assisted solution-phase preparation of flower-like Bi2WO6 and its visible-light-driven photocatalytic properties†
Xiao-Feng
Cao
a,
Lei
Zhang
a,
Xue-Tai
Chen
*a and
Zi-Ling
Xue
b
aState Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, P. R. China. E-mail: xtchen@netra.nju.edu.cn; Fax: +86-25-83314502
bDepartment of Chemistry, The University of Tennessee, Knoxville, Tennessee 37996-1600, USA
First published on 6th September 2010
Abstract
We report here the preparation of flower-like Bi2WO6via a simple, rapid, microwave-assisted solution-phase process. Powder X-ray diffraction (XRD), energy dispersive X-ray spectroscopy (EDS), field emission scanning electron microscopy (FE-SEM) and high resolution transmission electron microscopy (HRTEM) were employed to characterize the phase and morphologies of the products. The time-dependent experiments showed an Ostwald ripening mechanism in the crystal growth process. The as-prepared Bi2WO6 was able to efficiently degrade Rhodamine B (RhB) under visible light irradiation. Calcination was found to decrease the photocatalytic performance of flower-like Bi2WO6.
Introduction
Bismuth tungstate (Bi2WO6), one of the simplest Aurivillius phase oxides, has a layered structure in which the perovskite layer (WO4)2− lies between (Bi2O2)2+ layers.1–5 It has attracted a great deal of attention because of its excellent physical and chemical properties such as ferroelectric piezoelectricity, pyroelectricity, catalytic behaviour, oxide anion conducting, a non-linear dielectric susceptibility, and luminescent properties.6–11 Since the discovery of visible-light-driven photocatalytic activities of Bi2WO6 for O2 evolution12 and the degradation of organic compounds,13 a great deal of research has been conducted to study its photocatalytic performance under the visible light irradiation.8,10,14–18 As is well known, the shape, size and structure of the nanostructures can dramatically influence the physical and chemical properties of a material.19–21 Many research groups have carried out the studies of Bi2WO6 nano/microstructures, fabricating many kinds of Bi2WO6 with various morphologies such as nanoplates, nanoflake films, porous thin films, nanocages, nanofibrous mat-, flower-, tire-, and helix-like shapes.5,8,10,22–27 Several studies have also been performed on the Bi2WO6-based composite materials such as fluorinated Bi2WO6,28 C60/Bi2WO6,29Co3O4/Bi2WO6,30Bi2WO6/TiO2 hierarchical heterostructures,31 and AgBr–Ag–Bi2WO6 nanojunction system.32 These studies showed that controlling particle sizes and the second components of the nanostructures can significantly enhance their photocatalytic activities. Until now, several procedures such as solid-state reaction,12,13 amorphous complex precursor method,14 molten salt route33 and hydrothermal process8–11,16–18,23–25,34–35 have been used to prepare Bi2WO6 nano-/microcrystals.Recently, the use of microwaves, which can change the kinetics and selectivity of a chemical reaction, have been widely used in organic synthesis and nanomaterials preparation.36–38 Compared to traditional heating, microwave heating is simple, rapid, uniform, efficient, economical, and environmentally friendly.36–38 Fu39 and Shen's groups40 have independently employed microwave-assisted solvothermal process to prepare Bi2WO6 nanosheets and nanoplates. Huang et al.41 have synthesized 3D flower-like aggregation of Bi2WO6 nanoflakes in an acid precursor suspension by the microwave-assisted solvothermal process. They used NaOH solution to modulate the pH value and discussed the effect of pH value on the Bi2WO6 morphology and phase changes. However, the formation process of flower-like microstructure under the microwave heating was not revealed and the thermal stability of the as-prepared Bi2WO6 was not studied. Also the relationship of the photocatalytic efficiency and the morphologies was unknown.
In this work, we successfully prepared flower-like Bi2WO6 microstructures in a 5 min microwave-assisted solution-phase reaction process. Hexamethylene tetramine (HMT), a weak base and a good coordination agent, was used to investigate its effect on the morphology. The crystal growth process, BET surface area, optical absorption properties, thermal stabilities, visible-light-driven photocatalytic activity of as-prepared Bi2WO6 were studied.
Experimental
All reagents were purchased from Shanghai Chemical Company and used without further purification. All samples were prepared in a microwave system (2.45 GHz, 200 W, Discover S-Class, CEM). The system was equipped with in situ magnetic stirring. The exposure time and temperature were programmed. The automatic temperature-control system allowed continuous monitoring and control of the internal temperature of the reaction system (1 °C). The preset profile (desired time and temperature) was followed automatically by continuously adjusting the applied microwave power. In a typical preparation procedure, Bi(NO3)3·5H2O (1.0 mmol, AR), hexamethylene tetramine (HMT) (1.0 mmol, AR) were dissolved in HNO3 (1.2 M, 5 mL). Then Na2WO4 aqueous solution (0.1 M, 5 mL) was added dropwise to the above solution under continuous stirring, and white precipitates formed gradually. The reaction mixture was stirred at room temperature for 10 min and then transferred to a 35 mL round-bottom flask. After treating the mixture at 180 °C for 5 min under microwave irradiation, it was cooled to room temperature rapidly by air compressor. The product was collected, washed with deionized water and absolute ethanol, and dried in air at 60 °C for 6 h. It is necessary to point out that the reaction time can not exceed 7 min because the continual microwave heating may cause a tremendous rise of pressure in the reaction system. One possible reason is that HMT may decompose rapidly in acidic system under high temperature during a long heating process.The products were characterized by X-ray powder diffraction (XRD) with a Shimadzu XRD-6000 powder X-ray diffractometer with Cu-Kα radiation (λ = 1.5406 Å), and recorded with 2θ range from 10° to 80°. FE-SEM images and EDS were measured on a Hitachi S-4800 field emission scanning electron microanalyser employing an operating voltage of 5 kV or 25 kV. TEM images were obtained on a JEM-200CX transmission electronic microscope, employing an accelerating voltage of 200 kV. The FTIR spectra were recorded between 4000 and 400 cm−1 with a Bruker VECTOR-22 instrument. The UV-vis diffuse reflectance spectra were recorded on a UV-3600 (Shimadzu) using BaSO4 as the reference. The Brunauer–Emmett–Teller (BET) surface areas were measured at micromeritics ASAP2020 surface area and porosimetry system using adsorption data. The calcinations of the products were performed in a tube furnace with a temperature controller. The temperature programmed heating process maintained 3° min−1 in air.
The photocatalytic experiments were conducted in an XPA-7 photochemical reactor (Xujiang Electromechanical Plant, Nanjing, China) equipped with 500 W Xe lamp and a cutoff filter (λ > 420 nm). To measure the photocatalytic degradation activity of the product, 0.1 g Bi2WO6 was dispersed into 200 mL aqueous solutions containing Rhodamine B (RhB) at 1 × 10−5 M. The suspension was treated in an ultrasonic bath for 3 min and then magnetically stirred for 60 min in the dark to ensure the establishment of an adsorption-desorption equilibrium between the photocatalysts and RhB. After that, the solution was equally distributed in several quartz cuvettes, which were irradiated by Xe lamp light under magnetic stirring. At the desired time intervals, 5 mL suspension was sampled and centrifuged to remove the Bi2WO6 catalyst. The absorbance at 553 nm of the organic dye was monitored using a UV-3600 (Shimadzu) spectrophotometer.
Results and discussion
Fig. 1a shows the XRD pattern of the product prepared in the typical procedure. All diffraction peaks could be indexed to the orthorhombic phase of Bi2WO6 by comparison with the data from JCPDS card no. 79-2381. No peaks of any other phases or impurities were detected. Fig. 1b is the EDS spectrum of the typical sample. The peaks of Bi, W, and O can be found. No impurities peaks were detected except Cu and C, which were ascribed to the substrate and CO2 adsorbed by the sample. The other source of C may come from the absorbed HMT. Compared with the product prepared in the absence of HMT, the FTIR spectrum of the product obtained with HMT has an extra weak absorption at 1589 cm−1, which may be attributed to N–H vibration (ESI, Fig. S1).† This result indicated that the product contained trace amount of the absorbed HMT. Quantitative analysis shows that the atomic ratio of Bi to W is about 2.15: 1, which is very close to the Bi2WO6 stoichiometry.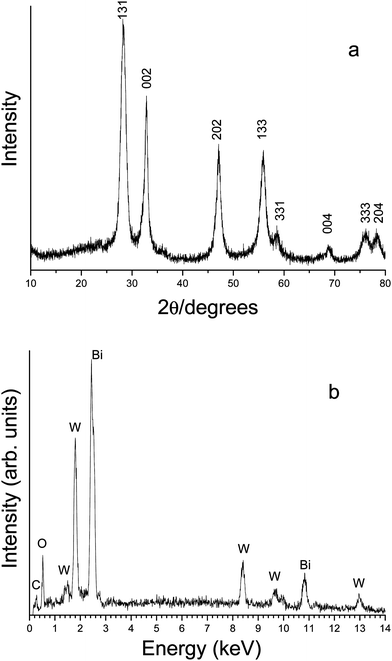 | ||
| Fig. 1 (a) XRD pattern and (b) EDS spectrum of the product prepared in the typical experiment. | ||
Fig. 2a is the low magnified FE-SEM image of the sample obtained in the typical procedure, indicating that the product was flower-like particles with average diameters ranging from 2 to 3 μm. The magnified FE-SEM images (Fig. 2b and 2c) show that the flower-like microstructures were constructed by nanosheets with thickness of about 15 nm. Fig. 2d is the TEM image of the product, which further proves the above microstructural information. In the HRTEM image (Fig. 2e) taken from the edge of the individual microstructure, the (131) planes were marked, with the spacing of the adjacent lattice planes of 0.319 nm, which is very close to the theoretical value of 0.315 nm.
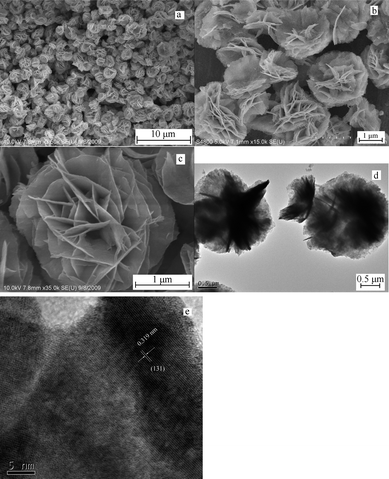 | ||
| Fig. 2 Typical FE-SEM, TEM and HRTEM images of the flower-like Bi2WO6. (a) low magnified FESEM image; (b) and (c) high magnified images; (d) TEM image of the product; (e) HRTEM image taken from the edge of the individual microstructure. | ||
In order to investigate the effect of reaction temperature on the morphology of the product, Bi2WO6 nano-/microcrystals were prepared at 160 and 140 °C, while the other conditions were kept constant. When the reaction temperature was reduced to 160 °C (Fig. 3a), the nanoplates and nanoparticles coexisted in the product. At 140 °C (Fig. 3b), the nanoparticles with size of ca. 20 nm were found. These observations suggested that the high temperature is favorable for the formation of the flower-like microstructure.
 | ||
| Fig. 3 FE-SEM images of Bi2WO6 obtained at different reaction temperatures for 5 min: (a) 160, (b) 140 °C. | ||
The amount of the reagents can greatly influence the morphology of the products. Controlled experiments were carried out under the same conditions except different amount of HMT or HNO3 were used. HMT is a highly water soluble, non-ionic cyclic tertiary amine. Usually, HMT can be used as the pH adjuster since it can decompose to form protonated ammonia. Changing the amount of HMT can affect pH value in the reaction system.42 Meanwhile, a HMT molecule containing four N atoms can act as a tetradentate ligand. The Bi(III) complex with HMT ligand has been reported.43Fig. 4a is the FE-SEM image of the product prepared in the absence of HMT, which shows that the sample was aggregation of thin nanosheets. Increasing the amount of HMT to 2 mmol (Fig. 4b) the product was the nanoparticles and no flower-like microstructures were found. HNO3 can also influence the morphology of the products. When no HNO3 was used in the reaction (Fig. 4c and 4d), the products were the self-assembled microstructures of nanoplates with the thickness of 15–30 nm.
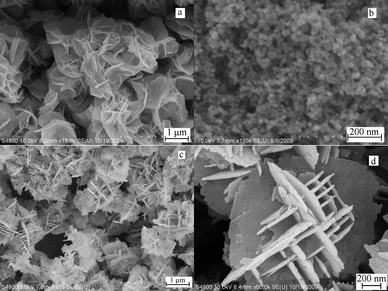 | ||
| Fig. 4 FE-SEM images of Bi2WO6 obtained with different amount of HMT or HNO3: (a) in the absence of HMT; (b) with 2 mmol HMT; (c) and (d) in the absence of HNO3. | ||
In order to understand the crystal growth behaviour a series of time-dependent experiments were performed. Fig. 5 shows the FE-SEM images of the products obtained at 180 °C for 1 min, 3 min and 4 min, respectively. When the reaction was carried out for 1 min, the product was the Bi2WO6 nanoparticles with the size distribution in the range of 20 to 30 nm (Fig. 5a). The broadness of the XRD diffraction peaks indicated the weak crystallinity (Fig. 5e). The BET surface area of the product was found to be 29.74 m2 g−1. When the reaction time was prolonged to 3 min, flower-like microstructures and nanoparticles coexisted in the product (Fig. 5b). Further prolonging the reaction time to 4 min, more flower-like microstructures appeared and the nanoparticles decreased (Fig. 5c). When the reaction time reached 5 min, the nanoparticles disappeared and the product was entirely flower-like microstructures (Fig. 1). The BET surface area of the product increased to 44.03 m2 g−1. With a longer reaction time, between 5 and 7 min, such as 6 min, the flower-like microstructures were preserved (Fig. 5d). The experiments results suggested the crystal growth behaviour was an obvious Ostwald ripening mechanism. At first, amorphous Bi2WO6 was formed after the W source was mixed with the Bi source at room temperature. Secondly the microwave heating process made the amorphous Bi2WO6 crystallized. Then part of crystalline nanoparticles grew to form flower-like microstructure. According to the Gibbs–Thomson law, the larger particles grew at the cost of the small ones. So the nanoparticles dissolved and more flower-like microstructures were generated until nanoparticles disappeared. Based on the above possible process, long reaction time can also yield the flower-like microstructures at low temperature (ESI, Fig. S2a and S2b).†
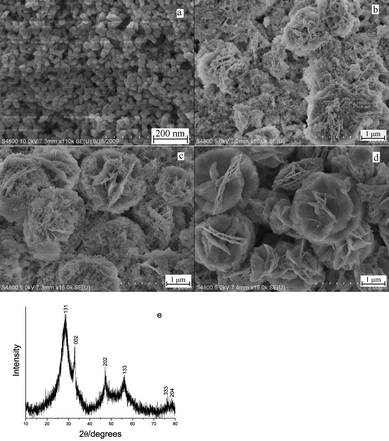 | ||
| Fig. 5 FE-SEM images of Bi2WO6 obtained at 180 °C with different reaction time: (a) 1 min; (b) 3 min; (c) 4 min; and (d) 6 min; (e) XRD pattern of the product obtained at 180 °C for 1 min. | ||
The thermal stability of as-prepared Bi2WO6 was also investigated. Fig. 6 shows the FE-SEM images of the flower-like Bi2WO6 calcined at different temperatures for 60 min. The images show the flower-like morphologies were kept until 450 °C. When the calcination temperature was increased to 500 °C, the particles became highly fused. Fig. 6b is the magnified FE-SEM image of the product calcined at 300 °C, which shows that numerous nanopores exist in the “flower petals”. This experimental phenomenon is in agreement with those previously reported.23 The calcined products had higher crystallinity than the un-calcined product and the XRD pattern exhibited higher intensity and narrower diffraction peaks (Fig. 6g).
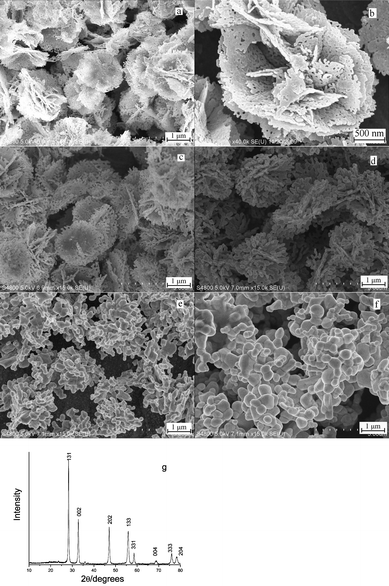 | ||
| Fig. 6 FE-SEM images of Bi2WO6 after calcinations at (a) and (b) 300; (c) 350; (d) 400; (e) 450; (f) 500 °C for 60 min; (g) XRD pattern of Bi2WO6 calcined at 300 °C for 60 min. | ||
The optical absorption properties of a semiconductor are relevant to the energy band structure, which is a key factor in determining its photocatalytic activity. The UV-vis diffuse reflectance spectrum of the flower-like Bi2WO6 was measured by using an UV-vis spectrometer. Fig. 7 shows the typical UV-vis diffuse reflectance spectrum. The absorption range of the sample extended from the UV region to ca. 455 nm. According to the calculation, the energy band gap of the product is ca. 2.73 eV, which is larger than Tang's result (2.69 eV),13 and could be attributed to the nanosize effect.10 This result indicates that the product may exhibit a high photocatalytic activity under the visible light irradiation.
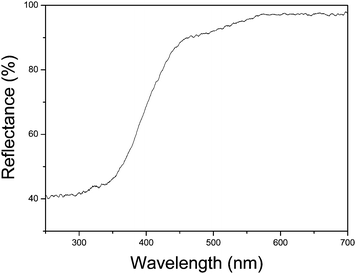 | ||
| Fig. 7 UV-vis diffuse reflectance spectrum of the flower-like Bi2WO6. | ||
Usually, RhB was chosen as a model pollutant to simulate the actual photocatalytic degradation of organic pollutants. The RhB aqueous solution shows an intense absorption band centered at 553 nm and the peak intensity is proportional to the concentration of RhB. Fig. 8 shows the temporal evolution of the absorption spectra during the photocatalytic degradation of RhB in the presence of flower-like Bi2WO6. As the irradiation time prolonged, the absorption peaks decreased and the position showed hypsochromic shifts. Based on the previous reports,11,44,45 the hypsochromic shifts comes from the de-ethylated degradation process in the stepwise manner. After 80 min, the absorption peaks disappeared and the red colour of RhB aqueous solution completely faded. The experiments showed the excellent photocatalytic activity of the flower-like microstructure Bi2WO6 for the degradation of RhB under visible light irradiation. The possible reason can be ascribed to the energy band structures and high BET surface area.
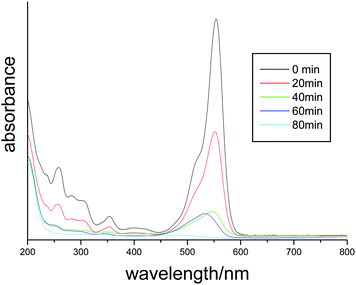 | ||
| Fig. 8 The temporal evolution of the absorption spectrum of the RhB solution in the presence of flower-like Bi2WO6 under exposure to visible light. | ||
In order to reveal the relationship between the microstructure and photocatalytic efficiency, the photocatalytic performances of different Bi2W2O6 samples were compared at the identical conditions under visible light irradiation. It was found that the degradation of RhB was very slow without photocatalyst under visible light irradiation (curve a in Fig. 9). The flower-like Bi2WO6 had the best photocatalytic efficiency, whose degradation efficiency of RhB was higher than 99.9% after 80 min (curve i in Fig. 9). The nanoparticles prepared at 180 °C for 1 min had weaker visible-light-driven photocatalytic property and the photocatalytic efficiency reached 59.4% after 80 min (curve g in Fig. 9), which could be attributed to the lower BET surface areas (29.74 m2 g−1) and more crystal defects acting as electron-hole recombination centers of the nanoparticles.23,37 Although the product prepared in the absence of HMT had similar BET surface areas (45.95 m2 g−1) with the flower-like Bi2WO6 (44.03 m2 g−1), the former showed slightly lower photocatalytic efficiency than the flower-like Bi2WO6 (curves h, i in Fig. 9). The difference in photocatalytic activity could be related to the different RhB absorption over the photocatalysts. The RhB adsorption percentage over the product prepared in the absence of HMT was 12.4% while the RhB adsorption percentage over the flower-like Bi2WO6 reached 24.9%. The lower absorption percentage decreased the probability of charges transfer between the catalyst and the dye molecules. The IR spectrum showed that there are trace amount of HMT molecules absorbed on the surface of the product prepared with HMT. The possible reason for the above different adsoption could be related to the adsorbed HMT molecules. HMT molecules could act as a tetrafunctional H-bond acceptor.46 It is probable that the H-bond existed between HMT and RhB molecules and more RhB molecules were adsorbed over the product prepared with HMT.
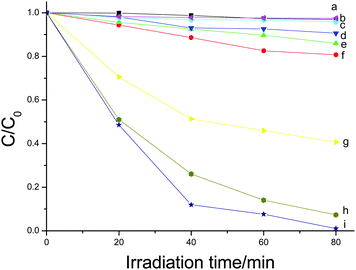 | ||
| Fig. 9 Photodegradation of RhB solution using different products as photocatalysts. (a) without catalyst; (b) the Bi2WO6 calcined at 500 °C; (c) the Bi2WO6 calcined at 450 °C; (d) the Bi2WO6 calcined at 400 °C; (e) the Bi2WO6 calcined at 350 °C; and (f) the Bi2WO6 calcined at 300 °C; (g) nanoparticles prepared at 180 °C for 1 min; (h) the Bi2WO6 prepared in the absence of HMT; and (i) the flower-like Bi2WO6. | ||
Calcination was found to decrease the photocatalytic performance of flower-like Bi2WO6. All of the calcined products had the lower photocatalytic activities than the original flower-like Bi2WO6. Furthermore, the higher calcined temperature corresponds to the lower activity (curves b, c, d, e, f in Fig. 9). This experiment phenomenon is contrary to the previous report.23 In Wang's research,23 the calcination can enhance the photocatalytic activity of the Bi2WO6. It was suggested that the better crystallinity of the calcined Bi2WO6 resulted in fewer defects acting as electron-hole recombination centers in their calcined Bi2WO6. In our experiments, the calcinations made the BET surface areas of the flower-like Bi2WO6 dramatically decreased and the calcined Bi2WO6 had the better crystallinity than the un-calcined Bi2WO6 (Fig. 6g). In general, the surface area of a catalyst can greatly affects its catalytic performance, and a high surface area corresponds to the good photocatalytic activity.47 In our experiments, the lower photocatalytic activity of the calcined Bi2WO6 can be related to the low BET surface areas.
Conclusions
In summery, we have successfully prepared flower-like Bi2WO6via a simple, rapid, economical, and efficient microwave-assisted solution-phase process. The experiments showed the HMT molecule and HNO3 played important roles in formation of flower-like microstructures. The formation of flower-like Bi2WO6 was an obvious Ostwald ripening process. The results showed the flower-like morphologies had a good thermal stability and could be maintained under the temperature of 450 °C. The energy band structures and high BET surface areas made the flower-like Bi2WO6 to possess excellent photocatalytic activities for the degradation of RhB under visible light irradiation. The controlled experiments showed the morphologies and calcined process can affect the photocatalytic efficiency significantly.Acknowledgements
This work was supported by National Basic Research Program of China (no. 2006CB806104 and 2007CB925102), National Science Foundation of China (no. 20721002), and the US National Science Foundation (CHE-0516928).References
- S. Lazure, R. Vannier, G. Nowogrocki, G. Mairesse, C. Muller, M. Anne and P. Strobel, J. Mater. Chem., 1995, 5, 1395–1403 RSC.
- K. R. Kendall, C. Navas, J. K. Thomas and H. C. zur Loye, Chem. Mater., 1996, 8, 642–649 CrossRef CAS.
- M. S. Islam, S. Lazure, R. Vannier, G. Nowogrocki and G. Mairesse, J. Mater. Chem., 1998, 8, 655–660 RSC.
- N. Kim, R. Vannier and C. P. Grey, Chem. Mater., 2005, 17, 1952–1958 CrossRef CAS.
- M. Shang, W. Z. Wang, J. Ren, S. M. Sun, L. Wang and L. Zhang, J. Mater. Chem., 2009, 19, 6213–6218 RSC.
- Y. H. Shi, S. H. Feng and C. S. Cao, Mater. Lett., 2000, 44, 215–218 CrossRef CAS.
- N. A. McDowell, K. S. Knight and P. Lightfoot, Chem.–Eur. J., 2006, 12, 1493–1499 CrossRef CAS.
- H. B. Fu, C. S. Pan, W. Q. Yao and Y. F. Zhu, J. Phys. Chem. B, 2005, 109, 22432–22439 CrossRef CAS.
- Y. Y. Li, J. P. Liu, X. T. Huang and G. Y. Li, Cryst. Growth Des., 2007, 7, 1350–1355 CrossRef CAS.
- C. Zhang and Y. F. Zhu, Chem. Mater., 2005, 17, 3537–3545 CrossRef CAS.
- D. K. Ma, S. M. Huang, W. X. Chen, S. W. Hu, F. F. Shi and K. L. Fan, J. Phys. Chem. C, 2009, 113, 4369–4374 CrossRef CAS.
- A. Kudo and S. Hijii, Chem. Lett., 1999, 1103–1104 CAS.
- J. W. Tang, Z. G. Zou and J. H. Ye, Catal. Lett., 2004, 92, 53–56 CrossRef CAS.
- J. G. Yu, J. F. Xiong, B. Cheng, Y. Yu and J. B. Wang, J. Solid State Chem., 2005, 178, 1968–1972 CrossRef CAS.
- H. B. Fu, L. W. Zhang, W. Q. Yao and Y. F. Zhu, Appl. Catal., B, 2006, 66, 100–110 CrossRef CAS.
- J. Wu, F. Duan, Y. Zheng and Y. Xie, J. Phys. Chem. C, 2007, 111, 12866–12871 CrossRef CAS.
- F. Amano, A. Yamakata, K. Nogami, M. Osawa and B. Ohtani, J. Am. Chem. Soc., 2008, 130, 17650–17651 CrossRef CAS.
- F. Amano, K. Nogami and B. Ohtani, J. Phys. Chem. C, 2009, 113, 1536–1542 CrossRef CAS.
- Y. G. Sun and Y. N. Xia, Science, 2002, 298, 2176–2179 CrossRef CAS.
- D. Wang, S. Lin, Y. Wu and C. M. Lieber, Nano Lett., 2003, 3, 1255–1259 CrossRef CAS.
- P. D. Yang, Nature, 2003, 425, 243–244 CrossRef CAS.
- M. Shang, W. Z. Wang and H. L. Xu, Cryst. Growth Des., 2009, 9, 991–996 CrossRef CAS.
- L. S. Zhang, W. Z. Wang, Z. G. Chen, L. Zhou, H. L. Xu and W. Zhu, J. Mater. Chem., 2007, 17, 2526–2532 RSC.
- L. S. Zhang, W. Z. Wang, L. Zhou and H. L. Xu, Small, 2007, 3, 1618–1625 CrossRef CAS.
- M. Shang, W. Z. Wang, S. M. Sun, L. Zhou and L. Zhang, J. Phys. Chem. C, 2008, 112, 10407–10411 CrossRef CAS.
- L. W. Zhang, Y. J. Wang, H. Y. Cheng, W. Q. Yao and Y. F. Zhu, Adv. Mater., 2009, 21, 1286–1290 CrossRef CAS.
- H. B. Fu, W. Q. Yao, L. W. Zhang and Y. F. Zhu, Mater. Res. Bull., 2008, 43, 2617–2625 CrossRef CAS.
- H. B. Fu, S. C. Zhang, T. G. Xu, Y. F. Zhu and J. M. Chen, Environ. Sci. Technol., 2008, 42, 2085–2091 CrossRef CAS.
- S. B. Zhu, T. G. Xu, H. B. Fu, J. C. Zhao and Y. F. Zhu, Environ. Sci. Technol., 2007, 41, 6234–6239 CrossRef CAS.
- Q. Xiao, J. Zhang, C. Xiao and X. K. Tan, Catal. Commun., 2008, 9, 1247–1253 CrossRef CAS.
- M. Shang, W. Z. Wang, L. Zhang, S. M. Sun, L. Wang and L. Zhou, J. Phys. Chem. C, 2009, 113, 14727–14731 CrossRef CAS.
- L. S. Zhang, K. H. Wong, Z. G. Chen, J. C. Yu, J. C. Zhao, C. Hu, C. Y. Chan and P. K. Wong, Appl. Catal., A, 2009, 363, 221–229 CrossRef CAS.
- A. P. Finlayson, V. N. Tsaneva, L. Lyons, M. Clark and B. A. Glowacki, Phys. Status Solidi A, 2006, 203, 327–335 CrossRef CAS.
- Y. Y. Li, X. T. Huang, J. P. Liu and H. H. Ai, J. Nanosci. Nanotechnol., 2009, 9, 1530–1534 CrossRef CAS.
- F. Amano, K. Nogami, R. Abe and B. Ohtani, J. Phys. Chem. C, 2008, 112, 9320–9326 CrossRef CAS.
- S. A. Galema, Chem. Soc. Rev., 1997, 26, 233–238 RSC.
- J. A. Gerbec, D. Magana, A. Washington and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 15791–15800 CrossRef CAS.
- G. A. Tompsett, W. C. Conner and K. S. Yngvesson, ChemPhysChem, 2006, 7, 296–319 CrossRef CAS.
- L. Wu, J. H. Bi, Z. H. Li, X. X. Wang and X. Z. Fu, Catal. Today, 2008, 131, 15–20 CrossRef CAS.
- H. D. Xie, D. Z. Shen, X. Q. Wang and G. Q. Shen, Mater. Chem. Phys., 2007, 103, 334–339 CrossRef CAS.
- S. S. Yao, J. Y. Wei, B. B. Huang, S. Y. Feng, X. Y. Zhang, X. Y. Qin, P. Wang, Z. Y. Wang, Q. Zhang, X. Y. Jing and J. Zhan, J. Solid State Chem., 2009, 182, 236–239 CrossRef CAS.
- A. Sugunan, H. C. Warad, M. Boman and J. Dutta, J. Sol-Gel Sci. Technol., 2006, 39, 49–56 CrossRef CAS.
- Y. C. Guo, S. R. Luan, Y. R. Chen, X. S. Zang, Y. Q. Jia, G. Q. Zhong and S. K. Ruan, J. Therm. Anal. Calorim., 2002, 68, 1025–1033 CrossRef CAS.
- T. Watanabe, T. Takizawa and K. Honda, J. Phys. Chem., 1977, 81, 1845–1851 CrossRef CAS.
- W. Zhao, C. C. Chen, X. Z. Li and J. C. Zhao, J. Phys. Chem. B, 2002, 106, 5022–5028 CrossRef CAS.
- S. B. Copp, S. Subramanian and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 1993, 32, 706–709 CrossRef.
- J. W. Tang, Z. G. Zou and J. H. Ye, Angew. Chem., Int. Ed., 2004, 43, 4463–4466 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR spectra of the products; FE-SEM images of Bi2WO6 obtained at 150 °C after different reaction time. See DOI: 10.1039/c0ce00031k |
| This journal is © The Royal Society of Chemistry 2011 |
