Synthesis of Au core Au/Ag alloy shell nanoparticles using branched Au nanoparticles as seeds
Xinling
Tang
a and
Masaharu
Tsuji
*ab
aDepartment of Applied Science for Electronics and Materials, Graduate School of Engineering Sciences, Kyushu University, Kasuga, 816-8580, Japan
bInstitute for Materials Chemistry and Engineering, Kyushu University, Kasuga, 816-8580, Japan. E-mail: tsuji@cm.kyushu-u.ac.jp; Fax: +81-092-583-7815; Tel: +81-092-583-7815
First published on 13th September 2010
Abstract
Novel Au core Au/Ag alloy shell particles denoted as Au@Au/Ag were prepared through the following two-step reduction method using branched Au nanoparticles as seeds. In the first step, branched Au seeds were prepared from an aqueous solution of an HAuCl4/ammonium formate (AF)/poly(vinylpyrrolidone) (PVP) mixture. In the second step, AgNO3 was reduced in N,N-dimethylformamide (DMF) in the presence of branched Au seeds and PVP. Then larger branched particles with smaller numbers of short branches were formed. On the basis of transmission electron microscopic (TEM), high-resolution (HR)-TEM, energy dispersed X-ray spectroscopic (EDS) and XRD data, crystal structures and their growth mechanisms were discussed. It was found that Au/Ag alloy shells were formed by simultaneous occurrence of melt of fine long branches in Au seeds and slow reduction of Ag+ to Ag0 during heating the DMF solution at 140 °C for 3 h. The fusion of fine Au branches was confirmed by observing shape change from fine branches to spherical particles on Au core particles in DMF without the presence of AgNO3.
1. Introduction
In recent years, heterostructured nanoparticles with core–shell and alloy structures attracted great interest owing to their combined multiple functions or unique optical and physical properties that are not possible from one nanomaterial alone.1–8 Noble metal Au and Ag have the same face centered cubic (fcc) crystal structure and similar lattice constants (Au: 0.408 nm and Ag: 0.409 nm). Based on these characteristics, significant advances have been made in developing synthetic methods to prepare various kinds of Au@Ag core–shell structures and Au/Ag alloys from Au/Ag reagents.4,9–20 In general, chemical, photochemical, and radiolytic reduction methods were used for the syntheses of Au@Ag and Au/Ag bimetallic particles.It has been known that shapes of Ag shells in Au@Ag particles depend on shapes of Au cores.14–18 Spherical Ag shells are generally formed from spherical Au cores. On the other hand, we have recently succeeded in the shape-selective epitaxial growth of cubic, bipyramidal, and one-dimensional rod-like Ag shells having {100} facets from octahedral, triangular plate-like, and decahedral Au cores having {111} facets in ethylene glycol (EG), respectively.14 In this case EG acts as both reductant and solvent. When reductant and solvent were changed from EG to DMF, octahedral, triangular plate-like, and decahedral Ag shells having {111} facets could be prepared from the corresponding Au cores.16 This implied that shapes of Ag shells depend on not only shapes of Au cores but also solvent and reductant.
In this article we studied effects of shapes of Au cores for the growth of Ag shells using dendritic branched Au nanoparticles as Au seeds in DMF. Then, we succeeded in the preparation of Au core Au/Ag alloy denoted as Au@Au/Ag particles. To the best of our knowledge, this is the first report on the synthesis of Au@Au/Ag particles. The growth mechanism of Au/Ag alloy shells over branched Au core particles is discussed on the basis of TEM, EDS, and XRD data. The present study demonstrates that melt of fine branches of Au core particles in the presence of Ag+ ions can be used as a new preparation technique of Au/Ag alloy shells on Au core particles.
2. Experimental
2.1 Materials
All the reagents used in this study were of analytical grade and used without further purification.2.2 Preparation procedure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in terms of monomer units), 5 mM of HAuCl4·4H2O, and 0.15 M AF were placed in a 100 ml three-necked flask. The mixed solution was heated from room temperature to 100 °C in an oil bath, then it was cooled to room temperature naturally. The as-prepared Au seeds were separated using centrifugal separation (10
000 in terms of monomer units), 5 mM of HAuCl4·4H2O, and 0.15 M AF were placed in a 100 ml three-necked flask. The mixed solution was heated from room temperature to 100 °C in an oil bath, then it was cooled to room temperature naturally. The as-prepared Au seeds were separated using centrifugal separation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min) and washed with water and ethanol solution three times.
000 rpm, 10 min) and washed with water and ethanol solution three times.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The final molar ratios of Au
000). The final molar ratios of Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag were 2
Ag were 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. This solution was heated in an oil bath at 140 °C for 3 hours, and then naturally cooled down to room temperature. The sample was collected and washed centrifugally (10
1, respectively. This solution was heated in an oil bath at 140 °C for 3 hours, and then naturally cooled down to room temperature. The sample was collected and washed centrifugally (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min) three times using distilled water and absolute ethanol to eliminate PVP and some other impurities in the product solution.
000 rpm, 10 min) three times using distilled water and absolute ethanol to eliminate PVP and some other impurities in the product solution.
2.3 Characterization of nanoparticles
Morphologies and crystal structures of Au branched core and product core–shell particles were characterized by using TEM, HR-TEM, and TEM-EDS at an accelerating voltage of 200 kV (JEOL JEM-2000XS and 2100F), and XRD (Rigaku RINT2100). XRD patterns were measured with the product films deposited on silicon substrates. Optical properties were characterized by using UV-visible (Vis) absorption spectroscopy (Shimadzu UV-3600).3. Results and discussion
Fig. 1a shows a typical TEM image of Au seed particles obtained from an HAuCl4·4H2O/AF/PVP mixture in aqueous solution. It should be noted that a new kind of branched Au particles are obtained. Au branched particles consist of spherical cores with average diameters of 100 ± 34 nm and many fine dendritic branches on the surfaces of cores. The average diameter of branches was 6 ± 1 nm and their lengths were about 30–100 nm. Au particles obtained in this work under oil-bath heating had finer and longer branches than those obtained under hydrothermal synthesis, where star-like dendritic particles were obtained.21 | ||
| Fig. 1 TEM image of (a) Au branched nanoparticles and (b) products obtained using Au branched particles as seeds. | ||
When these branched Au nanoparticles were added to the AgNO3/PVP/DMF solution as seeds, and heated at 140 °C for 3 h, larger nanoparticles with an average diameter of 141 ± 26 nm were prepared (Fig. 1b). It should be noted that the smaller numbers of short branches are produced by the addition of AgNO3. The average diameter and length of branches were 20 ± 5 nm and about 20–50 nm, respectively. These results indicate that the average size of nanoparticles in the cores increased by a factor of ∼1.4, whereas the diameter of branches increased by a factor of ∼3.3.
To examine crystal structures of the product particles, we have made TEM-EDS analyses. Fig. 2a–d show TEM and TEM-EDS data of product particles. It is clear from these data that spherical Au particles (red component) are located in the core parts and they are fully covered by the Ag component (green component). It should be noted that most of fine branches of Au particles observed in Fig. 1a disappeared in Fig. 2c and only a few short and thick Au branches remained around Au cores. These results suggest that branched Au core particles changed their morphologies during the preparation of Ag shell component in the second step.
![(a) TEM and (b–d) TEM-EDS data of the Au@Au/Ag nanostructures prepared by oil-bath heating of AgNO3/PVP/DMF for 3 hours at an [AgNO3]/[HAuCl4] molar ratio of 1/2.](/image/article/2011/CE/c0ce00018c/c0ce00018c-f2.gif) | ||
| Fig. 2 (a) TEM and (b–d) TEM-EDS data of the Au@Au/Ag nanostructures prepared by oil-bath heating of AgNO3/PVP/DMF for 3 hours at an [AgNO3]/[HAuCl4] molar ratio of 1/2. | ||
To obtain more detailed information on the product structure, TEM-EDS measurements and their line analyses were made for one typical core–shell particle (Fig. 3a–f). It is clear from Fig. 3c that spherical Au core component in the center is surrounded by a weak diffuse Au component having short and thick branches in comparison with those in Au branched cores. The crystal structure of Au component is clearly different from that of original Au seeds (cf.Fig. 1a). The average size of spherical Au core component is unchanged before and after the addition of AgNO3. However, by the addition of AgNO3, fine long Au branches observed in Au seeds disappear and smaller numbers of short and thick branches involving Au component remain. Fig. 3e shows the line analysis data of Au and Ag components along a red line in Fig. 3b (line A). Although there is no branch in the upper right, a short branch exists in the left under on this line, as shown by a red circle in Fig. 3a. If the product particles have Au@Ag core–shell structures, the Au component should decrease smoothly, as shown by two dotted blue lines in Fig. 3e on both sides, as reported for spherical Cu@Ag and Ag@Cu particles.6,22 However small amounts of Au components appear outside the Au cores as weak long tail components in Fig. 3e. It should be noted that a weak tail Au component exists in the 170–200 nm region, even though there is no branch in the upper right along line A (see Fig. 3a). The Ag component is located outside the center core, where weak Au components also exist. On the basis of these findings, Au cores are not surrounded by pure Ag shells but they are covered by Au/Ag alloy shells having ∼20 nm in thickness.
 | ||
| Fig. 3 (a) TEM, (b–d)TEM-EDS data of Au@Au/Ag core–shell nanostructures, and (e) and (f) distributions of Au and Ag components along the red (A) and blue (B) cross-section lines shown in (b), respectively. | ||
To examine the crystal structure of short branches, EDS analyses were made for a typical branch shown in a red circle in Fig. 3a corresponding to a weak component in the 20–50 nm region in Fig. 3e. Fig. 3f shows the line analysis data of Au and Ag components along the perpendicular direction (blue line B in Fig. 3b). The Ag component is not distributed outside the Au component but it is distributed on the same position as the Au component. These results indicate that short branches are also composed of Au/Ag alloys. On the basis of above facts, we concluded that Au@Au/Ag particles having short Au/Ag alloy branches are grown in the present conditions. When we measured Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag atomic ratios in several parts of Au/Ag alloy shell from EDS data, they were 27 ± 7
Ag atomic ratios in several parts of Au/Ag alloy shell from EDS data, they were 27 ± 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 ± 7%, respectively.
73 ± 7%, respectively.
Fig. 4a and b show a possible growth mechanism of Au@Au/Ag particles using dendritic branched Au particles as seeds. We have recently found that Au particles are fused by heating product solutions at 140 °C for 10–60 min and the fusion is enhanced in the presence of O2.23 A similar melt of Au particles may occur during heating DMF solution at 140 °C for 3 h in the present experiment. To examine whether branched Au particles really melt or not under our experimental conditions, they were heated at 140 °C in DMF without the addition of AgNO3. In this case, Au branched particles prepared in an aqueous solution were collected by centrifugation and they were dispersed in 15 ml DMF solution. Then the solution was heated to 140 °C and maintained for 3 hours at this temperature. Fig. 5a and b depict typical TEM images of Au cores before and after heating branched Au particles. It is noteworthy that all fine long Au branches disappeared and only some short and thick tubers and small spherical particles were formed on the surface of Au cores. This shape change of branched Au particles provides direct evidence that fine and long branches melt in DMF at 140 °C for 3 h. During heating little changes were observed for the spherical Au cores, indicating that spherical Au core parts of branched particles do not melt under the same temperature.
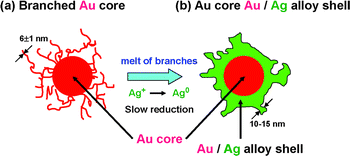 | ||
| Fig. 4 Growth mechanism of Au@Au/Ag nanostructure from branched Au core by the addition of AgNO3 under oil-bath heating at 140 °C for 3 h. | ||
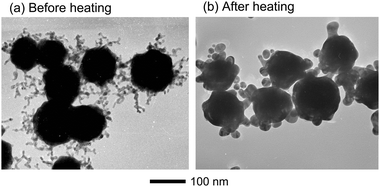 | ||
| Fig. 5 TEM images of (a) Au particles with long branches and (b) Au particles obtained after heating at 140 °C in DMF for 3 h without the addition of AgNO3. | ||
On the basis of the above findings, it is expected that fine and long branches more easily melt than large spherical Au cores in the present experimental conditions. In our recent experiment for the preparation of Ag nanocrystals in DMF, we found that the reduction rate of Ag+ at 140 °C is relatively slow.24,25 The melt of Au fine branches and the slow reduction of Ag+ to Ag0 occur simultaneously in Au seeds/AgNO3/PVP/DMF solutions, so that the Au/Ag alloy shells are grown on the surfaces of spherical Au cores in the second step. During the melt of Au fine branches and the reduction of Ag+ to Ag0 followed by nucleation and aggregation to form Ag component in Au/Ag shells, most of the fine branches change to diffuse spherical component around Au cores. Small amounts of branches do not melt completely to become spherical particles, so that they remain as short and thick Au rich Au/Ag branches as shown in the 20–50 nm region of Fig. 3e.
We found that long and fine Au branches with an average diameter 6 ± 1 nm selectively melt during the growth process of Ag shells. There are two possibilities why fine Au branches thermally melt at 140 °C. One is a size effect. It is known that the melting point of metallic nanoparticles depends on the size and it decreases with decreasing size.26 The decrease in the melting point is especially significant below the particle size of ∼30 nm and melt of small sizes of Au branches less than 10 nm is possible at 140 °C. The other reason is defects of crystal structures. If the crystallinity of Au branches is lower than that of larger spherical core, their melting point will be lower than that of core part.
When we attempted to measure selected area electron diffraction (SAED) patterns of branched Au particles, fine branches melted to spherical particles by the irradiation of focused electron beam, as observed in oil-bath heating at 140 °C for 3 h. Therefore, it was difficult to measure SAED pattern of branched Au particles to obtain crystal structures of branched parts. In order to obtain information on crystal structures of Au cores and Au@Au/Ag particles, their XRD patterns were measured (Fig. 6a and b). Strong diffraction peaks that can be assigned to the fcc phase of Au were observed in the branched core particles. These prominent diffraction peaks were indexed to the {100}, {200}, {220}, and {311} planes of Au seed particles. The peak intensity of {100} facet in Au is strongest indicating that favorable facet of branched Au particles is {100}. Owing to the same fcc crystal structure and similar lattice constants between Au and Ag, Au@Au/Ag particles show the similar diffraction peaks with Au seed particles. Sharp peaks observed in Au core and Au@Au/Ag particles without amorphous components suggest that these crystals are polycrystals. Thus, it was reasonable to assume that the melt of fine Au branches does not arise from low crystallinity of Au branches but from size effect.
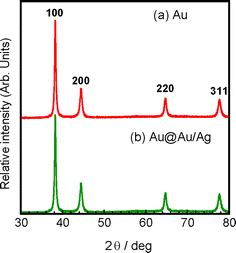 | ||
| Fig. 6 Typical XRD patterns of (a) Au particles and (b) Au@Au/Ag particles. | ||
Fig. 7 shows a typical HR-TEM image of a branched Au@Au/Ag particle. In a fused Au/Ag branched alloy shell, lattice fringes could be observed. The fringe spacing was about 0.29 nm corresponding to intervals of {110} facets of Ag rich Au alloy. It is therefore reasonable to assume that Ag rich Au/Ag alloy shells are composed of {110} facets.
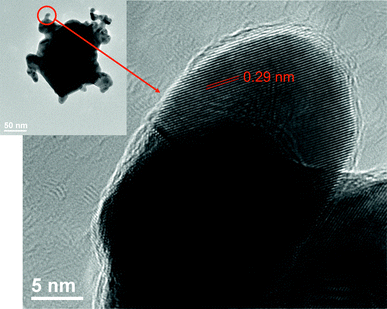 | ||
| Fig. 7 HR-TEM image of a branched Au@Au/Ag nanoparticle. | ||
The color of solutions of Au and Au@Au/Ag were brown and brick red as shown in Fig. 8a. The UV-Vis absorption spectra of the product solutions were measured to explore the information of optical properties of Au core and Au@Au/Ag core–shell nanostructures (Fig. 8b). For branched Au particles, a surface plasmon resonance (SPR) band with a strong peak at ca. 600 nm is observed. After the preparation of thin Au/Ag shells, the spectrum Au@Au/Ag particles becomes very broad with a peak at ca. 530 nm and the absorbance of the highest peak decreases by a factor of about 35%.
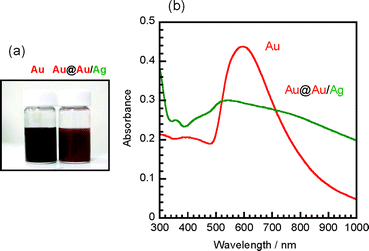 | ||
| Fig. 8 (a) Colors of solutions and (b) UV-Vis absorption spectra of Au and Au@Au/Ag nanoparticles. | ||
The SPR bands of Au, Ag, Au@Ag core–shell particles, and Au/Ag alloy particles have been studied extensively.9,11–16,19,27,28 According to these previous data, the peaks of SPR bands of spherical Au and Ag particles are observed at ca. 600 and 400 nm, respectively. On the other hand, the peaks of SPR bands of spherical Au@Ag and Au/Ag particles appear between 400 and 600 nm region, depending on the sizes and Au/Ag molar ratios of particles. When Ag shells are formed over Au cores, long tail bands are observed in the longer wavelength region.14 On the basis of these facts, it is reasonable to assume that the weak shoulder band in the 400–480 nm region arises from the Ag component in Au/Ag shells, whereas the main broad peak above ca. 500 nm arises from the Au component in Au cores. The appearance of a long tail band in the visible region arises from the formation of a core–shell structure.
4. Conclusions
In summary, Au@Au/Ag core–shell particles were prepared through a two-step reduction method using branched Au particles as seeded core. Their yield was high (∼100%) because no other shaped structures were observed. The present two-step method using simultaneous occurrence of melt of fine dendritic branches and slow reduction of Ag+ to Ag0 is a simple promising technique for the preparation of new Au@Au/Ag particles. The Au@Au/Ag particles having short branches provide high surface–area–volume ratio and the larger bimetallic interface and more surface defects. They will become promising materials for the application in surface plasmon resonance and catalysis.Acknowledgements
We thank Mr Takeshi Tanaka and Mrs Mika Matsunaga for their TEM-EDS and HR-TEM measurements and Prof. Junichi Yamaki and Shigeto Okada and Mrs Mika Matsunaga for their help in the measurements of XRD patterns. This work was supported by the Joint Project of Chemical Synthesis Core Research Institutions, Grant-in-Aid for Scientific Research from the Japanese MEXT (No. 22310060), and Kyushu University G-COE (novel carbon resource sciences).References
- N. Toshima, Pure Appl. Chem., 2000, 72, 317–325 CAS.
- M. N. Nadagouda and R. S. Varma, Cryst. Growth Des., 2007, 7, 2582–2587 CrossRef CAS.
- S. E. Habas, H. Lee, V. Radmilovic, G. A. Somorjai and P. Yang, Nat. Mater., 2007, 6, 692–697 CrossRef CAS.
- M. Tsuji, Core–shell particles, in Syntheses and Application of Metallic Nanoparticles, ed. T. Yonezawa, CMC, Tokyo, 2009, pp. 166–177 (in Japanese) Search PubMed.
- K. J. Major, C. De and S. O. Obare, Plasmonics, 2009, 4, 61–78 CrossRef.
- M. Tsuji, S. Hikino, Y. Sano and M. Horigome, Chem. Lett., 2009, 38, 518–519 CrossRef CAS.
- M. Tsuji, S. Hikino, R. Tanabe, M. Matsunaga and Y. Sano, CrystEngComm, 2010, 12 10.1039/c0ce00064g.
- H. T. Zhang, J. Ding, G. M. Chow, M. Ran and J. B. Yi, Chem. Mater., 2009, 21, 5222–5228 CrossRef CAS.
- J. H. Hodak, A. Henglein, M. Giersig and G. V. Hartland, J. Phys. Chem. B, 2000, 104, 11708–11718 CrossRef CAS.
- T. Shibata, B. A. Bunker, Z.-Y. Zhang, D. Meisel, C. F. Vardeman and J. D. Gezelter, J. Am. Chem. Soc., 2002, 124, 11989–11996 CrossRef CAS.
- M. Tsuji, N. Miyamae, K. Matsumoto, S. Hikino and T. Tsuji, Chem. Lett., 2005, 34, 1518–1519 CrossRef CAS.
- S. Xu, B. Zhao, W. Xu and Y. Fan, Colloids Surf., A, 2005, 257–258, 313–317 CrossRef CAS.
- J. H. Liu, A. Q. Wang, Y. S. Chi, H. P. Lin and C. Y. Mou, J. Phys. Chem. B, 2005, 109, 40–43 CrossRef CAS.
- M. Tsuji, N. Miyamae, S. Lim, K. Kimura, X. Zhang, S. Hikino and M. Nishio, Cryst. Growth Des., 2006, 6, 1801–1807 CrossRef CAS.
- S. P. Chandran, J. Ghatak, P. V. Satyam and M. Sastry, J. Colloid Interface Sci., 2007, 312, 498–505 CrossRef CAS.
- M. Tsuji, R. Matsuo, P. Jiang, N. Miyamae, D. Ueyama, M. Nishio, S. Hikino, H. Kumagae, K. S. N. Kamarudin and X.-L. Tang, Cryst. Growth Des., 2008, 8, 2528–2536 CrossRef CAS.
- M. Tsuji, M. Nishio, P. Jiang, N. Miyamae, S. Lim, K. Matsumoto, D. Ueyama and X. Tang, Colloids Surf., A, 2008, 317, 247–253 CrossRef CAS.
- F.-R. Fan, D.-Y. Liu, Y.-F. Wu, S. Duan, Z.-X. Xie, Z.-Y. Jiang and Z.-Q. Tian, J. Am. Chem. Soc., 2008, 130, 6949–6951 CrossRef CAS.
- C. M. Gonzalez, Y. Liu and J. C. Scaiano, J. Phys. Chem. C, 2009, 113, 11861–11867 CrossRef CAS.
- M. Tsuji, M. Ogino, M. Matsunaga, N. Miyamae, R. Matsuo, M. Nishio and Md. Jahangir Alam, Cryst. Growth Des., 2010, 10, 4085 CrossRef CAS.
- X.-L. Tang, P. Jiang, G.-L. Ge, M. Tsuji, S.-S. Xie and Y.-J. Guo, Langmuir, 2008, 24, 1763–1768 CrossRef CAS.
- M. Tsuji, S. Hikino and R. Tanabe, Chem. Lett., 2010, 39, 334–336 CrossRef CAS.
- M. J. Alam, M. Tsuji and M. Matsunaga, Bull. Chem. Soc. Jpn., 2010, 83, 92–100 CrossRef CAS.
- M. Tsuji, Y. Maeda, S. Hikino, H. Kumagae, M. Matsunaga, X.-L. Tang, R. Matsuo, M. Ogino and P. Jiang, Cryst. Growth Des., 2009, 9, 4700–4705 CrossRef CAS.
- M. Tsuji, M. Ogino, R. Matsuo, H. Kumagae, S. Hikino, T. Kim and S. H. Yoon, Cryst. Growth Des., 2010, 10, 296–301 CrossRef CAS.
- Ph. Buffat and J. P. Borel, Phys. Rev. A: At., Mol., Opt. Phys., 1976, 13, 2287–2298 CrossRef CAS.
- B. J. Wiley, S. H. Im, Z.-Y. Li, J. M. McLellan, A. Siekkinen and Y. Xia, J. Phys. Chem. B, 2006, 110, 15666–15675 CrossRef CAS.
- M. Hu, J. Chen, Z.-Y. Li, L. Au, G. V. Hartland, X. Li, M. Marquez and Y. Xia, Chem. Soc. Rev., 2006, 35, 1084–1094 RSC.
| This journal is © The Royal Society of Chemistry 2011 |
