DOI:
10.1039/C0CE00014K
(Paper)
CrystEngComm, 2011,
13, 177-181
Rectangular-plate like organosilica microcrystals based on silylated β-diketone and lanthanide ions†
Received
22nd March 2010
, Accepted 7th June 2010
First published on 27th August 2010
Abstract
We report here the preparation of luminescent organosilica microcrystals with rectangular-plate morphology by a facile method of reacting silylated β-diketone with lanthanide ions under reflux without any structure-directing agent. Factors affecting the formation of the morphology including reaction temperature and the molar ratio of lanthanide/β-diketone are discussed. FTIR, SEM, XRD, 29Si solid-state NMR spectroscopy and photoluminescence spectroscopy were employed to characterize the obtained microcrystals. Photoluminescence studies show the luminescence of the material is of high monochromatic purity. The 5D0 quantum efficiency of Eu3+ ions was estimated based on the decay time Eu3 + 5D0 excited level and the emission spectrum of the organosilica microcrystals.
Introduction
Intensive efforts have been made to develop lanthanide-based organic–inorganic hybrid materials for about a decade because they have potential applications in optical amplifiers, optical waveguides, OLEDs, etc.1–8 In these materials, lanthanide complexes have been embedded into sol–gel-derived matrices either by simple doping methods or by covalent grafting methods in which lanthanide complexes are immobilized into the matrices via covalent bonds. Materials obtained by the latter method show improved chemical stability, homogenous dispersion of both components and a higher concentration of lanthanide complexes compared those prepared by the former method. However, nearly all of the materials are amorphous solids with irregular morphology or microspherical morphology. Nanostructuring and tailoring over morphology in these hybrid organic–inorganic materials are of significant interest for the design of multi-functional materials, which have been realized by the use of external organic surfactant template.9 Recently, a new and effective approach for a controlled synthesis of organosilicas with crystalline structure through the design and hydrolysis of trialkoxyorganosilanes exhibiting hydrogen-bonding interactions has been reported.10 Studies indicate that the morphology and structure of the materials depend not only on the organic components, but also on the conditions of sol–gel process. However, most of the crystalline organosilicas have limited intrinsic functionalities, the development of this method to prepare hybrid silicas with new functionalities and targeted properties should open a much wider entry for creating new advanced functional materials.11
In this work, we report the synthesis of luminescent organosilica microcrystals with rectangular-plate morphology and tunable photoluminescence emissions in the visible and near infrared spectral region by a very simple method of reacting silylated β-diketonate12 (TTA-Si, Fig.1) with lanthanide ions in the absence of structure-directing agent. To the best of our knowledge, this is the first report on luminescent rectangular-plate like organosilica microcrystals without any structure-directing agent.
 |
| | Fig. 1
TTA-Si. | |
Results and discussion
The luminescent rectangular plate-like organosilica was obtained by a simple method of refluxing TTA-Si and EuCl3·6H2O in the mixture of THF and ethanol. Hydrolysis and condensation of TTA-Si has occurred to form organosilica network as revealed by FT-IR spectrum shown in Fig. 2 where the absorption band at 961 cm−1 corresponding to SiOCH2CH3 groups (Fig. 2a) has disappeared and a broad band at 1200–1000 cm−1 ascribing to Si–O–Si asymmetric stretching vibration appears. The 29Si solid-state NMR spectrum in Fig. 3 shows a smaller shoulder resonance at −57.5 with a major resonance around 66 ppm that can be attributed to the T2 (C–Si(OSi)2(OH)) and T3 (C–Si(OSi)3) unit, respectively, indicating the polycondensation and the formation of the siloxane network under the experimental conditions. No SiO4 unit (Q type) is observed between 90 and 120 ppm.
 |
| | Fig. 2
FTIR spectra of TTA-Si (a) and the luminescent hybrid organosilica obtained by reacting Eu3+ ions with TTA-Si under reflux for 4 h (b). The molar ratio of TTA-Si to Eu3+ ions is 4. | |
 |
| | Fig. 3
29Si NMR solid-state spectrum of hybrid organosilica obtained by reacting Eu3+ ions with TTA-Si under reflux for 4 h. The molar ratio of TTA-Si to Eu3+ ions is 4. | |
Interestingly, electron microscopy images (SEM) of the sample (Fig. 4a) show the material exhibits rectangular-plate morphology with the size of 0.5–2.0 μm, which is scarcely seen in the sol–gel-derived organic–inorganic hybrid materials. The X-ray powder diffraction pattern (XRD) exhibits sharp peaks that indicate rectangular-plate with crystalline structure (Fig. 5a). Similar results are obtained if Eu3+ ions are replaced with Nd3+ ions. Lu et al.13 have obtained organosilica with similar morphology by surfactant-assisted hydrolysis and self-assembly of biphenyl bridged organosilane. They believed the surfactant P123 plays a very important role to get uniform square-plate morphology. Here our method is very simple and no external template is involved. Although the detailed mechanism of their formation is still unclear, it can be reasonably assumed that the cooperative effects of hydrogen-bonding interactions among the TTA-Si bearing urea groups (see Fig. 1) and the interactions between lanthanide ions and β-diketonates were accounted for the formation of rectangular plate microcrystals. It has been documented that bis(trialkoxysilylated) organic molecules bearing bis(urea) groups tend to self-assemble through hydrogen-bonding interactions involvingthe urea units.10 Furthermore, the β-diketonatone moieties can coordinate to Eu3+ ions through the oxygen during the sol–gel process.12bIn order to verify the role of lanthanide ions, we also investigate the effect of the molar ratio of TTA-Si to Eu3+ (from 4 to 1) on the morphology of the resulting materials. The organosilica with rectangular-plate shape can be obtained when the molar ratio is ranging from 4 to 2, however, microparticles with size of 200 nm are obtained if the molar ratio is kept to be one (ESI, Fig. S1).† In this work, our attention is focused on the organosilica microcrystals with molar ratio of TTA-Si/Eu3+ being 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 because TTA-Si can sensitize the luminescence of Eu3+ ions. For a better understanding the formation of rectangular plates, we analyzed the precipitate formed at initial stage (ca. 0.5 h). The SEM images are shown Fig. 4b, the morphological development of rectangular plates can be clearly seen by comparing Fig. 4b with Fig. 4a, which is much clear if closer observation of the area marked with white circle in Fig. 4b.
1 because TTA-Si can sensitize the luminescence of Eu3+ ions. For a better understanding the formation of rectangular plates, we analyzed the precipitate formed at initial stage (ca. 0.5 h). The SEM images are shown Fig. 4b, the morphological development of rectangular plates can be clearly seen by comparing Fig. 4b with Fig. 4a, which is much clear if closer observation of the area marked with white circle in Fig. 4b.
 |
| | Fig. 4
SEM images of luminescent organosilica obtained by reacting Eu3+ ions with TTA-Si under reflux for 4 h (a) and for 0.5 h (b). The molar ratio of TTA-Si to Eu3+ is 4. | |
 |
| | Fig. 5
XRD patterns of the luminescent hybrid organosilica obtained by reacting Eu3+ ions with TTA-Si under reflux (a) and at room temperature (b) for 4 h. The molar ratio of TTA-Si to Eu3+ ions is 4. | |
The reaction temperature plays a vital role in the development of the rectangular plates. Reaction of TTA-Si with Eu3+ ions at room temperature leads to spherical particles of 50–500 nm in diameter rather than rectangular plate, as shown in Fig. 6. XRD pattern confirms that the particle is amorphous (Fig. 5b).
 |
| | Fig. 6
SEM image of the luminescent hybrid organosilica obtained by reacting Eu3+ ions with TTA-Si at room temperature for 4 h. The molar ratio of TTA-Si to Eu3+ ions is 4. | |
The material shows an intense red photoluminescence upon irradiation with UV light (Fig. 7 inset). As shown in Fig. 7, the excitation spectrum displays a broad excitation band and no intra-4f6 transitions can be observed. The broad band could be due to the π → π* transitions of the TTA moieties. The absence of intra-4f6 transitions in the excitation spectrum indicates that an effect energy transfer occurs from the ligands to the central Eu3+ ions. Excitation of the ligands (340 nm) leads to sharp emission peaks arising from transitions between 5D0 → 7FJ crystal-field components (J = 0, 1, 2, 3, 4) with the hypersensitive transition 5D0 → 7F2 as the most prominent line, suggesting that the europium(III) ion sites are indeed without a center of inversion.14 The 5D0 → 7F2 transition is a typical electric dipole transition and strongly varies with the local environment of Eu3+ ions. The typical red color of europium emission is mostly attributed to the strongest transition 5D0 → 7F2 centered at 612 nm. The 5D0 → 7F1 line is a parity-allowed magnetic dipole transition and is to a large extent independent of the local symmetry of the Eu3+ ions. If we define the branching ratio as the contribution (in %) of a given line to the total luminescence intensity of the whole spectrum and the sum of the branching ratio is 100%. 5D0 → 7F2 line contributes to 86% of the luminescence output.15 This indicates that the luminescence of the organosilica is of a high monochromatic purity. The decay curve is found to be mono-exponential and the luminescence decay time of the 5D0 level is 0.684 ± 0.002 ms (ESI, Fig. S2).† The data, coupled with the observation of only one major component in the 5D0 → 7F0 line indicate that only a single site in the luminescent organosilica. The quantum efficiency of the 5D0Eu3+ excited state and the number of water molecules (nw) coordinated to the first sphere of Eu3+ can be approximately estimated based on the emission spectrum and the decay time of the 5D0 excited level according to the produced procedure if an average index of refraction n equal to 1.5 was considered for the sample.16 The quantum efficiency of the 5D0 excited level is estimated to be 56.16% and all the water molecules (nw ≈ 0) have been repelled from the first coordination sphere of Eu3+. This means Eu3+ ions are well shielded from the TTA moieties which are immobilized on the organosilica network via covalent bonds.
 |
| | Fig. 7 Excitation (dot line) and emission (solid line) spectra of sample (TTA-Si/Eu3+ = 4). The excitation spectrum was obtained by monitoring the 5D0 → 7F2 emission at 612 nm, and the emission spectrum was obtained upon excitation at 340 nm. Both spectra were measured at r.t. in air. The inset shows the red-light emission under UV irradiation. | |
Eu3+ ions can be replaced by other lanthanide ions which emit in the near infrared region such as Nd3+ ions. The excitation and emission spectra are shown in Fig. 8. The excitation spectrum is similar to the organosilica containing Eu3+ ions, which could be ascribed to the absorption of the TTA moieties. This suggests an energy transfer occurs from the TTA groups to the Nd3+ ions. Excitation at the absorption of ligand at 340 nm leads to the characteristic emission of Nd3+, which consists of three sharp bands centered at 883 nm, 1067 nm, and 1344 nm, corresponding to the f–f transitions of 4F3/2 (emitting level) → 4I9/2, 4F3/2 → 4I11/2 and 4F3/2 → 4I13/2, respectively.
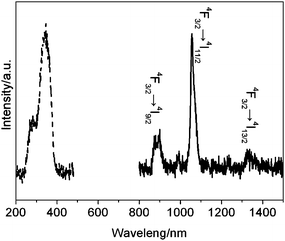 |
| | Fig. 8 Excitation (dot line) and emission (solid line) spectra of sample (TTA-Si/Nd3+ = 4). The excitation spectrum was obtained by monitoring the 4F3/2 → 4I11/2 emission at 1067 nm, and the emission spectrum was obtained upon excitation at 340 nm. Both spectra were measured at r.t. in air. | |
Conclusions
In summary, we have developed a very simple approach to synthesize highly luminescent hybrid organosilica microcrystals with tunable shape and luminescence properties. The molar ratio of lanthanide ions and TTA-Si as well as the reaction temperature are critical in obtaining the rectangular plate like morphology. The morphology and structure of hybrid organosilica can be finely tuned by changing the reaction temperature, by changing the molar ratio of TTA-Si and Eu3+ ions. The luminescence of the organosilica is of a high monochromatic purity in red spectral region and in the near infrared region depending on lanthanide ions involved. Due to the versatility of the silylated sensitizer and the lanthanide(III) ions, luminescence organosilica with various morphology and emission color can be obtained by this facile method.
Experimental
Materials
All solvents and reagents were used as received without further purification: EuCl3·6H2O, 2-thenoyltrifluoroacetone (TTA) and 3-isocyanatepropyltriethoxysilane were purchased from Aldrich. TTA-Si was synthesized and characterized according to the published procedure.12
Preparation of the materials
In a typical synthesis, to 0.5 mol L−1 of TTA-Si in THF, an appropriate amount of EuCl3 (or NdCl3) in ethanol was added and several pieces of quartz plates were immersed into the mixture to collect the samples, refluxing the mixture for 4–6 h led to a precipitate deposited on the quartz plates.
Characterizations
Infrared (IR) spectra were obtained on a Bruker Vector 22 spectrometer using KBr pellets for solid sample, from 400–4000 cm−1 at a resolution of 4 cm−1 (16 scans collected), about 2 mg of each compound was mixed with potassium bromide (Merck, spectroscopic grade) finely ground and pressed into pellets. 29Si solid-state NMR spectrum was obtained from a Varian InfintyPlus300. SEM images were obtained from a FE-SEM (Hitachi S-4300) at an acceleration voltage of 10 kV. X-Ray diffraction data were recorded on a Bruker D8 diffractometer with Cu-Kα radiation.
The steady-state luminescence spectra and the lifetime measurements were measured on an Edinburgh Instruments FS920P near-infrared spectrometer, with a 450 W xenon lamp as the steady-state excitation source, a double excitation monochromator (1800 lines mm−1), an emission monochromator (600 lines mm−1), a semiconductor cooled Hamamatsu RMP928 photomultiplier tube, and a liquid nitrogen cooled Hamamatsu R5509-72 near infrared photomultiplier tube.
Acknowledgements
This work is financially supported by the Key Project of Chinese Ministry of Education (208016), Program for New Century Excellent Talents in University (NCET-09-0113), the Scientific Program launched in 2008 by Hebei province (08965110D), National Natural Science Foundation of China (no. 20871040, 20873069, 20901022), Tianjin Natural Science Foundation (09JCYBJC05700), Hebei Natural Science Foundation (B2009000013) and Hebei Province Natural Science Foundation for Distinguished young Scholar (no. B2010000034).
References
- L. D. Carlos, R. A. S. Ferreira, V. de Zea Bermudez and S. J. L. Ribeiro, Adv. Mater., 2009, 21, 509–534 CrossRef CAS.
- K. Binnermans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS.
- P. Escribano, B. Julian-Lopez, J. Planelles-Arago, E. Cordoncillo, B. Viana and C. Sanchez, J. Mater. Chem., 2008, 18, 23–40 RSC.
-
(a) J. Feng, L. Zhou, S. Y. Song, Z. F. Li, W. Q. Fan, L. N. Sun, Y. N. Yu and H. J. Zhang, Dalton Trans., 2009, 6593–6598 RSC;
(b) X. M. Guo, H. D. Guo, L. S. Fu, L. D. Carlos, R. A. S. Ferreira, L. N. Sun, R. P. Deng and H. J. Zhang, J. Phys. Chem. C, 2009, 113, 12538–12545 CrossRef CAS.
-
(a) H. R. Li, N. N. Lin, Y. G. Wang, Y. Feng, Q. Y. Gan, H. J. Zhang, Q. L. Dong and Y. H. Chen, Eur. J. Inorg. Chem., 2009, 519–523 CrossRef CAS;
(b) N. N. Lin, H. R. Li, Y. G. Wang, Y. Feng, D. S. Qin, Q. Y. Gan and S. D. Chen, Eur. J. Inorg. Chem., 2008, 4781–4785 CrossRef CAS;
(c) H. R. Li, P. Liu, Y. G. Wang, L. Zhang, J. B. Yu, H. J. Zhang, B. Y. Liu and U. Schubert, J. Phys. Chem. C, 2009, 113, 3945–3949 CrossRef CAS;
(d) P. Liu, H. R. Li, Y. G. Wang, B. Y. Liu, W. J. Zhang, Y. J. Wang, W. D. Yan, H. J. Zhang and U. Schubert, J. Mater. Chem., 2008, 18, 735–737 RSC;
(e) Y. Feng, H. R. Li, Q. Y. Gan, B. Y. Liu and H. J. Zhang, J. Mater. Chem., 2010, 20, 972–975 RSC.
-
(a) B. Yan, Q. M. Wang and D. J. Ma, Inorg. Chem., 2009, 48, 36–44 CrossRef CAS;
(b) X. F. Qiao and B. Yan, J. Phys. Chem. B, 2008, 112, 14742–14750 CrossRef CAS.
- D. Dong, S. Jiang, Y. Men, X. Ji and B. Jiang, Adv. Mater., 2000, 12, 646–649 CrossRef.
- A. C. Franville, D. Zambon and R. Mahiou, Chem. Mater., 2000, 12, 428–435 CrossRef CAS.
-
(a) A. Sayari and S. Hamoudi, Chem. Mater., 2001, 13, 3151–3168 CrossRef CAS;
(b) M. P. Kapoor, Q. Yang and S. Inagaki, J. Am. Chem. Soc., 2002, 124, 15176–15177 CrossRef CAS.
-
(a) J. J. E. Moreau, L. Vellutini, M. Wong Chi Man, C. Bied, J. L. Bantignies, P. Dieudonné and J. L. Sauvajol, J. Am. Chem. Soc., 2001, 123, 7957–7958 CrossRef CAS;
(b) J. J. E. Moreau, L. Veuntini, M. Wong Chi Man, C. Bied, P. Dieudonne, J.-L. Bantignies and J.-L. Sauvajol, Chem.–Eur. J., 2005, 11, 1527–1537 CrossRef CAS;
(c) J. J. E. Moreau, B. P. Pichon, M. Wong Chi Man, C. Bied, H. Pritzkow, J.-L. Bantignies, P. Pieudonné and J.-L. Sauvajol, Angew. Chem., Int. Ed., 2004, 43, 203–206 CrossRef CAS; B. Menaa, M. Takahashi, P. Innocenzi and T. Yoko, Chem. Mater., 2007, 19, 1946–1953 CrossRef CAS.
-
(a) S. S. Nobre, C. D. S. Brites, R. A. S. Ferreira, V. de Zea Bermudez, C. Carcel, J. J. E. Moreau, J. Rocha, M. Wong Chi Man and L. D. Carlos, J. Mater. Chem., 2008, 18, 4172–4182 RSC;
(b) N. Liu, K. Yu, B. Smarsly, D. R. Dunphy, Y.-B. Jiang and C. J. Brinker, J. Am. Chem. Soc., 2002, 124, 14540–14541 CrossRef CAS;
(c) N. Liu, D. R. Dunphy, M. A. Rodriguez, S. Singer and C. J. Brinker, Chem. Commun., 2003, 1144–1145 RSC;
(d) L. Yang, H. S. Peng, K. Huang, J. T. Mague, H. X. Li and Y. F. Lu, Adv. Funct. Mater., 2008, 18, 1526–1535 CrossRef CAS.
-
(a) B. Yan, Y. Li and B. Zhou, Microporous Mesoporous Mater., 2009, 120, 317–324 CrossRef CAS;
(b) X. F. Qiao and B. Yan, Inorg. Chem., 2009, 48, 4714–4723 CrossRef CAS.
- J. B. Pang, L. Yang, D. A. Loy, H. S. Peng, H. S. Ashbaugh, J. Mague, C. J. Brinker and Y. F. Lu, Chem. Commun., 2006, 1545–1547 RSC.
- P. Lenaerts, K. Driesen, R. V. Deun and K. Binnemans, Chem. Mater., 2005, 17, 2148–2154 CrossRef CAS.
- K. Lunstroot, K. Driesen, P. Nockemann, C. Görller-Walrand, K. Binnemans, S. Bellayer, J. Le Bideau and A. Vioux, Chem. Mater., 2006, 18, 5711–5715 CrossRef CAS.
- M. Fernandes, V. de Zea Bermudez, R. A. Sá Ferreira, L. D. Carlos, A. Charas, J. Morgado, M. M. Silva and M. J. Smith, Chem. Mater., 2007, 19, 3892–3901 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM image of the luminescent hybrid organosilica, and decay curve of luminescent organosilica with rectangular-plate morphology. See DOI: 10.1039/c0ce00014k |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 because TTA-Si can sensitize the luminescence of Eu3+ ions. For a better understanding the formation of rectangular plates, we analyzed the precipitate formed at initial stage (ca. 0.5 h). The SEM images are shown Fig. 4b, the morphological development of rectangular plates can be clearly seen by comparing Fig. 4b with Fig. 4a, which is much clear if closer observation of the area marked with white circle in Fig. 4b.
1 because TTA-Si can sensitize the luminescence of Eu3+ ions. For a better understanding the formation of rectangular plates, we analyzed the precipitate formed at initial stage (ca. 0.5 h). The SEM images are shown Fig. 4b, the morphological development of rectangular plates can be clearly seen by comparing Fig. 4b with Fig. 4a, which is much clear if closer observation of the area marked with white circle in Fig. 4b.




