Solvothermal preparation and visible photocatalytic activity of polycrystalline β-In2S3 nanotubes†
Guodong
Liu
,
Xiuling
Jiao
,
Zhenhua
Qin
and
Dairong
Chen
*
Key Laboratory for Special Functional Aggregate Materials of Education Ministry, School of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P. R. China. E-mail: cdr@sdu.edu.cn
First published on 27th August 2010
Abstract
Ultra-thin tetragonal β-In2S3 nanotubes with the outer diameter of 10–20 nm, wall thickness of ca. 2.0 nm and length more than 1.0 μm have been successfully synthesized for the first time via a simple solution route. The polycrystalline nanotube is composed of nanocrystals with a size of 2.0 nm. The formation mechanism of the nanotubes was proposed, which went through the hydrolysis of In3+ cations, the decomposition of lauryl mercaptan and then the heterogeneous nucleation and growth of In2S3. The as-obtained β-In2S3 nanotubes show high photocatalytic performance on the decomposition of Rhodamine B (RhB) under solar irradiation.
1. Introduction
As an n-type III–VI semiconductor, body-centered tetragonal In2S3 (β-In2S3) with a band gap of 2.0–2.3 eV has received increasing attention due to its photoconductive and luminescence properties, which has already inspired applications in the preparation of green and red phosphors in the manufacture of picture tubes for color televisions, cells, and heterojunction for use in photovoltaic electric generators.1Comparing to its bulk solids, the β-In2S3 nanostructures have attracted much interest because of their unique chemical, physical, and optical properties. In the past decade, the β-In2S3 nanostructures with various controlled-shapes have been fabricated,2 and many preparative routes have been developed.3 Particularly, the syntheses of one dimensional (1D) nanostructures have been an interesting research topic for their unique electronic and optical properties. For example, the β-In2S3 nanorods have significant absorption in the early visible region and exhibit peculiar optoelectronic properties for quantum confinement, may be suitable alternatives to II–VI semiconductor nanocrystals in optoelectronic devices.4 To date, many 1D In2S3 nanostructures such as nanowires,5nanorods,6 and nanobelts7 have been prepared. For their syntheses, the methods might be classified into three categories: (1) CVD (chemical vapour deposition) by using organometallic as single-source8 or indium metal and H2S as precursors,9 (2) hard template route in which the silicon nanotubes,10 or AAO (Anodic aluminium oxide) membrane,11 or silica SBA-1512 as the templates to direct the growth of nanowires/nanorods, and (3) soft template method in which the surfactant molecules directing the crystal growth through the coating of the surfactant molecules on different crystal facets.13 However, the development of novel route to 1D In2S3 nanostructures still remain a great challenge, to the best of our knowledge, there is no report on the In2S3 nanotubes.
Single crystalline WS2 nanotubes were firstly synthesized in the 1990s, since then a series of inorganic nanotubular materials with polycrystalline structures have been prepared, which brought about major advances in chemistry and nanotechnology and pointed to numerous applications,14 and were likely to play critical roles in the improvement of the efficiencies of various electronic, optoelectronic, magnetic and other devices based on single nanoparticles or their composites. For the preparation of polycrystalline inorganic nanotubes, although many methods such as the deposition in a porous template (anodic alumina, zeolites, etc.) via precursor infiltration/wetting, electrochemical decoration, and ALD (atomic layer deposition) followed by template removal have been developed,15 to exploit new synthetic approaches to new kinds of metal sulfide nanotubes is still one of research focuses in the field of inorganic nanomaterials.
Herein, a simple solvothermal route to the polycrystalline β-In2S3 nanotubes by using In(NO3)3 as indium source, lauryl mercaptan as sulfur source and pyridine as solvent was introduced. In the present research, a possible growth mechanism based on the hydrolysis of In3+ cations and the reaction between the In3+ cations and S2− anions releasing from the decomposition of lauryl mercaptan was proposed. This synthesis shows some advantages on the preparation of nanomaterials such as amenable to scale up, simpleness and moderate, and the resultant nanotubes with the high aspect ratio. Furthermore, the product exhibits the wide UV-vis absorption region and high photocatalytic activity under solar irradiation.
2. Experimental
2.1 Synthesis
In a typical synthesis, into 10.0 mL pyridine 0.065 g (0.20 mmol) In(NO3)3·4H2O was added to form a transparent solution. After stirring for 0.5 h, 0.50 mL (2.0 mmol) lauryl mercaptan was added into the solution drop by drop, and the solution was continuously stirred for 0.5 h. Then the mixture was transferred into a 15 mL stainless-steel autoclave with the Teflon line. The autoclave was heated at 240 °C for 24 h and cooled to room temperature naturally. The resulting yellow precipitate was separated by centrifugation and washed repeatedly with ethanol under ultrasonic condition for several times and dried in air at 60 °C for 2 h.2.2 Characterization
X-Ray diffraction (XRD) patterns of the samples were recorded on an X-ray diffractometer (Rigaku D/Max 2200PC) with a graphite monochromator and Cu-Kα radiation (λ = 0.15418 nm) in the 2θ range of 10–80° at room temperature while the tube voltage and electric current were held at 40 kV and 20 mA. The morphology and microstructure of the products were determined by field emission scanning electron microscopy (FE-SEM, JSM-6700F), transmission electron microscopy (TEM, JEM-100CXII) with an accelerating voltage of 80 kV and high-resolution TEM (HR-TEM, GEOL-2010) with an accelerating voltage of 200 kV. Thermal gravimetric (TG) analysis was conducted on a thermal analyzer (TGA/SDTA, 851e METTLER) with the heating rate of 10.0 °C min−1 under an air flow of 20.0 mL min−1. The Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 5DX-FTIR spectrometer using the KBr pellet method in the range of 400–4000 cm−1. The elemental analysis was conducted on Vario EI III element analyzer. UV-vis spectrometer (Perkin-Elmer, Lambda-35) was used to characterize the UV-vis absorption spectra of the products. The Branauer-Emmet-Teller (BET) surface area was measured by nitrogen (N2) adsorption–desorption at 77 K using a QuadraSorb SI surface area analyzer after degassing the samples at 100 °C for 24 h.Visible photocatalytic activity was investigated through the decomposition of the Rhodamine B (RhB) in aqueous solution and the quartz beaker was used as the photoreactor vessel. The quartz beaker was exposed to bright sunlight on fine day in November with the temperature being kept at ca.15 °C, and the intensity of sunlight is about 1000 W m−2. Under vigorously stirring, 30.0 mg photocatalyst was added into the aqueous solution of RhB (initial concentration: 1.0 × 10−5 mol dm−3, 100.0 mL) and then the suspension was irradiated by sunlight for various durations. The characteristic absorption of RhB at 553 nm was chosen to monitor the photocatalytic degradation process. Samples were collected every 30 min to measure the RhB degradation by UV-vis spectra.
3. Results and discussion
Fig. 1 shows the XRD pattern of the product. All the reflections on the XRD pattern could be indexed to the body-centered tetragonal β-In2S3 structure (JCPDS no. 25-0390, a = 7.619 Å, c = 32.329 Å). No characteristic peaks were observed for the other impurities such as In2O3, InS and In(OH)3. The low intensity and broad shape of the diffraction peaks in XRD pattern should be due to the small crystalline size of the product.16,17 Based on the breadth of the (400) reflection, the crystalline size of the product was 4.8 nm calculated from the Scherrer equation. The EDS analysis gave the molar ratio In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, agreeing well with the stoichiometric proportion in β-In2S3 (Fig. 2d).
1.5, agreeing well with the stoichiometric proportion in β-In2S3 (Fig. 2d).
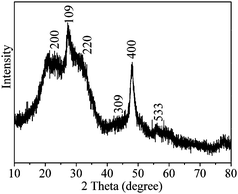 | ||
| Fig. 1 XRD pattern of the product. | ||
TEM image reveals the tubular structure of the obtained product with the outer diameter of 10–20 nm, wall thickness of ca. 2 nm and length more than 1.0 μm (Fig. 2b). Both of the TEM and FE-SEM images indicate the smooth surface and close end of the nanotubes. The corresponding HR-TEM image (Fig. 2c) shows that the nanotubes exhibit well-defined but discontinuous lattice fringes, indicating their polycrystalline nature. The composed nanoparticles have the size of ca. 2.0 nm, the difference from that based on the XRD pattern might be due to the oriented arrangement between some of the nanocrystals.
The FT-IR spectrum shows that the as-prepared β-In2S3 nanotubes have the bands around 2956, 2922 and 2851 cm−1, which can be ascribed to the C–H vibrations of pyridine. Obviously, the weak absorptions at 1576 and 1524 cm−1 should be attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations from the pyridine ring.18 All the absorptions and identified functional groups (ESI, Table S1)† demonstrate that the surface species of the nanocrystals are mainly pyridine molecules and a few of absorbed water.19Elemental analysis combined the TG analysis gave the contents of C, H, N, S, O, and In of 3.196, 1.023, 0.841, 27.56, 1.13, and 66.25 wt%, respectively. Then, it can be concluded that there are a few of absorbed water, and pyridine molecules on the surface of the nanotubes. On the TG curve, the weight loss occurred from room temperature to ca. 290 °C might be due to the removal of the surface absorbed water and pyridine molecules, and that from 290 °C to 385 °C resulted from the removal of the water from the surface hydroxyls. The weight loss from 385 °C to 800 °C should be ascribed to the conversion of In2S3 to In2O3, which is about 14.90% and is consistent with the theoretical value (14.74%).
N vibrations from the pyridine ring.18 All the absorptions and identified functional groups (ESI, Table S1)† demonstrate that the surface species of the nanocrystals are mainly pyridine molecules and a few of absorbed water.19Elemental analysis combined the TG analysis gave the contents of C, H, N, S, O, and In of 3.196, 1.023, 0.841, 27.56, 1.13, and 66.25 wt%, respectively. Then, it can be concluded that there are a few of absorbed water, and pyridine molecules on the surface of the nanotubes. On the TG curve, the weight loss occurred from room temperature to ca. 290 °C might be due to the removal of the surface absorbed water and pyridine molecules, and that from 290 °C to 385 °C resulted from the removal of the water from the surface hydroxyls. The weight loss from 385 °C to 800 °C should be ascribed to the conversion of In2S3 to In2O3, which is about 14.90% and is consistent with the theoretical value (14.74%).
Time-dependent experiments were conducted to investigate the formation process of the nanotubes. Before solvothermal reaction, the powders were obtained by vaporizing the solvent of the solution, washing with ethanol for several times and drying at 60 °C for 2 h, which are long nanofibers from the TEM observation (ESI, Fig. S1).† Its XRD pattern can not be indexed to any crystalline compound in the JCPDS database, but the elemental (ESI, Table S2)† and TG analyses (ESI, Fig. S2)† gave the molar ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In = 35.42
In = 35.42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74.97
74.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.026: 2.94
0.026: 2.94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.78
0.78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating the indium sulfide precursor was indium thiolate with a few of In–S replaced by In-OH due to the hydrolysis (In(C12H25S)2.94(OH)0.06), which is soluble in pyridine.
1, indicating the indium sulfide precursor was indium thiolate with a few of In–S replaced by In-OH due to the hydrolysis (In(C12H25S)2.94(OH)0.06), which is soluble in pyridine.
After solvothermal treatment for 0.5 h, the spheres with size of 10–150 nm appeared, whose size increased to ca. 1.0 μm as prolonging the reaction time to 1.0 h (Fig. 3a). Detailed FE-SEM observation revealed the relative smooth surface of the dense spheres (Fig. 3b). With the change of the morphology, the molar composition of the sample also changed to C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In = 1.565
In = 1.565![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.200
4.200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.644
0.644![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.832
0.832![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.39
3.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at the reaction time of 1 h (ESI, Table S2).† At the same time, with the disappearance of the vibrations from thiolate, a wide absorption band at 800–1500 cm−1 appeared in the IR spectrum, which could be attributed to the hydroxyls connected with In3+ (Fig. 5B, curve a). In addition, the absorption at 3280 cm−1 indicates the existence of N–H vibration of pyridine connected with surface hydroxyls (Fig. 5B).18 These results indicate that the hydrolysis reaction of In3+ has occurred during the first hour of the solvothermal process in the alkaline solution and indium hydroxide with some pyridine molecules was formed.
1 at the reaction time of 1 h (ESI, Table S2).† At the same time, with the disappearance of the vibrations from thiolate, a wide absorption band at 800–1500 cm−1 appeared in the IR spectrum, which could be attributed to the hydroxyls connected with In3+ (Fig. 5B, curve a). In addition, the absorption at 3280 cm−1 indicates the existence of N–H vibration of pyridine connected with surface hydroxyls (Fig. 5B).18 These results indicate that the hydrolysis reaction of In3+ has occurred during the first hour of the solvothermal process in the alkaline solution and indium hydroxide with some pyridine molecules was formed.
 | ||
| Fig. 3 TEM, FE-SEM images of products prepared by solvothermal treatments for (a, b) 1 h, (c, d) 3 h, (e) 6 h, (f) 12 h, (f) 18 h, and (g) 24 h. | ||
 | ||
| Fig. 4 FT-IR spectra (a) and TG curve (b) of obtained β-In2S3 nanotubes. | ||
 | ||
| Fig. 5 XRD patterns (A) and IR spectra (B) of the samples prepared by solvothermal treatments for (a) 1 h, (b) 1.5 h, (c) 3 h, (d) 6 h, (e) 12 h, and (f) 24 h. | ||
Then the surface of the microspheres grew coarse (ESI, Fig. S3)† and nanosheets were formed on it which gradually enlarged with the time prolonging, and a small amount of nanoparticles were also observed with the microspheres (Fig. 3c). The curled nanosheets were observed at the reaction time of 6 h due to the large surface tension (the framed part in Fig. 3e and the enlarged one in the inset). At this stage, the hydroxyls connected with In3+ reduced from the IR spectrum, and the contents of In and S gradually increased with the C, H, N, and O contents decreasing (ESI, Table S2).† The XRD patterns (Fig. 5A) showed that β-In2S3 started to form, whose XRD reflections gradually enhanced with the reaction proceeding. After solvothermal treatment for 12 h, the microspheres changed very fluffy and long nanotubes were formed on the spheres' surface (Fig. 3f), and the molar ratio C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In = 0.83
In = 0.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.80
1.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10
0.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.49
1.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.28
0.28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was obtained based on the elemental analyses and TG curves (ESI, Table S2 and Fig. S2).† Combined with the IR spectrum, it could be concluded that In2S3 was formed with some absorbed water and organics. At last, the long β-In2S3 nanotubes were formed with only a few of small nanosheets remained at the center of the original spheres (Fig. 3h). XRD pattern, elemental analysis and IR spectrum revealed that the product was phase pure β-In2S3 with a small amount of pyridine molecules absorbed on the particle surface.
1 was obtained based on the elemental analyses and TG curves (ESI, Table S2 and Fig. S2).† Combined with the IR spectrum, it could be concluded that In2S3 was formed with some absorbed water and organics. At last, the long β-In2S3 nanotubes were formed with only a few of small nanosheets remained at the center of the original spheres (Fig. 3h). XRD pattern, elemental analysis and IR spectrum revealed that the product was phase pure β-In2S3 with a small amount of pyridine molecules absorbed on the particle surface.
Based on above experimental results, the formation mechanism of the β-In2S3 nanotubes is illustrated in Fig. 6. At first, the In3+ cations hydrolyzed and some pyridine molecules coordinated to In3+ to form the hydroxide with some coordinated organics under solvothermal conditions, which aggregated to microspheres. Then, the In2S3 nanosheets formed based on the aggregation of the nanoparticles, and the high surface energy of the nanosheets further resulted in their curling, and nanotubes were formed. It is known that thiol can decompose to release H2S at high temperature.20 Herein, the supernatant solution of the suspension after solvothermal treated for 24 h was detected using lead acetate test paper, and the change of the paper color revealed that there are S2− anions in the system. Therefore, it can be concluded that lauryl mercaptan decomposed under the solvothermal conditions to release S2−, and S2− further reacted with In3+ in the system to form In2S3, and then the heterogeneous nucleation occurred on the surface of the microspheres. Because of the slow decomposition rate of lauryl mercaptan under the present conditions and relative fast growth rate of In2S3, the formation of In2S3 was very slow which depended on the decomposition of lauryl mercaptan. With the time prolonging, the microspheres gradually dissolved and In2S3 increased due to the small solubility of In2S3 comparing to indium hydroxide.21 At last, the β-In2S3 nanotubes were formed.
 | ||
| Fig. 6 Schematic illustration of the proposed growth mechanism for In2S3 nanotubes. | ||
Obviously, thiol is critical during the formation of indium sulfide. It provides the source of sulfur in a special form. It was also found from the further experiments that the molar ratio of thiol to In excess of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was necessary to ensure the formation of phase-pure In2S3, which was consistent with James's report.22 When stoichiometric thiol/In(NO3)3 = 3/2 was used, only the mixture of InS, In2O3, and In(OH)3 was obtained (ESI, Fig. S4).† It is speculated that low thiol concentration in the system can not provided enough S2− in the solvothermal reaction and then InS as well as hydroxide and oxide of indium were formed.
1 was necessary to ensure the formation of phase-pure In2S3, which was consistent with James's report.22 When stoichiometric thiol/In(NO3)3 = 3/2 was used, only the mixture of InS, In2O3, and In(OH)3 was obtained (ESI, Fig. S4).† It is speculated that low thiol concentration in the system can not provided enough S2− in the solvothermal reaction and then InS as well as hydroxide and oxide of indium were formed.
As a comparison, other sulfur sources, such as Na2S and thiourea, were also used to replace thiol in the same solvothermal system. Only irregular In2S3 nanoparticles with sizes respectively concentrated at ca. 30 nm and 45 nm were obtained, whose surface species is similar to the In2S3 nanotubes with a relative large amount of hydroxyls (ESI, Fig. S5 and S6).† In this state, the concentrations of S2− and In3+ are so large that rapid homogeneous nucleation occurred in the system, then the In2S3 nanoparticles were obtained.
As a promising semiconductive material, the UV-vis absorption of as-prepared β-In2S3 nanotubes was studied at room temperature. From the UV-vis absorption spectrum of the nanotubes shown in Fig. 7A, a strong peak around 264.0 nm was observed, whose band edge was 510.0 nm. As well known, the band gap (Eg) in bulk In2S3 is reported to be between 2.0 and 2.2 eV with the corresponding UV band from 620.0 nm to 550.0 nm.23 Obvious blue shift is observed comparing with the bulk In2S3 materials. The nanotubes exhibited the wall thickness about 2.0 nm, which was much smaller than the Bohr excition radius of In2S3 (33.8 nm),24 and the strong quantum confinement effect of the excitonic transition is expected for β-In2S3 nanotubes.
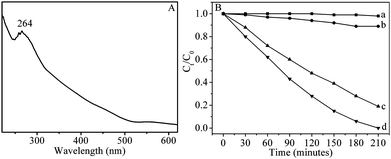 | ||
| Fig. 7 (A) UV-vis absorption spectrum of as-synthesized β-In2S3 nanotubes. (B) Photodegradation of RhB (a) without catalyst, with (b) P25, (c) In2S3 nanoparticles, and (d) In2S3 nanotubes. | ||
The In2S3 nanocrystals with strong absorption from the visible to UV region may be the good candidate for photocatalytic degradation of organic pollutants,25 thus the photocatalytic degradation of RhB with the as-prepared In2S3 nanotubes as photocatalyst was investigated, and the commercial Degussa P25 was used as the reference under the same conditions. The absorption peaks corresponding to RhB gradually diminished with the exposure time was extended, when the In2S3 nanotubes was used as the photocatalyst (ESI, Fig. S7a).† After irradiation by sunlight for 210 min, the characteristic absorption of RhB disappeared. It can be seen from Fig. 7B that the RhB hardly decomposed after irradiated by the sunlight without catalyst, and the In2S3 nanotubes show much better photocatalytic activities than commercial P25. Furthermore, to demonstrate the influence of the sample's morphology on its photocatalytic efficiency, the degradation process of RhB with the In2S3 nanoparticles shown in Figure S5b as photocatalyst was studied, indicating a higher catalytic activity of the nanotubes than that of the nanoparticles. Further experiments revealed that β-In2S3 nanotubes are stable during the catalytic process and can be repeatedly used for many times, more than 99% of the initial catalytic efficiency still remained even after the nanotubes were used for 6 times (ESI, Fig. S7b).† It is known that the factors including crystal size, surface area, crystallinity, particle size, and morphology can affect the photocatalytic activity of photocatalyst. N2 adsorption experiment gives the BET surface areas of 31.1, 72.0, and 50.4 m2 g−1 for In2S3 nanoparticles, In2S3 nanotubes, P25, and no adsorption of RhB on the In2S3 nanocrystal's surface is detected. Further experiments indicated that 30.0 mg nanotubes and 70.0 mg nanoparticles, which have the similar surface areas of 2.16 m2 g−1, showed different catalytic activities, and the In2S3 nanotubes displayed slight higher photocatalytic activity than nanoparticles. The higher photocatalytic activity of In2S3 nanocrystals comparing to commercial Degussa P25 might relate to their wide UV-vis absorption region (Figure S8) and several deep trap states or defects in their structure. Ultra-thin wall of nanotubes can reduce the time of photogenerated electron to migrate to the surface and slow down the recombination rate of photogenerated electron and hole. Furthermore, the nanotube's novel morphology might cause new defect sites different from the nanoparticles, which increased active sites and led to excellent catalytic performance. In addition, the presence of N in pyridine on the surface of nanotubes may hinder the interaction between the oxygen vacancies and electrons due to its partially occupied N 2p band, and then enhance the catalytic property.26
4. Conclusions
In summary, the polycrystalline β-In2S3 nanotubes with the diameter of 10–20 nm and length of 1–10 μm were synthesized via one-step solvothermal method using In(NO3)3·4H2O and lauryl mercaptan as indium source and sulfur source respectively. Under the solvothermal condition, the In3+ cations firstly hydrolyzed to form the indium hydroxide, and then S2− was released due to the pyrolysis of thiol which reacted with the free In3+ cations to form In2S3. The heterogenous nucleation and growth of In2S3 occurred with the pyrolysis of thiol and the dissolution of the indium hydroxide, and the In2S3 nanotubes were formed at last. The higher photocatalytic activity comparing to commercial Degussa P25 might be due to the small crystals in the In2S3 nanotubes,25 and novel structures.Acknowledgements
This work is supported by the Major State Basic Research Development Program of China (973 program, no. 2010CB933504) and the Excellent Youth Foundation of Shandong Scientific Committee (JQ200903).References
- L. Aldon, C. Branci, L. Ziegeler, J. Roth, P. Schaaf, H. Metzner, J. Olivier-Fourcade, J. J. C. umas and M. Uhrmacher, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 11303 CrossRef CAS; S. Choe, T. Bang, N. Kim, H. Kim, C. Lee, M. Jin, S. Oh and W. Kim, Semicond. Sci. Technol., 2001, 16, 98 CrossRef CAS; T. P. Niesen and M. R. DeGuire, J. Electroceram., 2001, 6, 169 CrossRef CAS; A. Timoumi, H. Bouzouita, M. Kanzari and B. Rezig, Eur. Phys. J.: Appl. Phys., 2006, 33, 77 CrossRef CAS; R. Lucena, I. Aguilera, P. Palacios, P. Wahnón and J. C. Conesa, Chem. Mater., 2008, 20, 5125 CrossRef CAS.
- W. Han, L. Yi, N. Zhao, A. Tang, M. Gao and Z. Tang, J. Am. Chem. Soc., 2008, 130, 13152 CrossRef CAS; L. Liu, H. Liu, H. Kou, Y. Wang, Z. Zhou, M. Ren, M. Ge and X. He, Cryst. Growth Des., 2009, 9, 113 CrossRef CAS; R. Lucena, I. Aguilera, P. Palacios, P. Wahnón and J. C. Conesa, Chem. Mater., 2008, 20, 5125 CrossRef CAS; Y. Xing, H. Zhang, S. Song, J. Feng, Y. Lei, L. Zhao and M. Li, Chem. Commun., 2008, 1476 RSC; L. Chen, Z. Zhang and W. Wang, J. Phys. Chem. C, 2008, 112, 4117 CrossRef CAS; Z. Li, X. Tao, Z. Wu, P. Zhang and Z. Zhang, Ultrason. Sonochem., 2009, 16, 221 CrossRef CAS; S. D. Naik, T. C. Jagadale, S. K. Apte, R. S. Sonawane, M. V. Kulkarni, S. I. Patil, S. B. Ogale and B. B. Kale, Chem. Phys. Lett., 2008, 452, 301 CrossRef CAS; H. Bai, L. Zhang and Y. Zhang, Mater. Lett., 2009, 63, 823 CrossRef CAS.
- S. Avivi, O. Palchik, V. Palchik, M. A. Slifkin, A. M. Weiss and A. Gedanken, Chem. Mater., 2001, 13, 2195 CrossRef CAS; S. Yu, L. Shu, Y. Wu, J. Yang, Y. Xie and Y. Qian, J. Am. Ceram. Soc., 2004, 82, 457 CrossRef; X. Cao, L. Gu, L. Zhuge, W. Qian, C. Zhao, X. Lan, W. Sheng and D. Yao, Colloids Surf., A, 2007, 297, 183 CrossRef CAS; F. Cao, W. Shi, R. Deng, S. Song, Y. Lei, S. Wang, S. Su and H. Zhang, Solid State Sci., 2010, 12, 39 CrossRef CAS; K. Xue, D. Chen and X. Jiao, Inorg. Chem., 2010, 49, 1191 CrossRef CAS.
- M. A. Franzman and R. L. Brutchey, Chem. Mater., 2009, 21, 1790 CrossRef CAS.
- A. Datta, G. Sinha, S. K. Panda and A. Patra, Cryst. Growth Des., 2009, 9, 427 CrossRef CAS.
- M. F. Cansizoglu, R. Engelken, H. Seo and T. Karabacak, ACS Nano, 2010, 4, 733 CrossRef CAS; W. Lee, S. Min, G. Cai, R. S. Mane, T. Ganesh, G. Koo, J. Chang, S. Baek, S. Lee and S. Han, Electrochim. Acta, 2008, 54, 714 CrossRef CAS.
- W. Du, J. Zhu, S. Li and X. Qian, Cryst. Growth Des., 2008, 8, 2130 CrossRef CAS.
- M. Afzaal, M. A. Malik and P. O'Brien, Chem. Commun., 2004, 334 RSC.
- R. Rao, H. Chandrasekaran, S. Gubbala, M. K. Sunkara, C. Daraio, S. Jin and A. M. Rao, J. Electron. Mater., 2006, 35, 941 CrossRef CAS.
- C. Liang, Y. Shimizu, T. Sasaki, H. Umehara and N. Koshizaki, J. Mater. Chem., 2004, 14, 248 RSC.
- J. Shi, C. Chen, Y. Lin, W. Hsu, Y. Chen and P. Wu, Nanoscale Res. Lett., 2009, 4, 1059 CrossRef CAS.
- X. Liu, B. Tian, C. Yu, B. Tu, Z. Liu, O. Terasaki and D. Zhao, Chem. Lett., 2003, 32, 824 CrossRef CAS.
- A. Datta, S. Gorai, D. Ganguli and S. Chaudhuri, Mater. Chem. Phys., 2007, 102, 195 CrossRef CAS.
- H. Fan, U. Gosele and M. Zacharias, Small, 2007, 3, 1660 CrossRef CAS; Y. Zhao and L. Jiang, Adv. Mater., 2009, 21, 3621 CrossRef CAS; R. Tenne1 and G. Seifert, Annu. Rev. Mater. Res., 2009, 39, 387 CrossRef CAS.
- Z. Tang and N. A. Kotov, Adv. Mater., 2005, 17, 951 CrossRef CAS; L. Zhao, T. Lu, M. Zacharias, J. Yu, J. Shen, H. Hofmeister, M. Steinhart and U. Göele, Adv. Mater., 2006, 18, 363 CrossRef CAS; H. Shin, D. Jeong, J. Lee, M. Sung and J. Kim, Adv. Mater., 2004, 16, 1197 CrossRef CAS.
- K. Park, K. Jang and S. Son, Angew. Chem., Int. Ed., 2006, 45, 4608 CrossRef CAS.
- Y. Xiong, Y. Xie, G. Du, X. Tian and Y. Qian, J. Solid State Chem., 2002, 166, 336 CrossRef CAS.
- X. Xing, S. Chen and S. Me, The Workable Index of IR Spectra (in Chinese). Tianjin Sci. Technol. Press, Tianjin 1992 Search PubMed.
- A. F. Lindmark and R. C. Fay, Inorg. Chem., 1983, 22, 2000 CrossRef CAS; S. Bhattacharya, N. Seth, D. K. Srivastava, V. D. Gupta, H. Nöth and M. Thomann-Albach, J. Chem. Soc., Dalton Trans., 1996, 2815 RSC.
- B. Yang, S. Tian and S. Zhao, Fuel Process. Technol., 2006, 87, 673 CrossRef CAS.
- R. T. Olsson, G. Salazar-Alvarez, M. S. Hedenqvist, U. W. Gedde, F. Lindberg and S. J. Savage, Chem. Mater., 2005, 17, 5109 CrossRef CAS.
- J. Tabernor, P. Christian and P. O'Brien, J. Mater. Chem., 2006, 16, 2082 RSC.
- D. K. Nagesha, X. Liang, A. A. Mamedov, G. Gainer, M. A. Eastman, M. Giersig, J. Song, T. Ni and N. A. Kotov, J. Phys. Chem. B, 2001, 105, 7490 CrossRef CAS.
- W. Chen, J. O. Bovin, A. G. Joly, S. Wang, F. Su and G. Li, J. Phys. Chem. B, 2004, 108, 11927 CrossRef CAS.
- Y. He, D. Li, G. Xiao, W. Chen, Y. Chen, M. Sun, H. Huang and X. Fu, J. Phys. Chem. C, 2009, 113, 5254 CrossRef CAS.
- J. Graciani, A. Nambu, J. Evans, J. Rodriguez and J. F. Sanz, J. Am. Chem. Soc., 2008, 130, 12056 CrossRef CAS; J. Graciani, L. J. Álvarez, J. A. Rodriguez and J. F. Sanz, J. Phys. Chem. C, 2008, 112, 2624 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: Assignment of the IR absorptions of the product, TEM images, elemental analysis data and TG curves of the samples obtained at different times, SEM images of the 1.5 h sample, TEM image and XRD pattern of the product obtained with the molar ratio thiol to In(NO3)3 of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, TEM images, XRD patterns, IR spectra and particle size distribution diagrams of In2S3 obtained using Na2S or thiourea as sulfur source, UV-vis absorption curves of the RhB solutions as a function of time in the presence of In2S3 nanotubes under exposure to sunlight, photocatalytic efficiency of the product as a function of recycling times. See DOI: 10.1039/c0ce00084a 2, TEM images, XRD patterns, IR spectra and particle size distribution diagrams of In2S3 obtained using Na2S or thiourea as sulfur source, UV-vis absorption curves of the RhB solutions as a function of time in the presence of In2S3 nanotubes under exposure to sunlight, photocatalytic efficiency of the product as a function of recycling times. See DOI: 10.1039/c0ce00084a |
| This journal is © The Royal Society of Chemistry 2011 |

