DOI:
10.1039/C0CE00309C
(Paper)
CrystEngComm, 2011,
13, 243-250
Using alcohols as alkylation reagents for 4-cyanopyridinium and N,N′-dialkyl-4,4′-bipyridinium and their one-dimensional iodoplumbates†
Received
16th June 2010
, Accepted 8th July 2010
First published on 3rd September 2010
Abstract
Solvothermal reactions of PbI2 with 4-cyanopyridine (4-cypy) or 4,4′-bipyridine (4,4′-bipy) and alcohols (ethanol, propanol, iso-propanol, benzyl alcohol, α,α′-dihydroxy-p-xylene) in the presence of PbI2, I2 and a trace amount of water in acetonitrile gave rise to a family of 1D iodoplumbate complexes of 4-cyanopyridinium and N,N′-dialkyl-4,4′-bipyridinium including {[EC]4[(Pb2I6)2]·2MeCN}n (EC+ = N-ethyl-4-cyanopyridium) (1), {[PC][PbI3]}n (PC+ = N-propyl-4-cyanopyridium) (2), {[iPC]3[(PbI3)(Pb2I6)]}n (iPC+ = N-isopropyl-4-cyanopyridium) (3), {[BzC][PbI3]}n (BzC+ = N-benzyl-4-cyanopyridium) (4), {[Cxy]2[(Pb2I6)2]}n (Cxy2+ = 1,4-bis(4-cyanopyridium)-xylene) (5), {[EV]1.5[Pb3I9]}n (EV2+ = N,N′-diethyl-4,4′-bipyridinium) (6), {[PV]1.5[Pb3I9]}n (PV2+ = N,N′-dipropyl-4,4′-bipyridinium) (7), and {[iPV]2[Pb4I12]}n (iPV2+ = N,N′-diisopropyl-4,4′-bipyridinium) (8). The resulting 4-cyanopyridinium and viologen cations were generated in situ via the cleavage of C–O bond of alcohols followed by alkylation of 4-cypy or 4,4′-bipy. X-Ray analysis revealed that compounds 1–8 contain one-dimensional anionic [PbI3]nn− (1–5, 8) and [Pb3I9]n3n− (6 and 7) chains that are enclosed into the different cationic channels formed from the 4-cyanopyridinium and N,N′-dialkyl-4,4′-bipyridinium. In addition, the optoelectronic and dielectric properties of 1–8 were also investigated.
Introduction
The alkylation of pyridyl groups is of importance because the resulting products, pyridinium cations, have many applications in advanced materials such as catalytic,1agrochemical,2surfactant,3 and optoelectronic materials.4 The traditional approach to pyridinium cations is to react pyridine derivatives with halohydrocarbons. In recent years, some reports indicated that alcohols, instead of halohydrocarbons, could be used as the alkylation reagents under the presence of metal salts and strong acids.5 On the other hand, the pyridinium cations have been widely used as templates to tailor out different types of low-dimensional metal-halide-pyridinium complexes.6 Among them, the low-dimensional pyridinium iodoplumbate hybrids have received much attention due to their intriguing architectures and their potential applications in the optical and electronic fields.7 The normal approach to iodoplumbate-pyridinium complexes is to combine PbI2 with the preformed pyridinium salts directly in certain solvents, and in many cases, NaI, KI or HI is added to increase the solubility of the products and to promote crystallization.8,6a,7g Because the readily-made pyridinium salts are limited in number, the above method can work only on a small scale, especially for iodoplumbate-pyridinium complexes.
In this article, we have developed a new approach to one-dimensional (1D) iodoplumbate-pyridinium complexes viasolvothermal reactions of 4-cyanopyridine (4-cypy) or 4,4′-bipyridine (4,4′-bipy) with various alcohols in the presence of PbI2, I2 and a trace amount of water in acetonitrile. A set of 1D iodoplumbate complexes of 4-cyanopyridinium (C+/C2+) and viologen (V2+) including {[EC]4[(Pb2I6)2]·2MeCN}n (EC+ = N-ethyl-4-cyanopyridium) (1), {[PC][PbI3]}n (PC+ = N-propyl-4-cyanopyridium) (2), {[iPC]3[(PbI3)(Pb2I6)]}n (iPC+ = N-isopropyl-4-cyanopyridium) (3), {[BzC][PbI3]}n (BzC+ = N-benzyl-4-cyanopyridium) (4), {[Cxy]2[(Pb2I6)2]}n (Cxy2+ = 1,4-bis(4-cyanopyridium)-xylene) (5), {[EV]1.5[Pb3I9]}n (EV2+ = N,N′-diethyl-4,4′-bipyridinium) (6), {[PV]1.5[Pb3I9]}n (PV2+ = N,N′-dipropyl-4,4′-bipyridinium) (7), and {[iPV]2[Pb4I12]}n (iPV2+ = N,N′-diisopropyl-4,4′-bipyridinium) (8) were isolated there from. Considering that the low dielectric constant materials (low-k materials) is of importance in modern semiconductor industry,9 and most of the low-k materials are based on inorganosilica10 and organosilica11 complexes, aromatic polymers,12 fluorinated polymers,13polyimides,14 low-dimensional nanomaterials,15copper and aluminium composites,16 we have also investigated dielectric properties of 1–8 in order to screen out suitable low-k complexes for potential applications. Herein, we report their syntheses, structures along with their optoelectronic and dielectric properties.
Experimental
Materials and physical measurements
All starting materials were purchased from commercial sources and used without further purification. Solvents were purchased as reagent grade and distilled prior to use. The elemental analyses for C, H, and N were performed on a Carlo–Erba CHNO–S microanalyzer. The IR spectra were recorded on a Varian 1000 FT–IR spectrometer as KBr disks (4000–400 cm−1). Solid-state UV-vis-NIR spectra were measured with a Shimadzu UV-3150 spectrometer at room temperature in the range 200∼2000 nm. The permittivity measurements were performed on a Novocontrol Concept 80 broadband dielectric spectrometer.
Preparation of complexes 1–8
{[EC]4[(Pb2I6)2]·2MeCN}n (1).
To a Pyrex glass tube (15 cm in length, 7 mm in inner diameter) was added PbI2 (23 mg, 0.05 mmol), I2 (13 mg, 0.05 mmol), 4-cypy (10 mg, 0.1 mmol), water (3 μL), ethanol (0.5 mL) and acetonitrile (1.5 mL). The tube was sealed and heated in an oven at 150 °C for 35 h and then cooled to room temperature at a rate of 5 °C 100 min−1 to form brown-yellow rod crystals of 1, which were collected by filtration, washed with ethyl acetate, and dried in air. Yield: 11 mg (30% based on PbI2). Anal. calcd for C36H42N10Pb4I12: C 14.58, H 1.43, N 4.72%. Found: C 14.81, H 1.45, N 4.98%. IR (KBr, cm−1): 3107 w, 3045 m, 2973 w, 2932 w, 2244 w, 1732 w, 1638 m, 1561 m, 1506 w, 1452 s, 1384 w, 1331 w, 1298 m, 1231 m, 1158 m, 1085 w, 1053 w, 971 w, 838 s, 760 w, 722 w, 670 w, 559 s.
{[PC][PbI3]}n (2).
The brown rod crystals of 2 were prepared by a similar method used in the synthesis of 1 except that ethanol was replaced by propanol (0.15 g, 2.50 mmol). Yield: 6 mg (17% based on PbI2). Anal. calcd for C9H11I3N2Pb: C 14.70, H 1.51, N 3.81%. Found: C 14.76, H 1.51, N 3.75%. IR (KBr, cm−1): 3104 m, 3083 m, 3043 s, 2964 m, 2931 m, 2873 w, 2244 w, 1637 s, 1561 m, 1507 w, 1453 s, 1384 w, 1333 w, 1295 m, 1214 m, 1156 m, 1085 w, 1052 w, 846 m, 829 m, 670 w, 560 m.
{[iPC]3[(PbI3)(Pb2I6)]}n (3).
The orange rod crystals of 3 were prepared by a similar method used in the synthesis of 2 except that propanol was replaced by isopropanol (0.20 g, 3.33 mmol). Yield: 18 mg (16% based on PbI2). Anal. calcd for C27H33I9N6Pb3: C 14.70, H 1.51, N 3.81%. Found: C 14.84, H 1.52, N 3.73%. IR (KBr, cm−1): 3109 m, 3074 m, 3045 m, 2979 m, 2932 w, 2873 w, 2817 w, 2244 w, 1923 w, 1732 w, 1683 w, 1635 s, 1562 m, 1504 m, 1454 s, 1374 m, 1320 w, 1297 w, 1249 s, 1178 m, 1137 s, 1073 w, 1049 m, 952 w, 891 w, 842 s, 760 w, 726 w, 671 w, 566 s, 426 w.
{[BzC][PbI3]}n (4).
The orange rod crystals of 4 were prepared by a similar method used in the synthesis of 2 except that propanol was replaced by benzyl alcohol (0.15 g, 1.40 mmol). Yield: 16 mg (42% based on PbI2). Anal. calcd for C13H11I3N2Pb: C 19.94, H 1.42, N 3.58%. Found: C 20.11, H 1.45, N 3.69%. IR (KBr, cm−1): 3098 m, 3075 w, 3038 m, 2983 w, 2245 w, 1634 m, 1558 m, 1495 m, 1450 s, 1335 w, 1286 m, 1204 w, 1139 m, 1080 w, 1051 w, 1028 w, 1002 w, 855 w, 817 m, 769 w, 726 m, 702 m, 598 m, 543 m, 492 w.
{[Cxy]2[(Pb2I6)2]}n (5).
The red rod crystals of 5 were prepared by a similar method used in the synthesis of 2 except that propanol was replaced by α,α′-dihydroxy-p-xylene (14 mg, 0.1 mmol). Yield: 4 mg (10% based on PbI2). Anal. calcd for C40H32I12N8Pb4: C 16.14, H 1.08, N 3.76%. Found: C 16.28, H 1.07, N 3.83%. IR (KBr, cm−1): 3103 w, 3039 m, 2963 w, 2934 w, 2244 w, 1923 w, 1635 s, 1559 m, 1506 m, 1450 s, 1363 w, 1285 w, 1212 m, 1126 m, 1084 w, 1050 w, 1021 w, 965 w, 826 m, 734 w, 700 w, 609 w, 552 m.
{[EV]1.5[Pb3I9]}n (6).
The dark red rod crystals of 6 were prepared by a similar method used in the synthesis of 1 except that 4-cypy was replaced by 4,4′-bipy (7.8 mg, 0.05 mmol). Yield: 16 mg (45% based on PbI2). Anal. calcd for C21H27I9N3Pb3: C 12.10, H 1.31, N 2.02%. Found: C 12.17, H 1.30, N 1.95%. IR (KBr, cm−1): 3109 w, 3083 w, 3046 m, 2966 w, 2928 w, 1918 w, 1635 s, 1557 m, 1500 w, 1441 s, 1377 w, 1340 w, 1290 w, 1234 w, 1214 w, 1172 m, 1084 m, 969 w, 864 w, 822 m, 767 w, 713 w, 561 w, 507 w.
{[PV]1.5[Pb3I9]}n (7).
The dark red block crystals of 7 were prepared by a similar method used in the synthesis of 6 except that ethanol was replaced by propanol (0.5 mL). Yield: 22 mg (63% based on PbI2). Anal. calcd for C24H33I9N3Pb3: C 13.55, H 1.56, N 1.98%. Found: C 13.78, H 1.51, N 2.14%. IR (KBr, cm−1): 3085 w, 3046 m, 2958 m, 2928 w, 2871 w, 1922 w, 1635 s, 1556 m, 1501 m, 1440 s, 1384 w, 1338 m, 1283 w, 1214 m, 1172 m, 1099 w, 1049 w, 830 m, 805 m, 714 w, 573 w, 505 w.
{[iPV]2[Pb4I12]}n (8).
The black-red block crystals of 8 were prepared by a similar method used in the synthesis of 6 except that ethanol was replaced by isopropanol (0.5 mL). Yield: 12 mg (35% based on PbI2). Anal. calcd for C32H44I12N4Pb4: C 13.55, H 1.56, N 1.98%. Found: C 13.41, H 1.54, N 1.87%. IR (KBr, cm−1): 3112 w, 3080 w, 3050 m, 2976 w, 2927 w, 1632 s, 1557 m, 1496 w, 1439 s, 1376 m, 1249 m, 1160 m, 1079 m, 1045 w, 824 m, 737 w, 712 w, 534 w.
X-Ray data collection and structure determination
Single crystals of 1–8 were obtained directly from the above preparations. Diffraction intensities of 1–8 were collected on a Rigaku Mercury CCD X-ray diffractometer (Mo-Kα, λ = 0.71073 Å). Each single crystal was mounted at the top of a glass fiber at 293 K for 4 and 223 K for others in a stream of gaseous nitrogen. Cell parameters were refined on all observed reflections by using the program CrystalClear (Rigaku and MSc, Ver. 1.3, 2001). The collected data were reduced by the program CrystalClear, and an absorption correction (multi-scan) was applied. The reflection data were also corrected for Lorentz and polarization effects.
The crystal structures of 1–8 were solved by direct methods and refined on F2 by full-matrix least-squares techniques with SHELXTL-97 program.17 For 7, two propyl groups on a N,N′-dipropyl-4,4′-bipyridinium cation were disordered over two positions with the occupancy factors of 0.45/0.55 for C17–C18/C17′–C18′ and C21/C21′, respectively. For 8, the Pb centers and some I atoms were disordered over two positions with the occupancy factors of 0.83/0.17 for Pb1/Pb1′, Pb2/Pb2′, Pb3/Pb3′, Pb4/Pb4′, I1/I1′, I2/I2′, I4/I4′, I8/I8′, I9/I9′ and I11/I11′. All non-hydrogen atoms were refined anisotropically while all hydrogen atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms. Presence of heavy elements like Pb and I may unfavourably affect the crystallographic results, even if absorption correction is applied. Checkcif results showed several Hirshfeld-test failures, unexpected deviations from expected ADP's, unusual bond distances, etc. However, the geometric details of the organic part may not affect too much the significance of these structures. A summary of key crystallographic information for 1–8 is given in Table 1. Selected bond lengths and angles for 1–8 are listed in Table 2.
| Compound |
1
|
2
|
3
|
|
R = Σ‖Fo| − |Fc‖/Σ|Fo|.
wR = {Σw(Fo2 − Fc2)2/Σw(Fo2)2}1/2.
GOF = {Σ[w((Fo2 − Fc2)2)/(n − p)}1/2, where n = number of reflections and p = total numbers of parameters refined.
|
| Formula |
C36H42N10Pb4I12 |
C9H11N2PbI3 |
C27H33N6Pb3I9 |
|
FW/g mol−1 |
2966.44 |
735.10 |
2205.29 |
| Crystal system |
Monoclinic |
Monoclinic |
Trigonal |
| Space group |
C2/c |
P21/c |
P-3
|
|
a/Å |
16.743(3) |
7.8610(11) |
19.208(3) |
|
b/Å |
14.793(3) |
12.7769(19) |
19.208(3) |
|
c/Å |
28.814(6) |
16.133(2) |
7.8129(16) |
|
β/° |
94.97(3) |
97.655(4) |
|
|
γ/° |
|
|
120.00 |
|
V/Å3 |
7110(2) |
1606.0(4) |
2496.4(8) |
|
Z
|
4 |
4 |
2 |
|
T/K |
223(2) |
223(2) |
223(2) |
|
D
c/g cm−3 |
2.810 |
3.040 |
2.934 |
|
F(000) |
5256.0 |
1280 |
1920 |
|
μ (Mo-Kα/mm−1) |
14.686 |
16.249 |
15.680 |
| Total no. of reflections |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517 517 |
9048 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 015 |
| No. of unique reflections |
7997 |
3651 |
3804 |
| No. of observed reflections |
4774 |
2354 |
2813 |
|
R
a (I > 2.00σ (I)) |
0.0725 |
0.0650 |
0.0403 |
|
wR
b
|
0.1437 |
0.2498 |
0.0640 |
| GOFc |
1.045 |
1.153 |
0.933 |
| Δρmax/Δρmin/e Å−3 |
1.692/−2.210 |
2.708/−2.791 |
2.803/−2.054 |
| Compound |
4
|
5
|
6
|
| Formula |
C13H11N2PbI3 |
C40H32N8Pb4I12 |
C21H27N3Pb3I9 |
|
FW/g mol−1 |
783.14 |
2976.34 |
2085.16 |
| Crystal system |
Monoclinic |
Monoclinic |
Monoclinic |
| Space group |
P21/c |
P21/c |
P21/c |
|
a/Å |
8.0356(16) |
7.7261(15) |
12.026(2) |
|
b/Å |
14.126(3) |
21.194(4) |
14.039(3) |
|
c/Å |
16.065(3) |
19.674(4) |
24.770(5) |
|
β/° |
93.41(3) |
94.01(3) |
90.44(3) |
|
V/Å3 |
1820.3(6) |
3213.7(11) |
4181.9(14) |
|
Z
|
4 |
2 |
4 |
|
T/K |
293(2) |
223(2) |
223(2) |
|
D
c/g cm−3 |
2.858 |
3.076 |
3.312 |
|
F(000) |
1376 |
2584 |
3588 |
|
μ (Mo-Kα/mm−1) |
14.346 |
16.243 |
18.708 |
| Total no. of reflections |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293 293 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 948 948 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 117 |
| No. of unique reflections |
3323 |
7200 |
9316 |
| No. of observed reflections |
2709 |
4202 |
6458 |
|
R
a (I > 2.00σ (I)) |
0.0528 |
0.0372 |
0.0785 |
|
wRb |
0.1100 |
0.0596 |
0.1435 |
| GOFc |
1.023 |
0.815 |
1.097 |
| Δρmax/Δρmin/e Å−3 |
1.170/−0.967 |
1.339/−1.338 |
1.444/−1.771 |
| Compound |
7
|
8
|
| Formula |
C24H33N3Pb3I9 |
C32H44N4Pb4I12 |
|
FW/g mol−1 |
2127.23 |
2836.31 |
| Crystal system |
Monoclinic |
Monoclinic |
| Space group |
P21/c |
P21/c |
|
a/Å |
12.328(3) |
16.182(3) |
|
b/Å |
14.341(3) |
21.323(4) |
|
c/Å |
24.982(5) |
17.966(4) |
|
β/° |
90.24(3) |
103.44(3) |
|
V/Å3 |
4416.7(17) |
6029(2) |
|
Z
|
4 |
4 |
|
T/K |
223(2) |
223(2) |
|
D
c/g cm−3 |
3.199 |
3.125 |
|
F(000) |
3684 |
4912 |
|
μ (Mo-Kα/mm−1) |
17.717 |
17.305 |
| Total no. of reflections |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 775 775 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 681 681 |
| No. of unique reflections |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 019 019 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380 380 |
| No. of observed reflections |
7579 |
4568 |
|
R
a (I > 2.00σ (I)) |
0.0722 |
0.0986 |
|
wRb |
0.1244 |
0.1842 |
| GOFc |
1.109 |
1.062 |
| Δρmax/Δρmin/e Å−3 |
1.256/−2.152 |
1.554/−1.421 |
Table 2 Selected bond lengths (Å) and angles (°) for complexes 1–8a
|
Symmetry codes for 1: A: −x + 1/2, −y + 1/2, −z; B: −x, y, −z − 1/2. Symmetry codes for 2: A: −x + 2, −y + 1, −z + 1; B: −x + 1, −y + 1, −z + 1. Symmetry codes for 3: A: −y + 1, x − y, z; C: −y + 1, x − y, z + 1; F: −x + 2, −y, −z + 2; M: −x + 2, −y, −z + 3. Symmetry codes for 4: A: −x + 1, −y + 1, −z; B: −x + 2, −y + 1, −z; C: x + 1, y, z. Symmetry codes for 5: A: x + 1, y, z; B: x − 1, y, z. Symmetry codes for 6: A: −x + 1, −y + 2, −z; B: x + 1, y, z; C: −x + 2, −y + 2, −z. Symmetry codes for 7: A: −x, −y + 2, −z; B: x − 1, y, z; C: −x − 1, −y + 2, −z. Symmetry codes for 8: A: x − 1, y, z.
|
| Complex 1 |
| av. Pb(1)–I |
3.2335(13) |
av. Pb(2)–I |
3.2265(12) |
| av. Pb(3)–I |
3.2263(12) |
|
|
| I(1)–Pb(1)–I(3) |
172.99(3) |
I(2)–Pb(1)–I(2B) |
174.90(5) |
|
I(6A)–Pb(2)–I(1B) |
178.16(3) |
I(5)–Pb(2)–I(2B) |
176.36(3) |
| I(4)–Pb(2)–I(3) |
174.19(3) |
I(5)–Pb(3)–I(5A) |
180.0 |
| I(4)–Pb(3)–I(4A) |
180.0 |
I(6A)–Pb(3)–I(6) |
180.0 |
| Complex 2 |
| av. Pb(1)–I |
3.2244(15) |
av. Pb(2)–I |
3.2258(15) |
| I(1)–Pb(1)–I(1B) |
180.0 |
I(3)–Pb(1)–I(3B) |
180.0 |
| I(2)–Pb(1)–I(2B) |
180.0 |
I(1)–Pb(2)–I(1A) |
180.0 |
| I(3)–Pb(2)–I(3A) |
180.0 |
I(2)–Pb(2)–I(2A) |
180.0 |
| Complex 3 |
| av. Pb(1)–I |
3.2227(7) |
av. Pb(2)–I |
3.2265(8) |
| Pb(3)–I(3) |
3.2235(7) |
Pb(4)–I(3) |
3.2390(7) |
|
I(1A)–Pb(1)–I(2) |
178.139(18) |
I(2)–Pb(2)–I(1C) |
179.051(17) |
| I(3)–Pb(3)–I(3F) |
180.0 |
I(3M)–Pb(4)–I(3) |
180.0 |
| Complex 4 |
| av. Pb(1)–I |
3.2272(11) |
av. Pb(2)–I |
3.2482(12) |
| I(2)–Pb(1)–I(2A) |
180.0 |
I(3)–Pb(1)–I(3A) |
180.0 |
|
I(1A)–Pb(1)–I(1) |
180.0 |
I(1B)–Pb(2)–I(1) |
180.0 |
|
I(2B)–Pb(2)–I(2) |
180.0 |
I(3C)–Pb(2)–I(3A) |
180.0 |
| Complex 5 |
| av. Pb(1)–I |
3.2335(10) |
av. Pb(2)–I |
3.2259(10) |
| I(5)–Pb(1)–I(2) |
174.70(2) |
I(6A)–Pb(1)–I(1) |
175.284(19) |
| I(3)–Pb(1)–I(4) |
175.96(2) |
I(5B)–Pb(2)–I(2) |
174.52(2) |
| I(6)–Pb(2)–I(1) |
178.04(2) |
I(3)–Pb(2)–I(4B) |
175.51(2) |
| Complex 6 |
| av. Pb(1)–I |
3.2315(14) |
av. Pb(2)–I |
3.2740(17) |
| av. Pb(3)–I |
3.2485(15) |
|
|
| I(4)–Pb(1)–I(5A) |
177.75(3) |
I(7)–Pb(1)–I(5) |
168.81(4) |
| I(8)–Pb(1)–I(6) |
174.63(4) |
I(9)–Pb(2)–I(8) |
179.48(4) |
|
I(7C)–Pb(3)–I(4) |
173.71(4) |
I(9B)–Pb(3)–I(6) |
166.18(4) |
| I(1)–Pb(3)–I(6C) |
171.94(4) |
|
|
| Complex 7 |
| av. Pb(1)–I |
3.2370(13) |
av. Pb(2)–I |
3.1474(14) |
| av. Pb(3)–I |
3.2489(13) |
|
|
| I(7)–Pb(1)–I(5A) |
165.73(3) |
I(4)–Pb(1)–I(5) |
177.91(3) |
| I(8)–Pb(1)–I(6) |
172.28(3) |
I(8)–Pb(2)–I(9) |
174.98(3) |
| I(4)–Pb(3)–I(7C) |
173.67(3) |
I(9B)–Pb(3)–I(6) |
166.52(3) |
| I(1)–Pb(3)–I(6C) |
168.91(4) |
|
|
| Complex 8 |
| av. Pb(1)–I |
3.230(4) |
av. Pb(2)–I |
3.283(4) |
| av. Pb(3)–I |
3.227(4) |
av. Pb(4)–I |
3.283(4) |
| I(3)–Pb(1)–I(10A) |
172.30(11) |
I(11A)–Pb(1)–I(2) |
177.64(11) |
| I(1)–Pb(1)–I(12A) |
174.14(12) |
I(3)–Pb(1)–I(12A) |
101.63(9) |
| I(6)–Pb(2)–I(2) |
108.31(9) |
I(3)–Pb(2)–I(4) |
114.32(9) |
| I(2)–Pb(2)–I(4) |
158.30(10) |
I(6)–Pb(2)–I(1) |
155.12(9) |
| I(3)–Pb(2)–I(5) |
154.10(8) |
I(1)–Pb(2)–I(5) |
120.52(9) |
| I(6)–Pb(3)–I(9) |
174.03(10) |
I(4)–Pb(3)–I(8) |
174.05(12) |
| I(9)–Pb(3)–I(5) |
100.36(10) |
I(7)–Pb(3)–I(5) |
175.62(8) |
| I(9)–Pb(4)–I(11) |
110.81(13) |
I(8)–Pb(4)–I(11) |
160.38(12) |
| I(9)–Pb(4)–I(12) |
155.76(11) |
I(12)–Pb(4)–I(7) |
119.75(10) |
| I(8)–Pb(4)–I(10) |
107.49(10) |
I(7)–Pb(4)–I(10) |
154.86(10) |
Results and discussion
Synthetic and spectral aspects
Complexes 1–8 were generated in situ under solvothermal conditions, in which the C+/C2+ and V2+ cations were formed via cleavage of the C–O bond of alcohols followed by alkylation of 4-cypy or 4,4′-bipy (Scheme 1). In these reactions, elemental iodine played a key role in realizing the synthetic processes. The possible mechanisms had been suggested in ESI, Scheme S1.†Elemental iodine may react with water to produce HI, which reacts with alcohols to form iodohydrocarbons. The resulting iodohydrocarbons further react with 4-cypy or 4,4′-bipy to generate a series of C+/C2+ or V2+ cations (Fig. 1). In the case of α,α′-dihydroxy-p-xylene, an unprecedented Cxy2+ dication was produced (Fig. 1e). With these C+/C2+ or V2+ cations in solution, they worked as various templates to induce Pb2+ and I− to self-assemble into 1D anionic [PbI3]nn− (1–5, 8) and [Pb3I9]n3n− (6 and 7) chains.
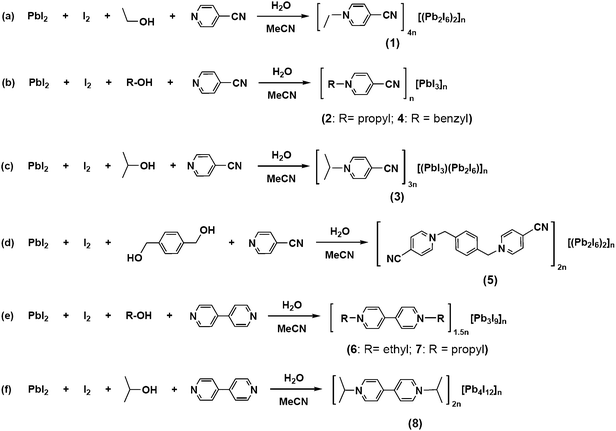 |
| | Scheme 1 The reactions for the synthesis of complexes 1–8 | |
 |
| | Fig. 1 View of the in situ generated C+/C2+ and V2+ cations in 1–8. Atom colour codes: C = grey; H = white; N = cyan (the same hereinafter). | |
Compounds 1–8 are air- and moisture-stable, and insoluble in common solvents but soluble in DMF and DMSO. The elemental analysis was consistent with their chemical formula. The IR spectra of 1–8 show medium peaks at about 3050 cm−1 and strong or medium peaks in the range of 1650–1450 cm−1 indicating the existence of pyridyl groups; the medium peaks at ca. 2244 cm−1 in 1–5 are assigned to be the terminal cyanide stretching vibration; and the medium peaks in the range of 3000–2800 cm−1 denotes the existence of alkyl groups in 1–8.18 The identities of the eight compounds were further confirmed by single-crystal diffraction analysis.
Crystal structure of 1
Complex 1 crystallizes in the monoclinic space groupC2/c, and its asymmetric unit consists of one [Pb2I6]2− dianion, two discrete EC+ cations, and one and one-half MeCN molecules. 1 features its b-type [PbI3]nn− chain, which is constructed by PbI6 octahedra being linked by sharing I–I–I faces (Fig. 2, top). Pb1 is sitting on one mirror plane while a two-fold axis goes through Pb3. Looking along the Pb → Pb vector of the chain, the six I atoms in each PbI6 octahedron are not fully overlapped with their corresponding ones of the neighbouring octahedra (Fig. 2b) because each PbI6 octahedron is slightly distorted (Table 2). The mean Pb⋯Pb contact in the chain is 4.009 Å, which excludes any metal-metal interactions. In the crystal of 1, the EC+ cations surround the 1D anionic chains through static forces and stacked along the [101] direction to form rhombic channels where the 1D iodoplumbate chains are encapsulated (Fig. 3).
![View of the three types of [PbI3]nn− chains observed in 1–5 and 8. Atom colour codes: Pb = blue; I = pink (the same hereinafter).](/image/article/2011/CE/c0ce00309c/c0ce00309c-f2.gif) |
| | Fig. 2 View of the three types of [PbI3]nn− chains observed in 1–5 and 8. Atom colour codes: Pb = blue; I = pink (the same hereinafter). | |
Crystal structure of 2–5
Complexes 2, 4 and 5 crystallize in the monoclinic space groupP21/c and the asymmetric units of 2 and 4 contain half a [Pb2I6]2− dianion, and one PC+ or BzC+ cation, while that of 5 contains one [Pb2I6]2− dianion and two one-halves of Cxy2+ dications. Complex 3 crystallizes in the trigonal space groupP![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , and the asymmetric unit consists of one-third of a [Pb2I6]2− dianion, and one-sixth of the other [Pb2I6]2− dianion, and one discrete iPC+ cation. Complexes 2–5 possess the a-type [PbI3]nn− chain, which is constructed by interconnections of nearly perfect PbI6 octahedra. Looking along the Pb → Pb vector of the chain, the six I atoms in each PbI6 octahedron is overlapped with their corresponding ones of the neighbouring octahedral, forming a six-vanes windmill-like configuration (Fig. 2a). Each PbI6 unit in 2–5 adopts an almost perfect octahedron and the mean Pb⋯Pb contact in the chain varies from 3.817 Å to 4.018 Å. The arrangement of the resulting pyridinium cations around the inorganic chains in 2–5 deserves comments. For 2 and 4, the PC+ or BzC+ cations stack along the a-axis to form 1D rhombic channels where the a-type [PbI3]nn− chains are enveloped (ESI, Fig. S1†). In the case of 3, the iPC+ cations stack along the c-axis, forming a Kagomé-shaped channels where the a-type [PbI3]nn− chains are included (Fig. 4). In addition, the similar chains are also observed in triangle channels constructed by three Kagomé-shaped channels. While in 5, each four Cxy2+ dications stack along the a-axis to form a honeycomb-like channels where the a-type [PbI3]nn− chains are located (ESI, Fig. S2†).
, and the asymmetric unit consists of one-third of a [Pb2I6]2− dianion, and one-sixth of the other [Pb2I6]2− dianion, and one discrete iPC+ cation. Complexes 2–5 possess the a-type [PbI3]nn− chain, which is constructed by interconnections of nearly perfect PbI6 octahedra. Looking along the Pb → Pb vector of the chain, the six I atoms in each PbI6 octahedron is overlapped with their corresponding ones of the neighbouring octahedral, forming a six-vanes windmill-like configuration (Fig. 2a). Each PbI6 unit in 2–5 adopts an almost perfect octahedron and the mean Pb⋯Pb contact in the chain varies from 3.817 Å to 4.018 Å. The arrangement of the resulting pyridinium cations around the inorganic chains in 2–5 deserves comments. For 2 and 4, the PC+ or BzC+ cations stack along the a-axis to form 1D rhombic channels where the a-type [PbI3]nn− chains are enveloped (ESI, Fig. S1†). In the case of 3, the iPC+ cations stack along the c-axis, forming a Kagomé-shaped channels where the a-type [PbI3]nn− chains are included (Fig. 4). In addition, the similar chains are also observed in triangle channels constructed by three Kagomé-shaped channels. While in 5, each four Cxy2+ dications stack along the a-axis to form a honeycomb-like channels where the a-type [PbI3]nn− chains are located (ESI, Fig. S2†).
![View of the b-type [PbI3]nn− chains enclosed into rhombic channels assembled by N-ethyl-4-cyanopyridium cations in 1 (looking along the [101] direction). All H atoms are omitted for clarity.](/image/article/2011/CE/c0ce00309c/c0ce00309c-f3.gif) |
| | Fig. 3 View of the b-type [PbI3]nn− chains enclosed into rhombic channels assembled by N-ethyl-4-cyanopyridium cations in 1 (looking along the [101] direction). All H atoms are omitted for clarity. | |
![View of the a-type [PbI3]nn− chains enclosed into Kagomé-shaped channels assembled by iPC+ cations in 3 (looking along the c-axis). All H atoms are omitted for clarity.](/image/article/2011/CE/c0ce00309c/c0ce00309c-f4.gif) |
| | Fig. 4 View of the a-type [PbI3]nn− chains enclosed into Kagomé-shaped channels assembled by iPC+ cations in 3 (looking along the c-axis). All H atoms are omitted for clarity. | |
Crystal structures of 6 and 7
Complexes 6 and 7 crystallize in the monoclinic space groupP21/c and the asymmetric unit consists of one [Pb3I9]3− trianion, and one and one-half EV2+ or PV2+ dications. 6 or 7 possesses an uncommon 1D [Pb3I9]n3n− chain that is constructed by edge-sharing PbI6 octahedra (Fig. 5a). Such a chain is similar to that observed in (Et2NH2)3Pb3I9·0.5H2O.8h Intriguingly, a group of six EV2+ or PV2+ cations forms a chair-like cyclohexane cavity that encompasses the [Pb6I24]12− species (Fig. 5b). Such a [Pb6I24]12− species links its neighbouring ones to form a 1D [Pb3I9]n3n− chain extending along the a-axis (Fig. 5a).
![(a) View of the 1D step-shaped [Pb3I9]n3n− chain in 6 and 7 (top). Each octahedron represents the PbI6 unit (bottom). (b) View of the [Pb6I24]12− species enclosed by a chair-like cyclohexane cavity constructed through a group of six EV2+ in 6.](/image/article/2011/CE/c0ce00309c/c0ce00309c-f5.gif) |
| | Fig. 5 (a) View of the 1D step-shaped [Pb3I9]n3n− chain in 6 and 7 (top). Each octahedron represents the PbI6 unit (bottom). (b) View of the [Pb6I24]12− species enclosed by a chair-like cyclohexane cavity constructed through a group of six EV2+ in 6. | |
Crystal structure of 8
Complex 8 crystallizes in the monoclinic space groupP21/c. The asymmetric unit consists of one [Pb4I12]4−tetraanion, and one and two haves of iPV2+. The four Pb centers show different coordination geometries. Pb1 and Pb3 adopt a PbI6 octahedral geometry (O) while Pb2 and Pb4 take a trigonal prism geometry (TP). The PbI6 units interconnect alternately via sharing the I–I–I faces in the manner [O-TP-O-TP] to form a rare c-type [PbI3]nn− chain (Fig. 2c). Because Pb centers show different coordination geometries, all the I atoms in the octahedral PbI6 can not overlap those of the trigonal prismatic PbI6 unit. The iPV2+ dications (Fig. 1h) stack along the a axis, thereby forming a honeycomb-like channels where these c-type [PbI3]nn− chains are encircled (Fig. 6).
![View of the c-type [PbI3]nn− chains enclosed into honeycomb-like channels assembled by iPV2+ cations in 8 (looking along the a-axis). All H atoms are omitted for clarity.](/image/article/2011/CE/c0ce00309c/c0ce00309c-f6.gif) |
| | Fig. 6 View of the c-type [PbI3]nn− chains enclosed into honeycomb-like channels assembled by iPV2+ cations in 8 (looking along the a-axis). All H atoms are omitted for clarity. | |
Optoelectronic and dielectric properties of 1–8
The optical diffuse-reflection spectra of crystalline solids 1–8 were measured at room temperature. The absorption (α/S) data were calculated from the reflectance using the Kubelka–Munk function.19 The energy band gaps (Eonset) obtained by extrapolation of the linear portion of the absorption edges were estimated to be 2.22 eV (1), 1.99 eV (2), 2.12 eV (3), 1.79 eV (4), 1.85 eV (5), 1.85 eV (6), 1.82 eV (7), and 1.61 eV (8) (Fig. 7), indicating a semiconductor nature. At the same time, the values of energy band gaps of 1–8 are all red-shifted with respect to that of the layered compound PbI2 (2.30 eV),20 which suggests that the introduction of pyridinium cations into the framework of PbI2 may increase the activities of electrons due to the charge transfer effect.21
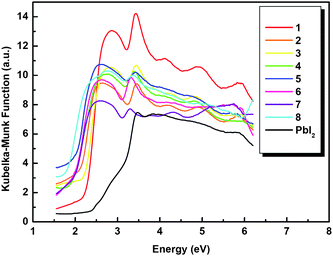 |
| | Fig. 7 Solid-state optical diffuse-reflection spectra of 1–8 and PbI2 derived from diffuse reflectance data at ambient temperature. | |
The dielectric behaviours of 1–8 had been measured based on continuous variation of frequency at 298 K. As shown in Fig. 8(a), the dielectric constants of 1–8 in the range of 100 Hz to 106 Hz were nearly frequency-independent with the values being estimated to be 4.57 (1), 6.88 (2), 5.98 (3), 5.92 (4), 4.74 (5), 6.55 (6), 7.22 (7), and 9.64 (8), respectively, which were all smaller than the observed value of PbI2 (10.60). It is noted that the values of 1 and 5 were close to that of SiO2 (4.3).9 The permittivity (real part) of the iodoplumbate-V2+ complexes (6–8) were generally larger than those of iodoplumbate-C+/C2+ ones (1–5). Complex 8 exhibited the maximum permittivity value compared with 1–7. From 1 Hz to 106 Hz, the dielectric loss of 1–8 and PbI2 was gradually close to zero, and 5 showed the maximum value of 0.03 (average) compared with other complexes in the range of 100 Hz to 106 Hz.
 |
| | Fig. 8 (a) Frequency dependence of permittivity (εr) of 1–8 and PbI2 at 298 K. (b) Frequency dependence of dielectric loss (εi/εr) of 1–8 and PbI2 at 298 K. The εr and εi are the real and imaginary parts of permittivity, respectively. | |
Conclusions
In summary, we have demonstrated an efficient approach to a series of 1D 4-cyanopyridinium and viologen iodoplumbates 1–8 by solvothermal reactions of alcohols with 4-cypy/4,4′-bipy, PbI2, I2 and a small amount of water in acetonitrile, in which the 4-cyanopyridinium and viologen cations were generated in situ. The optoelectronic and dielectric properties of 1–8 were explored. It was found that the introduction of C+/C2+ and V+ cations into bulk PbI2 could decrease the energy band gaps and dielectric constant, obviously. It is anticipated that the as-synthesized complexes may be have potential application as low-k materials in the microelectronic field. The aforementioned synthetic methodology may be applied to prepare other low-dimensional charge transfer pyridinium-iodometalates with new structures, better optoelectronic performances and low dielectric constant characteristics.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (20525101, 20871088 and 90922018), the Nature Science Key Basic Research of Jiangsu Province for Higher Education (09KJA150002), the Specialized Research Fund for the Doctoral Program of higher Education of Ministry of Education (20093201110017), the State Key Laboratory of Organometallic Chemistry of Shanghai Institute of Organic Chemistry (08–25), the Qin-Lan and the “333” Projects of Jiangsu Province, and the “Soochow Scholar” Program and the Program for Innovative Research Team of Suzhou University, and the Innovative Research Program for Postgraduates in Universities of Jiangsu Province (CX09B_020Z). The authors also greatly thanked the helpful comments from the editor and the reviewers
References
-
(a) V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 1731 CrossRef CAS;
(b) G. Dyker, Angew. Chem., Int. Ed., 1999, 38, 1698 CrossRef;
(c) T. Naota, H. Takaya and S. I. Murahashi, Chem. Rev., 1998, 98, 2599 CrossRef CAS;
(d) B. A. Arndtsen, R. G. Bergman, T. A. Mobley and T. H. Peterson, Acc. Chem. Res., 1995, 28, 154 CrossRef;
(e) C.-H. Jun, Chem. Soc. Rev., 2004, 33, 610 RSC;
(f) D. Milstein and B. Rybtchinski, Angew. Chem., Int. Ed., 1999, 38, 870 CrossRef;
(g) Y. Lei, A. D. Wrobleski, J. E. Golden, D. R. Powell and J. Aubé, J. Am. Chem. Soc., 2005, 127, 4552 CrossRef CAS;
(h) A. Maercker, Angew. Chem., Int. Ed. Engl., 1987, 26, 972 CrossRef;
(i)
J. Tsuji, Transition Metal Reagents and Catalysts, Wiley, New York, 2000 Search PubMed.
-
(a) P.-h. Szu, X. He, L. Zhao and H.-w. Liu, Angew. Chem., Int. Ed., 2005, 44, 6742 CrossRef CAS;
(b) F. Casado, L. Pisano, M. Farriol, I. Gallardo, J. Marquet and G. Melloni, J. Org. Chem., 2000, 65, 322 CrossRef CAS;
(c) G. W. Kabalka, M.-L. Yao, S. Borella and L. K. Coins, Organometallics, 2007, 26, 4112 CrossRef CAS;
(d) Y. Nishibayashi, A. Shinoda, Y. Miyake, H. Matsuzawa and M. Sato, Angew. Chem., Int. Ed., 2006, 45, 4835 CrossRef CAS;
(e) S. Jang, L. M. Atagi and J. M. Mayer, J. Am. Chem. Soc., 1990, 112, 6413 CrossRef CAS;
(f) F. Ozama, T. Ishiyama, S. Yamamoto, S. Kawagishi and M. Hiromi, Organometallics, 2004, 23, 1698 CrossRef CAS.
-
(a) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS;
(b) G. Mann, J. F. Hartwig, M. S. Driver and C. Fernández-Rivas, J. Am. Chem. Soc., 1998, 120, 827 CrossRef CAS;
(c) M. O. Frederick, J. A. Mulder, M. R. Tracey, R. P. Hsung, J. Huang, K. C. M. Kurtz, L. Shen and C. J. Douglas, J. Am. Chem. Soc., 2003, 125, 2368 CrossRef CAS;
(d) A. Klapars, S. Parris, K. W. Anderson and S. L. Buchwald, J. Am. Chem. Soc., 2004, 126, 3529 CrossRef CAS;
(e) K. Muñiz, J. Am. Chem. Soc., 2007, 129, 14542 CrossRef CAS;
(f) W. C. P. Tsang, N. Zheng and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 14560 CrossRef CAS.
-
(a)
M. E. Halpern, Phase-Transfer Catalysis, ACS publication, Washington, DC, 1997 Search PubMed;
(b) G.-S. Luo, Y.-J. Wang, Y.-C. Lu and D. Huang, Ind. Eng. Chem. Res., 2007, 46, 3656 CrossRef;
(c) T. Ooi, K. Doda and K. Maruoka, J. Am. Chem. Soc., 2003, 125, 2054 CrossRef CAS;
(d) Y. Du, Y. Wu, A.-H. Liu and L.-N. He, J. Org. Chem., 2008, 73, 4709 CrossRef CAS;
(e) R. Annunziata, M. Benaglia, M. Cinquini, F. Cozzi and G. Tocco, Org. Lett., 2000, 2, 1737 CrossRef CAS.
-
(a) J.-K. Cheng, Y.-G. Yao, J. Zhang, Z.-J. Li, Z.-W. Cai, X.-Y. Zhang, Z.-N. Chen, Y.-B. Chen, Y. Kang, Y.-Y. Qin and Y.-H. Wen, J. Am. Chem. Soc., 2004, 126, 7796 CrossRef CAS;
(b) J.-J. Hou, C.-H. Guo and X.-M. Zhang, Inorg. Chim. Acta, 2006, 359, 3991 CrossRef CAS;
(c) G. Xu, G.-C. Guo, M.-S. Wang, Z.-J. Zhang, W.-T. Chen and J.-S. Huang, Angew. Chem., Int. Ed., 2007, 46, 3249 CrossRef CAS.
-
(a) Z. Tang and A. M. Guloy, J. Am. Chem. Soc., 1999, 121, 452 CrossRef CAS;
(b) A. Ohno, Y. Ishikawa, N. Yamazaki, M. Okamura and Y. Kawai, J. Am. Chem. Soc., 1998, 120, 1186 CrossRef CAS;
(c) Z. Tang, A. P. Litvinchuk, H.-G. Lee and A. M. Guloy, Inorg. Chem., 1998, 37, 4752 CrossRef CAS;
(d) F. Neve, A. Crispini and O. Francescangeli, Inorg. Chem., 2000, 39, 1187 CrossRef CAS;
(e) E. Cariati, R. Macchi, D. Roberto, R. Ugo, S. Galli, N. Masciocchi and A. Sironi, Chem. Mater., 2007, 19, 3704 CrossRef CAS;
(f) H.-H. Li, Z.-R. Chen, J.-Q. Li, C.-C. Huang, Y.-F. Zhang and G.-X. Jia, Eur. J. Inorg. Chem., 2006, 2447 CrossRef CAS;
(g) H.-H. Li, Z.-R. Chen, J.-Q. Li, H.-B. Zhan, W.-X. Zhang, C.-C. Huang, C. Ma and B. Zhao, J. Solid State Chem., 2006, 179, 1415 CrossRef CAS;
(h) Z. Glavcheva, H. Nakanishi, S. Okada and H. Umezawa, Mater. Lett., 2004, 58, 2466 CrossRef CAS;
(i) F. Neve and A. Crispini, CrystEngComm, 2003, 5, 265 RSC;
(j) H.-G. Zhu, Z. Yu, H. Cai, X.-Z. You, J. J. Vittal and G.-K. Tan, Chem. Lett., 1999, 289 CrossRef CAS.
-
(a) D. B. Mitzi, S. Wang, C. A. Feild, C. A. Chess and A. M. Guloy, Science, 1995, 267, 1473 CAS;
(b) D. B. Mitzi, C. A. Feild, W. T. A. Harrison and A. M. Guloy, Nature, 1994, 369, 467 CrossRef CAS;
(c) T. E. Gier and G. D. Stucky, Nature, 1991, 349, 508 CrossRef CAS;
(d) P. G. Lacroix, R. Clement, K. Nakatani, J. Zyss and I. Ledoux, Science, 1994, 263, 658 CrossRef CAS;
(e) T. Ishihara, J. Takahashi and T. Goto, Phys. Rev. B: Condens. Matter, 1990, 42, 11099 CrossRef CAS;
(f) Z. Glavcheva, H. Umezawa, S. Okada and H. Nakanishi, Mater. Lett., 2004, 58, 2466 CrossRef CAS;
(g) A. M. Guloy, Z. Tang, P. B. Miranda and V. I. Srdanov, Adv. Mater., 2001, 13, 833 CrossRef CAS;
(h)
T. Ishihara and T. Goto, Nonlinear Optics of Organics and Semiconductors, Springer-Verlag, Berlin, 1989 Search PubMed;
(i) Z.-J. Zhang, G.-C. Guo, G. Xu, M.-L. Fu, J.-P. Zou and J.-S. Huang, Inorg. Chem., 2006, 45, 10028 CrossRef CAS;
(j) Z.-J. Zhang, S.-C. Xiang, Y.-F. Zhang, A.-Q. Wu, L.-Z. Cai, G.-C. Guo and J.-S. Huang, Inorg. Chem., 2006, 45, 1972 CrossRef CAS;
(k) J.-P. Li, L.-H. Li, L.-M. Wu and L. Chen, Inorg. Chem., 2009, 48, 1260 CrossRef CAS;
(l) S. Mishra, E. Jeanneau, S. Daniele, G. Ledoux and P. N. Swamy, Inorg. Chem., 2008, 47, 9333 CrossRef CAS.
-
(a) S. Wang, D. B. Mitzi, C. A. Field and A. Guloy, J. Am. Chem. Soc., 1995, 117, 5297 CrossRef CAS;
(b) H. Krautscheid and F. Vielsack, Angew. Chem., Int. Ed. Engl., 1995, 34, 2035 CrossRef CAS;
(c) H. Krautscheid and F. Vielsack, J. Chem. Soc., Dalton Trans., 1999, 2731 RSC;
(d) H. Krautscheid, C. Lode, F. Vielsack and H. Vollmer, J. Chem. Soc., Dalton Trans., 2001, 1099 RSC;
(e) H.-H. Li, Z.-R. Chen, L.-Q. Guo, K.-N. Ding, J.-Q. Li, C.-C. Huang and Z.-L. Cai, Aust. J. Chem., 2007, 60, 595 CrossRef CAS;
(f) C. P. Raptopoulou, A. Terzis, G. A. Mousdis and G. C. Papavassiliou, Z. Naturforsch. B, 2002, 57, 645 CAS;
(g) X.-H. Zhu, N. Mercier, P. Frère, P. Blanchard, J. Roncali, M. Allain, C. Pasquier and A. Riou, Inorg. Chem., 2003, 42, 5330 CrossRef CAS;
(h) R. D. Willett and K. R. Maxcy, Inorg. Chem., 2002, 41, 7024 CrossRef;
(i) H.-H. Li, Z.-R. Chen, L.-C. Cheng, J.-B. Liu, X.-B. Chen and J.-Q. Li, Cryst. Growth Des., 2008, 8, 4355 CrossRef CAS;
(j) N. Mercier, S. Poiroux, A. Riou and P. Batail, Inorg. Chem., 2004, 43, 8361 CrossRef CAS.
-
(a) W. Volksen, R. D. Miller and G. Dubois, Chem. Rev., 2010, 110, 56 CrossRef CAS;
(b)
International Technology Roadmap for Semiconductors-Interconnect, 2006, Update 2006 Search PubMed.
-
(a) Z. Li, S. Li, H. Luo and Y. Yan, Adv. Funct. Mater., 2004, 14, 1019 CrossRef CAS;
(b) R. A. Farrell, N. Petkov, K. Cherkaoui, H. Amenitsch, J. D. Holmes, P. K. Hurley and M. A. Morris, ChemPhysChem, 2008, 9, 1524 CrossRef CAS.
-
(a) W. Wang, D. Grozea, A. Kim, D. D. Perovic and G. A. Ozin, Adv. Mater., 2010, 22, 99 CrossRef CAS;
(b) B. D. Hatton, K. Landskron, W. Whitnall, D. D. Perovic and G. A. Ozin, Adv. Funct. Mater., 2005, 15, 823 CrossRef CAS;
(c) K. Su, D. R. Bujalski, K. Eguchi, G. V. Gordon, D.-L. Ou, P. Chevalier, S. Hu and R. P. Boisvert, Chem. Mater., 2005, 17, 2520 CrossRef CAS;
(d) C. M. Lew, Z. Li, S. Li, S.-J. Hwang, Y. Liu, D. I. Medina, M. Sun, J. Wang, M. E. Davis and Y. Yan, Adv. Funct. Mater., 2008, 18, 3454 CrossRef CAS.
-
(a) X.-Y. Zhao and H.-J. Liu, Polym. Int., 2010, 59, 597 CAS;
(b) S. J. Martin, J. P. Godschalx, M. E. Mills, E. O. Shaffer II and P. H. Townsend, Adv. Mater., 2000, 12, 1769 CrossRef CAS.
- G.-D. Fu, Z. Yuan, E.-T. Kang, K.-G. Neoh, D. M. Lai and A. C. H. Huan, Adv. Funct. Mater., 2005, 15, 315 CrossRef CAS.
- X. Jin and H. Ishii, J. Appl. Polym. Sci., 2006, 100, 4240 CrossRef CAS.
-
(a) C.-M. Leu, Y.-T. Chang and K.-H. Wei, Chem. Mater., 2003, 15, 3721 CrossRef CAS;
(b) Y.-H. Zhang, S.-G. Lu, Y.-Q. Li, Z. M. Dang, J. H. Xin, S.-Y. Fu, G.-T. Li, R.-R. Guo and L.-F. Li, Adv. Mater., 2005, 17, 1056 CrossRef CAS.
-
(a) E. Kondoh, E. Ukai and S. Aruga, Phys. Status Solidi C, 2008, 5, 1219 Search PubMed;
(b) W. Ling, A.-J. Gu, G.-Z. Liang and L. Yuan, Polym. Compos., 2010, 31, 307 Search PubMed;
(c) H. Treichel and C. Goonetilleke, Adv. Eng. Mater., 2001, 3, 461 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112.
-
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed., Wiley, New York, 1986 Search PubMed.
-
W. W. Wendlandt and H. G. Hecht, Reflectance Spectroscopy, Interscience Publishers, New York, 1966 Search PubMed.
- X. H. Zhu, Z. R. Wei, Y. R. Jin and A. P. Xiang, Cryst. Res. Technol., 2007, 42, 456 CrossRef CAS.
- L. Cusack, X. Marguerettaz, S. N. Rao, J. Wenger and D. Fitzmaurice, Chem. Mater., 1997, 9, 1765 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: the possible mechanisms on generating complexes 1–8, 3D packing diagrams of 2, 4, 5 and 7, and frequency dependence of permittivity (imaginary part) of 1–8. CCDC reference numbers 776895–776899 and 765929–765931. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ce00309c |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517
517![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015
015![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293
293![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 948
948![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117
117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 775
775![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 681
681![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 019
019![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380
380

![View of the three types of [PbI3]nn− chains observed in 1–5 and 8. Atom colour codes: Pb = blue; I = pink (the same hereinafter).](/image/article/2011/CE/c0ce00309c/c0ce00309c-f2.gif)
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , and the asymmetric unit consists of one-third of a [Pb2I6]2− dianion, and one-sixth of the other [Pb2I6]2− dianion, and one discrete iPC+ cation. Complexes 2–5 possess the a-type [PbI3]nn− chain, which is constructed by interconnections of nearly perfect PbI6 octahedra. Looking along the Pb → Pb vector of the chain, the six I atoms in each PbI6 octahedron is overlapped with their corresponding ones of the neighbouring octahedral, forming a six-vanes windmill-like configuration (Fig. 2a). Each PbI6 unit in 2–5 adopts an almost perfect octahedron and the mean Pb⋯Pb contact in the chain varies from 3.817 Å to 4.018 Å. The arrangement of the resulting pyridinium cations around the inorganic chains in 2–5 deserves comments. For 2 and 4, the PC+ or BzC+ cations stack along the a-axis to form 1D rhombic channels where the a-type [PbI3]nn− chains are enveloped (ESI, Fig. S1†). In the case of 3, the iPC+ cations stack along the c-axis, forming a Kagomé-shaped channels where the a-type [PbI3]nn− chains are included (Fig. 4). In addition, the similar chains are also observed in triangle channels constructed by three Kagomé-shaped channels. While in 5, each four Cxy2+ dications stack along the a-axis to form a honeycomb-like channels where the a-type [PbI3]nn− chains are located (ESI, Fig. S2†).
, and the asymmetric unit consists of one-third of a [Pb2I6]2− dianion, and one-sixth of the other [Pb2I6]2− dianion, and one discrete iPC+ cation. Complexes 2–5 possess the a-type [PbI3]nn− chain, which is constructed by interconnections of nearly perfect PbI6 octahedra. Looking along the Pb → Pb vector of the chain, the six I atoms in each PbI6 octahedron is overlapped with their corresponding ones of the neighbouring octahedral, forming a six-vanes windmill-like configuration (Fig. 2a). Each PbI6 unit in 2–5 adopts an almost perfect octahedron and the mean Pb⋯Pb contact in the chain varies from 3.817 Å to 4.018 Å. The arrangement of the resulting pyridinium cations around the inorganic chains in 2–5 deserves comments. For 2 and 4, the PC+ or BzC+ cations stack along the a-axis to form 1D rhombic channels where the a-type [PbI3]nn− chains are enveloped (ESI, Fig. S1†). In the case of 3, the iPC+ cations stack along the c-axis, forming a Kagomé-shaped channels where the a-type [PbI3]nn− chains are included (Fig. 4). In addition, the similar chains are also observed in triangle channels constructed by three Kagomé-shaped channels. While in 5, each four Cxy2+ dications stack along the a-axis to form a honeycomb-like channels where the a-type [PbI3]nn− chains are located (ESI, Fig. S2†).
![View of the b-type [PbI3]nn− chains enclosed into rhombic channels assembled by N-ethyl-4-cyanopyridium cations in 1 (looking along the [101] direction). All H atoms are omitted for clarity.](/image/article/2011/CE/c0ce00309c/c0ce00309c-f3.gif)
![View of the a-type [PbI3]nn− chains enclosed into Kagomé-shaped channels assembled by iPC+ cations in 3 (looking along the c-axis). All H atoms are omitted for clarity.](/image/article/2011/CE/c0ce00309c/c0ce00309c-f4.gif)
![(a) View of the 1D step-shaped [Pb3I9]n3n− chain in 6 and 7 (top). Each octahedron represents the PbI6 unit (bottom). (b) View of the [Pb6I24]12− species enclosed by a chair-like cyclohexane cavity constructed through a group of six EV2+ in 6.](/image/article/2011/CE/c0ce00309c/c0ce00309c-f5.gif)
![View of the c-type [PbI3]nn− chains enclosed into honeycomb-like channels assembled by iPV2+ cations in 8 (looking along the a-axis). All H atoms are omitted for clarity.](/image/article/2011/CE/c0ce00309c/c0ce00309c-f6.gif)


