Development of inositol-based antagonists for the D-myo-inositol 1,4,5-trisphosphate receptor†‡
Neil S.
Keddie§
a,
Yulin
Ye§
b,
Tashfeen
Aslam
a,
Tomas
Luyten
c,
Davide
Bello
a,
Clive
Garnham
d,
Geert
Bultynck
c,
Antony
Galione
d and
Stuart J.
Conway
*b
aSchool of Chemistry and Centre for Biomolecular Sciences, University of St Andrews, North Haugh, St Andrews, Fife, KY16 9ST, UK
bDepartment of Chemistry, Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford, OX1 3TA, UK. E-mail: stuart.conway@chem.ox.ac.uk; Fax: +44 (0)1865 285002; Tel: +44 (0)1865 285109
cLaboratory of Molecular Signalling, Campus Gasthuisberg O/N1, K.U. Leuven, Herestraat 49, Bus 802, B-3000 Leuven, Belgium
dDepartment of Pharmacology, University of Oxford, Mansfield Road, Oxford, OX1 3QT, UK
First published on 11th October 2010
Abstract
The syntheses of four D-myo-inositol 1,4,5-trisphosphate (InsP3) derivatives, incorporating phosphate bioisosteres at the 5-position, are reported. The methyl phosphate ester and sulfate derivatives retain InsP3receptor (InsP3R) agonist activity; the compounds that possess a methylphosphonate or a carboxymethyl moiety are InsP3R antagonists.
D-myo-Inositol 1,4,5-trisphosphate (InsP3, 1, Fig. 1) is a ubiquitous intracellular second messenger that mediates a plethora of cellular functions via interaction with defined receptors (InsP3Rs).1,2 These receptors are ligand-gated Ca2+-releasing channels that are located predominantly on the endo- or sarcoplasmic reticulum (ER/SR). The central signalling role of InsP3 has promoted many chemical syntheses of the natural compound and numerous unnatural derivatives.3,4 With only a few exceptions,5–8 these compounds have all displayed full agonist activity at InsP3Rs. However, to enable chemical dissection of the InsP3signalling pathway, the development of selective InsP3R antagonists is an essential aim. Although some antagonists of this receptor do exist, these compounds are not selective for the InsP3Rs over other Ca2+ channels and pumps. For example, 2-aminoethoxydiphenyl borate (2-APB), the most commonly used InsP3R antagonist,9 displays a number of biological actions, including inhibition of SERCA pumps10 and inhibition of store operated Ca2+ entry.11,12
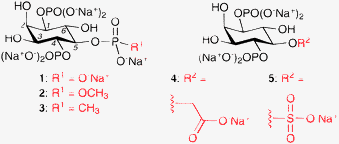 | ||
| Fig. 1 The structures of InsP3 (1) and its four 5-position derivatives, 2–5. | ||
Most existing InsP3R antagonists are not structurally related to InsP3; indeed, only one InsP3 derivative (3) has been reported to act as an InsP3R antagonist and no biological data were provided for this compound.7,8 However, it seems probable that InsP3-based antagonists are most likely to be selective for InsP3Rs over other cellular targets. The elucidation of the X-ray crystal structure of the mouse type 1 InsP3R in complex with InsP3 enables the rational design of InsP3R ligands.13 This structure shows that InsP3 binds between two flexible lobes, the α-domain and the β-domain, leading to proposals of a “clam-shell-like” mode of receptor activation.14 The 1- and 5-position phosphates of InsP3 bind to the α-domain, whereas the 4-position phosphate binds predominantly to the β-domain. The structure confirms the findings of previous structure–activity studies that the 4- and 5-position phosphate groups are the most important for InsP3-receptor interactions.3 We previously hypothesised that reduction of charge at the 4-position, by introducing a phosphate isostere, would lead to compounds that can bind to the InsP3Rs. The reduced charge of these compounds would lead to them being unable to evoke the conformational change required for receptor activation, hence they would acts as competitive antagonists.15 None of the 4-position-modified compounds synthesised exhibited agonist or antagonist activity at InsP3Rs, leading to the conclusion that the 4-position phosphate group is essential for InsP3R binding.15 Furthermore, we hypothesised that the conformational change undergone by InsP3Rs upon InsP3 binding is modest, and mediated by interaction of the 5-position phosphate of InsP3 with its receptor. To test this hypothesis, our attention turned to the development of InsP3 derivatives that contain phosphate bioisosteres at the 5-position (Fig. 1). Again, phosphate isosteres that possess reduced charge, compared to a phosphate group, were utilised. However, more polar isosteres than used previously have been employed, to maximise the likelihood of these compounds binding to the polar InsP3 binding pocket of the InsP3Rs.
Herein, we report the synthesis of four 5-position-modified InsP3 derivatives (Fig. 1). It is shown that, while compounds with a methyl phosphate ester (2) or a sulfate group (5) at the 5-position retain InsP3R agonist activity, compounds with a methylphosphonate (3) and a carboxymethyl (4) derivative act as InsP3R antagonists.
The synthesis of compounds 3–5 commenced from myo-inositol, and employed a well-established sequence of transformations to afford the chiral, non-racemic, camphor derivative 7 (Scheme 1).16,17 Removal of the chiral auxiliary under acidic conditions followed by treatment with dibutyltin oxide and benzyl bromide furnished the desired 3-O-benzyl regioisomer (8) in high yield. Phosphitylation and oxidation of the 1- and 4-position hydroxyl groups gave the protected bisphosphate. The allyl group was removed by treatment with PdCl2 in methanol, yielding the key intermediate 9. The methyl phosphonate was installed using conditions reported by Dreef et al. (Scheme 2).7,8 In our hands, this reaction was capricious and the greatest success was achieved when using freshly prepared reagents and rigorously dry conditions. Hydrogenolysis in the presence of sodium bicarbonate afforded the methyl phosphonate 3 as its presumed pentakis sodium salt. The carboxymethyl derivative 4 was synthesised by cleavage and oxidation of the allyl group,18,19 followed by hydrogenolysis as before. The sulfate 5 was afforded by reaction of 9 with sulfur trioxide in pyridine, followed by hydrogenolysis.
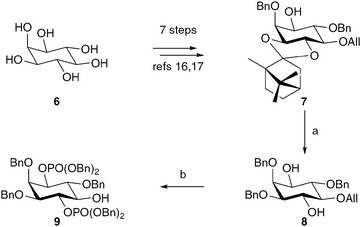 | ||
| Scheme 1 The synthesis of the key intermediate 9. Reagents and conditions: a. i. AcCl, MeOH, CH2Cl2, 83%; ii. Bu2SnO, BnBr, TBABr, 3 Å molecular sieves, MeCN, 79%; b. i. 1. (BnO)2PNiPr2, 1H-tetrazole, CH2Cl2; 2. mCPBA, CH2Cl2, 81%; ii. PdCl2, MeOH, 80–84%. | ||
![The synthesis of the 5-position-modified InsP3 derivatives 3–5. Reagents and conditions: a. i. 1. Bis[6-(trifluoromethyl)benzotriazol-1-yl] methylphosphonate, pyridine; 2. BnOH, 36–60%; ii. Pd black, H2, tBuOH, H2O, NaHCO3, 93%. b. i. RuCl3·H2O, NaIO4, CCl4, MeCN, H2O, 44%; ii. Pd black, H2, tBuOH, H2O, NaHCO3, 98%. c. i. SO3, pyridine, 52%; ii. tBuOH, H2O, NaHCO3, 94%.](/image/article/2011/CC/c0cc03003a/c0cc03003a-s2.gif) | ||
| Scheme 2 The synthesis of the 5-position-modified InsP3 derivatives 3–5. Reagents and conditions: a. i. 1. Bis[6-(trifluoromethyl)benzotriazol-1-yl] methylphosphonate, pyridine; 2. BnOH, 36–60%; ii. Pd black, H2, tBuOH, H2O, NaHCO3, 93%. b. i. RuCl3·H2O, NaIO4, CCl4, MeCN, H2O, 44%; ii. Pd black, H2, tBuOH, H2O, NaHCO3, 98%. c. i. SO3, pyridine, 52%; ii. tBuOH, H2O, NaHCO3, 94%. | ||
The methyl phosphate ester 2 was synthesised from intermediate 11, which was prepared as described previously.20 Deprotection of the TIPS group, using TBAF, furnished a 1,4-diol. This compound was phosphitylated and oxidised to give the protected bisphosphate 12 (Scheme 3). Oxidative cleavage of the PMB ether, followed by phosphitylation and oxidation, gave the fully protected methyl phosphate ester 13. Hydrogenolysis, as above, furnished 2 as the presumed pentakis sodium salt.
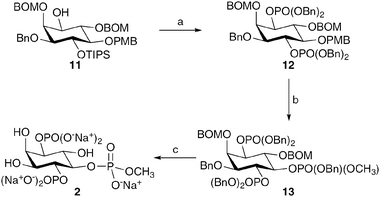 | ||
| Scheme 3 The synthesis of the 5-position-modified InsP3 derivative 2. Reagents and conditions: a. i. TBAF, THF, 89%; ii. 1. (BnO)2PNiPr2, 1H-tetrazole, CH2Cl2; 2. mCPBA, CH2Cl2, 89%; b. i. CAN, MeCN, H2O, 75%; ii. 1. (BnO)(OCH3)PNiPr2, 1H-tetrazole, CH2Cl2; 2. mCPBA, CH2Cl2, 92%; c. Pd black, H2, tBuOH, H2O, NaHCO3, 51%. | ||
The ability of 2–5 to mobilise Ca2+ or inhibit InsP3-induced Ca2+ release (IICR) was investigated using either a unidirectional 45Ca2+ flux assay in permeabilised cells or a Ca2+ fluorescence assay in sea urchin egg homogenate. In the 45Ca2+ assay, either MEF cells or L15 cells were employed.¶ Using the 45Ca2+ assay in MEF cells, the methyl phosphate ester (2) acts as an InsP3R agonist, with an EC50 = 9.7 μM (InsP3EC50 = 5.2 μM in the same system, see ESI‡). The sulfate 5 also acts as a weak InsP3R agonist, evoking InsP3R-mediated Ca2+ release when applied at 100 and 300 μM in permeabilised L15 cells (see ESI‡). The methylphosphonate 3 showed little activity when applied to permeabilised L15 cells alone. However, application of 3 (300 μM) before and during application of InsP3 (0.25 μM) caused approximately 40% reduction in the amount of IICR (Fig. 2).
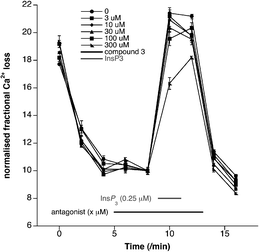 | ||
| Fig. 2 Fractional loss plots of 45Ca2+ in L15 cells when treated with the methylphosphonate analogue 3. The cells were permeabilised, leading to an initial loss of 45Ca2+ (0–3 min). Application of InsP3 evokes a large loss of 45Ca2+ as a result of InsP3R activation. This loss of 45Ca2+ is reduced by ∼40% in the presence of the methylphosphonate 3 (300 μM), indicating that this compound is behaving as an InsP3R antagonist. | ||
The biological activity of 3 and 4 was also investigated using the sea urchin egg homogenate-based assay.21–23|| In this assay both 3 and 4 behaved as InsP3R antagonists (Fig. 3). The methylphosphonate (3) gave 37% inhibition of IICR when applied at a concentration of 1.67 mM. The carboxymethyl derivative 4 demonstrated 58% inhibition of IICR when applied at a concentration of 5.00 mM. In both cases, the compounds evoked no Ca2+ release when applied in the absence of InsP3. Additionally, preliminary data (not shown) indicate that 4 has no significant effect on cyclic adenosine diphosphate ribose-induced Ca2+ release, which occurs viaryanodine receptors. These data indicate that 4 is acting at InsP3Rs and not non-selectively at other Ca2+ channels or pumps. Taken together, our data show that 3 and 4 are acting as InsP3R antagonists. These data represent the first conclusive proof that InsP3 derivatives can act as antagonists at InsP3Rs.
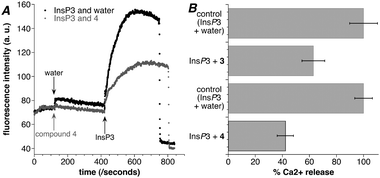 | ||
| Fig. 3 Compounds 3 and 4 inhibit IICR. (A) A representative trace obtained from sea urchin egg homogenate loaded with the fluorescent Ca2+ dye Fluo-3. Application of InsP3 (400 nM) evokes an increase in fluorescence due to Ca2+ release from InsP3Rs (black trace). Application of compound 4 (5.00 mM) alone does not cause Ca2+ release, however, application of InsP3 (400 nM) in the presence of 4 evokes significantly reduced Ca2+ release, demonstrating that 4 is an InsP3R antagonist (grey trace). (B) A comparison of the Ca2+ release evoked by InsP3 alone and in the presence of compound 3 or compound 4. The Ca2+ release caused by InsP3 (400 nM) alone is taken as 100% (top bar). When InsP3 (400 nM) and compound 3 (1.67 mM) were applied to the sea urchin egg homogenate, a smaller increase in fluorescence (63%, n = 4) was observed (middle bar), consistent with reduced Ca2+ release from InsP3Rs. When InsP3 and compound 4 (5.00 mM) were applied a similarly reduced increase in fluorescence was seen (42%, n = 4, bottom bar). These data show that both 3 and 4 are acting as InsP3R antagonists. Error bars show SEM. | ||
The structural similarity of the methyl phosphate ester 2 and the methylphosphonate 3, which differ by one oxygen atom yet possess opposing activity at InsP3Rs, potentially provides unique insight into the mechanism of InsP3R activation and inhibition. Our current hypothesis is that all compounds bind to the InsP3R ligand-binding domain in a similar orientation to InsP3. The methyl phosphate ester (2) and the sulfate (5) derivatives have the same number of atoms as a phosphate group (such as in InsP3) from which to make polar contacts. However, the methylphosphonate (3) and the carboxylate (4) have one less polar atom at the 5-position. It is possible that this reduced interaction with the receptor renders the compounds unable to evoke the conformation change required for InsP3R activation, and hence 3 and 4 act as competitive antagonists at InsP3Rs.
In conclusion, four InsP3 derivatives with phosphate bioisosteres at the 5-position have been synthesised. Biological evaluation of these compounds has revealed the methyl phosphate ester (2) and sulfate (5) derivatives retain InsP3R agonist activity. However, the methylphosphonate (3) and carboxymethyl (4) derivatives behave as InsP3R antagonists. These studies provide the first proof that InsP3 derivatives can act as antagonists at InsP3Rs. The compounds developed herein will likely prove useful tools for the study of InsP3Rs and provide new insight into the mechanism of InsP3R activation and inhibition.
The authors thank the University of St Andrews (DB) and the BBSRC (NSK, TA) for funding. SJC thanks St Hugh's College, Oxford, for research support.
Notes and references
- M. J. Berridge, M. D. Bootman and H. L. Roderick, Nat. Rev. Mol. Cell Biol., 2003, 4, 517 CrossRef CAS.
- M. J. Berridge, Biochim. Biophys. Acta, 2009, 1793, 933 CAS.
- B. V. L. Potter and D. Lampe, Angew. Chem., Int. Ed. Engl., 1995, 34, 1933 CrossRef CAS.
- S. J. Conway and G. J. Miller, Nat. Prod. Rep., 2007, 24, 687 RSC.
- A. M. Rossi, A. M. Riley, S. C. Tovey, T. Rahman, O. Dellis, E. J. A. Taylor, V. G. Veresov, B. V. L. Potter and C. W. Taylor, Nat. Chem. Biol., 2009, 5, 631 CrossRef CAS.
- S. T. Safrany, R. A. Wilcox, C. Liu, D. Dubreuil, B. V. L. Potter and S. R. Nahorski, Mol. Pharmacol., 1993, 43, 499 CAS.
- C. E. Dreef, J. P. Jansze, C. J. J. Elie, G. A. van der Marel and J. H. van Boom, Carbohydr. Res., 1992, 234, 37 CrossRef CAS.
- C. E. Dreef, W. Schiebler, G. A. van der Marel and J. H. van Boom, Tetrahedron Lett., 1991, 32, 6021 CrossRef CAS.
- T. Maruyama, T. Kanaji, S. Nakade, T. Kanno and K. Mikoshiba, Jpn. J. Biochem., 1997, 122, 498 Search PubMed.
- J. G. Bilmen, L. L. Wootton, R. E. Godfrey, O. S. Smart and F. Michelangeli, Eur. J. Biochem., 2002, 269, 3678 CrossRef CAS.
- M. D. Bootman, T. J. Collins, L. Mackenzie, H. L. Roderick, M. J. Berridge and C. M. Peppiatt, FASEB J., 2002, 16, 1145 CrossRef CAS.
- C. M. Peppiatt, T. J. Collins, L. Mackenzie, S. J. Conway, A. B. Holmes, M. D. Bootman, M. J. Berridge, J. T. Seo and H. L. Roderick, Cell Calcium, 2003, 34, 97 CrossRef CAS.
- I. Bosanac, J. R. Alattia, T. K. Mal, J. Chan, S. Talarico, F. K. Tong, K. I. Tong, F. Yoshikawa, T. Furuichi, M. Iwai, T. Michikawa, K. Mikoshiba and M. Ikura, Nature, 2002, 420, 696 CrossRef CAS.
- C. W. Taylor, P. C. A. da Fonseca and E. P. Morris, Trends Biochem. Sci., 2004, 29, 210 CrossRef CAS.
- D. Bello, T. Aslam, G. Bultynck, A. M. Z. Slawin, H. L. Roderick, M. D. Bootman and S. J. Conway, J. Org. Chem., 2007, 72, 5647 CrossRef CAS.
- G. F. Painter, S. J. A. Grove, I. H. Gilbert, A. B. Holmes, P. R. Raithby, M. L. Hill, P. T. Hawkins and L. R. Stephens, J. Chem. Soc., Perkin Trans. 1, 1999, 923 RSC.
- S. J. Conway, J. Gardiner, S. J. A. Grove, M. K. Johns, Z. Y. Lim, G. F. Painter, D. Robinson, C. Schieber, J. W. Thuring, L. S. M. Wong, M. X. Yin, A. W. Burgess, B. Catimel, P. T. Hawkins, N. T. Ktistakis, L. R. Stephens and A. B. Holmes, Org. Biomol. Chem., 2010, 8, 66 RSC.
- P. H. J. Carlsen, T. Katsuki, V. S. Martin and K. B. Sharpless, J. Org. Chem., 1981, 46, 3936 CrossRef CAS.
- C. S. Liu and B. V. L. Potter, J. Org. Chem., 1997, 62, 8335 CrossRef CAS.
- N. S. Keddie, G. Bultynck, T. Luyten, A. M. Z. Slawin and S. J. Conway, Tetrahedron: Asymmetry, 2009, 20, 857 CrossRef CAS.
- D. L. Clapper, T. F. Walseth, P. J. Dargie and H. C. Lee, J. Biol. Chem., 1987, 262, 9561 CAS.
- A. Galione, S. Patel and G. C. Churchill, Biol. Cell, 2000, 92, 197 CrossRef CAS.
- A. J. Morgan and A. Galione, Methods, 2008, 46, 194 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Additional biological data, compound characterisation data and NMR spectra for compounds 2–5. See DOI: 10.1039/c0cc03003a |
| § These authors contributed equally to this work. |
| ¶ MEF cells are immortalised wild-type mouse embryonic fibroblast cells expressing normal levels of InsP3R1 and InsP3R3. L15 cells are mouse L-fibroblast cells stably over-expressing InsP3R1 by ∼8–10 fold. |
| || Sea urchin eggs possess only one subtype of InsP3R. |
| This journal is © The Royal Society of Chemistry 2011 |
