A metal complex that imitates a micelle†‡
Nicola
Giri
and
Stuart L.
James
Centre for the Theory and Application of Catalysis, School of Chemistry and Chemical Engineering, Queen's University Belfast, David Keir Building, Stranmillis Road, Belfast BT9 5AG, UK. E-mail: s.james@qub.ac.uk
First published on 12th May 2010
Abstract
A metal complex with a micelle-like, core–shell structure adopts higher nuclearity in water than in organic solvents, thereby imitating also the growth of a micelle, but through covalent rather than non-covalent aggregation.
The concept of ‘metal-complex-as-amphiphile’, in which metal complexes act as components of metallo-micelles, is well established.1,2 However, an alternative concept, that of ‘metal-complex-as-micelle’, in which a single metal complex imitates the structure and behaviour of a complete micelle, has not yet been described. Here we report an example of such a metal complex. The structure is shown generically in Fig. 1a, and can be seen as a hybrid of a metal complex and a micelle, since it is based on amphiphilic ligands which form a hydrophobic interior surrounded by hydrophilic head groups. Importantly, the stability of this type of complex in water is therefore not only due to metal coordination but also hydrophobic interactions. For the complex reported here, we show that the structure it adopts is indeed directed by hydrophobic interactions. In particular, in pure water the complex is dinuclear with bridging ligands. However, if the hydrophobic interactions are negated or overcome by lowering the solvent polarity or pH, the complex switches to a mononuclear state, with its ligands changing from bridging to chelating. In this way the complex therefore imitates a true micelle by growing or shrinking in response to its medium, but by a non-micelle-like mechanism which requires breaking and remaking of coordination bonds.
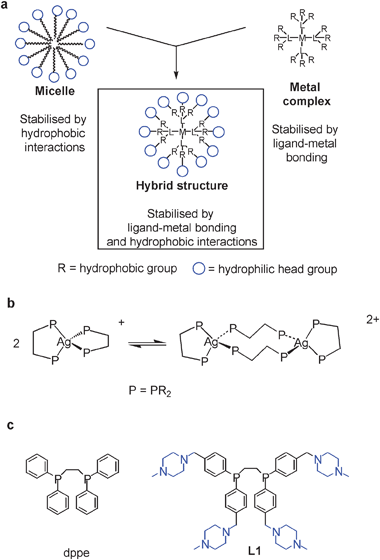 | ||
| Fig. 1 (a) Schematic of micelles, metal complexes and hybrid structures, (b) equilibrium between mono-silver and di-silver complexes of diphosphines and (c) structures of dppe and L1. | ||
The system described here is based around types of silver complex formed by bidentate phosphines such as dppe, bis(diphenylphosphino)ethane. The complex [Ag(dppe)2]+ is normally mononuclear (i.e. both ligands chelating) but can become dinuclear for certain ligand variants or upon crystallisation (Fig. 1b).3 Therefore the monomer–dimer equilibrium appears to be finely balanced for this type of complex. In the present work, the complex was given a pseudo-micellar structure by using an amphiphilic variant of dppe. Specifically, hydrophilic piperazine groups were added to the para positions of the diphosphine's hydrophobic aryl groups. The amphiphilic ligand (L1, Fig. 1c) can therefore generate complexes with hydrophobic cores surrounded by hydrophilic head groups.
The behaviours of free, uncoordinated L1 and its silver(I) complex are summarised in Fig. 2. Free L1 forms micelles in water, behaving as a non-ionic surfactant,3 as revealed by the following observations: (i) a critical micelle concentration (CMC) of 5 mM was determined using the dye inclusion technique,5 corroborated by DLS (dynamic light scattering), (ii) DLS shows particles of hydrodynamic diameter 5.0 nm in D2O (20 mM, pH 9.0, Fig. 3b) with low polydispersity whereas chloroform solutions showed no aggregation, (iii) the size of the micelles in water was not influenced by ionic strength since 10 mM NaCl solutions gave identical results to those in deionized water, (iv) concentration-dependent line broadening is seen in 1H and 31P-NMR spectra in D2O with upfield 31P shifts relative to those in CDCl3 (Fig. 3a), and (v) a cloud point (33 °C) was observed in water which is a phenomenon well known for related non-ionic surfactants.6
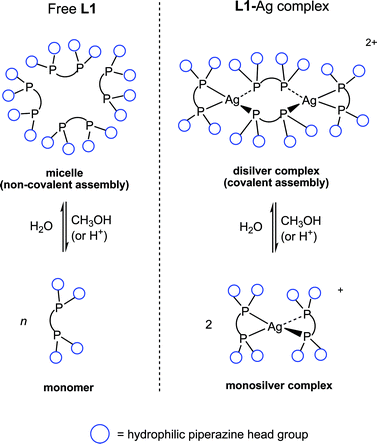 | ||
| Fig. 2 Schematic diagram to show the structures adopted by the free ligand and its silver complex change in response to the medium. | ||
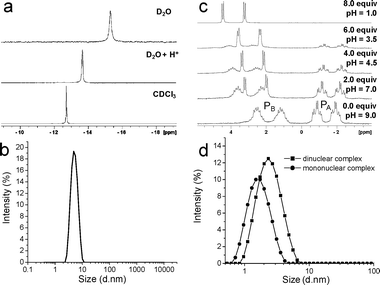 | ||
| Fig. 3 (a) 31P NMR spectra for L1, (b) dynamic light scattering of L1 micelles in water, (c) 31P NMR spectra showing interconversion between mono- and di-nuclear silver complexes with addition of HOTf, and (d) dynamic light scattering for the mono- and di-nuclear complexes. | ||
Addition of methanol (to 30% v/v) or acid (HO3SCF3) to aqueous L1 disrupted the micelles as shown by DLS and sharpening of NMR signals. Micelle disruption by methanol is expected because it lowers the hydrophobicity of the medium. Micelle disruption by acid is also expected since protonation generates positive charges on the piperazine groups, increasing the water-solubility of L1 as well as electrostatic repulsion between the piperazine head groups in the micelles.4 The free ligand therefore serves to illustrate typical micellar behaviour.
For the metal complex, in pure D2O a 2 ∶ 1 mixture of L1 and AgOTf was found to adopt the dinuclear structure [Ag2(L1)4]2+ as revealed by 31P-NMR spectroscopy. The spectrum consists of two pairs of multiplets at δ = −1.5 (PA) and 1.9 (PB) which can be assigned to the chelating and bridging ligands respectively (Fig. 3c). PA consists of a pair of triplets of doublets from which both P–Ag and PA–PB coupling constants could be extracted (1J(109Ag–31PA) = 225 Hz and 2J(PA–PB) = 42 Hz). The PB signal is complicated by coupling to both Ag centres and the pattern was not readily analysable. However, based on the separation between the two symmetrical halves of the multiplet, 1J(109Ag–31PB) was estimated to be 290 Hz. The magnitudes of the silver–phosphorus couplings are diagnostic of AgP4 coordination centres.7 The assignments were consistent with a 31P COSY NMR spectrum which showed PA and PB to be mutually coupled. Further substantiation came from literature values for the analogous disilver complex formed by 1,2-bis(di-4-pyridylphosphino)ethane.3c The 1H-NMR spectrum also showed the expected two ligand environments.
Addition of methanol or acid to the disilver complex converted it to the mononuclear bis-chelate [Ag(L1)2]+ (Fig. 2, right) as indicated by simpler NMR spectra and DLS (Fig. 3c, d). At pH 1.0 with four equivalents of HO3SCF3 per L1 (one for each piperazine group) a single 31P environment coupled to 107Ag and 109Ag was seen (δP = 3.8 ppm, 1J109Ag−31P = 263 Hz). The data are similar to those for the analogous dppe complex [Ag(dppe)2]+ (4.4 ppm, 266 Hz3a).
Molecular models of the mono- and di-silver complexes are shown in Fig. 4. They exhibit micelle-like core–shell structures, each complex having a hydrophobic interior surrounded by hydrophilic head groups. Importantly, the models also show that some of the hydrophobic interior of the monosilver complex remains exposed to the solvent and that in the disilver complex more of this hydrophobic surface is shielded from the solvent. This helps to understand adoption of the dinuclear structure in pure water as being driven by hydrophobic interactions.
![Modelled structures of [Ag(L1)2]+ (above) and [Ag2(L1)4]2+ (below) showing their pseudo-micellar structures. Piperazine substituents = blue, Ag = white, and P = yellow. For clarity H-atoms are not shown.](/image/article/2011/CC/c0cc00648c/c0cc00648c-f4.gif) | ||
| Fig. 4 Modelled structures of [Ag(L1)2]+ (above) and [Ag2(L1)4]2+ (below) showing their pseudo-micellar structures. Piperazine substituents = blue, Ag = white, and P = yellow. For clarity H-atoms are not shown. | ||
Overall, as shown in Fig. 2, there are similarities between the behaviour of the free ligand and its Ag complex. In pure water, where hydrophobic interactions are present, free L1 forms micelles and its silver complex is large (dinuclear). When hydrophobic interactions are absent (in organic solvents or low pH) free L1 is monomeric and its silver complex is correspondingly small (mononuclear). The driving forces for the changes in structure are the same for the free ligand and metal complex. For both species methanol decreases the hydrophobic interactions. Similarly, protonation destabilises both the micelle and the disilver complex by causing head group–head group repulsions between the piperazine groups. However, an important difference is that whereas the micelle is a non-covalent assembly of individual molecules, the disilver complex is a single, covalently bonded molecule. Changing between mono- and di-nuclear complexes requires breaking and then remaking of coordination bonds. Therefore, the silver complex can be seen to imitate the growth of a true micelle but through covalent rather than non-covalent aggregation.
In summary, we report that hydrophobic interactions can be important in directing the structures adopted by metal complexes which have micelle-like core–shell configurations. Previous work on the aggregation of metal complexes by hydrophobic interactions can be described as ‘metal-complex-as-amphiphile’, because of the head-group-and-tail structure of the complexes and the fact that non-covalent aggregation preserves the original molecular species. However, the situation here is better described as ‘metal-complex-as-micelle’.8 This description relates to the structural similarity of the complexes to micelles (core–shell) as well as the fact that the larger species stabilised by hydrophobic interactions is a single metal complex and not a non-covalent aggregate.
The concept may also be relevant to other metal complexes based on hydrophobic ligands to which hydrophilic substituents have been added (to render them water soluble). Examples of such complexes can be found in homogeneous catalysis9 and medicinal chemistry.3c,10 It also points to a general way of making coordination systems which respond to their medium in ways typically associated with micelles. Work is currently underway with further discrete and polymeric systems.
We are grateful to A. P. de Silva for valuable discussions.
Notes and references
- (a) F. M. el Torki, R. H. Schmehl and W. F. Reed, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 349 RSC; (b) M. Yashiro, K. Matysumoto and S. Yoshikawa, Chem. Lett., 1989, 985 CAS; (c) D. W. Bruce, J. D. Holbrey, A. R. Tajbakhsh and G. J. T. Tiddy, J. Mater. Chem., 1993, 3, 905 RSC; (d) D. W. Bruce, I. R. Denby, G. J. T. Tiddy and J. M. Watkins, J. Mater. Chem., 1993, 3, 911 RSC; (e) J. D. Holbrey, G. J. T. Tiddy and D. W. Bruce, J. Chem. Soc., Dalton Trans., 1995, 1769 RSC; (f) B. Donnio, Curr. Opin. Colloid Interface Sci., 2002, 7, 371 CrossRef CAS; (g) J. Bowers, M. J. Danks and D. W. Bruce, Langmuir, 2003, 19, 292 CrossRef CAS; (h) P. Ghosh, T. K. Khan and P. K. Bharadwaj, Chem. Commun., 1996, 189 RSC; (i) I. A. Fallis, P. C. Griffiths, P. M. Griffiths, D. E. Hibbs, M. B. Hursthouse and A. L. Winnington, Chem. Commun., 1998, 665 RSC; (j) U. Maran, H. Conley, M. Frank, A. M. Arif, A. M. Orendt, D. Britt, V. Hlady, R. Davis and P. J. Stang, Langmuir, 2008, 24, 5400 CrossRef CAS; (k) C. Ferroud, H. Borderies, E. Lasri, A. Guy and M. Port, Tetrahedron Lett., 2008, 49, 5972 CrossRef CAS; (l) G. Zhou, J. He and I. Harruna, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4204 CrossRef CAS; (m) A. Warshawsky, I. Rogachev, Y. Patil, L. Baszkin, A. Weiner and J. Gressel, Langmuir, 2001, 17, 5621 CrossRef CAS; (n) E. Valls, A. Solsona, J. Suades, R. Mathieu, F. Comelles and C. López-Iglesias, Organometallics, 2002, 21, 2473 CrossRef CAS.
- For metal-containing covalent polymers adopting micellar structures see: T. Gädt, N. S. Ieong, G. Cambridge, M. A. Winnik and I. Manners, Nat. Mater., 2009, 8, 144 Search PubMed.
- (a) S. J. Berners-Price, C. Brevard, A. Pagelot and P. J. Sadler, Inorg. Chem., 1985, 24, 4287; (b) Y. Huahui, Z. Lansun, X. Yunjie and Z. Qianer, Chin. J. Inorg. Chem., 1992, 8, 65; (c) S. J. Berners-Price, R. J. Bowen, P. J. Harvey, P. C. Healy and G. A. Koutsantonis, J. Chem. Soc., Dalton Trans., 1998, 1743 RSC; (d) V. Saboonchian, G. Wilkinson, B. Hussainbates and M. B. Hursthouse, Polyhedron, 1991, 10, 737 CrossRef CAS; (e) S. L. James, Chem. Soc. Rev., 2009, 38, 1744 RSC.
- For overviews of micellar assembly containing the points raised here see: (a) R. J. Stokes and D. F. Evans, Fundamentals of Interfacial Engineering, Wiley-VCH, Inc., 1996, ch. 5 Search PubMed; (b) J. H. Fendler, Membrane Mimetic Chemistry, Wiley-Interscience, 1982 Search PubMed.
- K. G. Furton and A. Norelus, J. Chem. Educ., 1993, 70, 254 CrossRef CAS.
- R. Strey, R. Schomäcker, D. Roux, F. Nallet and U. Olsson, J. Chem. Soc., Faraday Trans., 1990, 86, 2253 RSC.
- Methods in Stereochemical Analysis, in Phosphorus-31 NMR Spectroscopy in Stereochemical Analysis, ed. J. G. Verkade and L. D. Quin, VCH Publishers Inc, Deerfield Beach, FL, 1987, vol. 8 Search PubMed.
- The complexes should not be confused with actual micelles however, the latter being held together entirely by non-covalent interactions with different thermodynamic implications. The complexes here can be thought of as a type of ‘single-molecule micelle’, see C. N. Moorefield and G. R. Newkome, C. R. Chim., 2003, 6, 715 Search PubMed , but which are constitutionally dynamic and responsive.
- K. H. Shaughnessy, Chem. Rev., 2009, 109, 643 CrossRef CAS; J. W. Ellis, K. N. Harrison, P. A. T. Hoye, A. G. Orpen, P. G. Pringle and M. B. Smith, Inorg. Chem., 1992, 31, 3026 CrossRef CAS; D. J. Darensbourg, J. B. Robertson, D. L. Larkins and J. H. Reibenspies, Inorg. Chem., 1999, 38, 2473 CrossRef CAS; I. T. Horváth, R. V. Kastrup, A. A. Oswald and E. J. Mozeleski, Catal. Lett., 1989, 2, 85 CrossRef CAS.
- E. Benelita, C. Levine, I. Ubarretxena-Belandia, A. Varela-Ramirez, R. J. Aguilera, R. Ovalle and M. Contel, Eur. J. Inorg. Chem., 2009, 3421; J. J. Liu, P. Galettis, A. Farr, A. Maharaj, H. Samarasiha, A. C. McGechan, B. C. Baguley, R. J. Bowen, S. J. Berners-Price and M. J. McKeage, J. Inorg. Biochem., 2008, 102, 303 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Synthesis of L1, experimental details, details of modelling. See DOI: 10.1039/c0cc00648c |
| This journal is © The Royal Society of Chemistry 2011 |
