Silver-catalyzed furoquinolines synthesis: from nitrogen effects to the use of silver imidazolate polymer as a new and robust silver catalyst†‡
Evelyne
Parker
a,
Nicolas
Leconte
b,
Thomas
Godet
a and
Philippe
Belmont
*ab
aUniversity of Lyon and CNRS, UMR 5246 ICBMS, 43 Boulevard du 11 Novembre 1918, 69622 Villeurbanne (Lyon), France
bInstitut Curie and CNRS, UMR 176 CSVB, Institut Curie, 26 rue d'Ulm, 75248 Paris cedex 05, France. E-mail: Philippe.Belmont@curie.fr; Web: http://www.curie.fr/equipe/388/lang/_gb Fax: +33 (0)1.56.24.66.31; Tel: +33 (0)1.56.24.68.24
First published on 23rd August 2010
Abstract
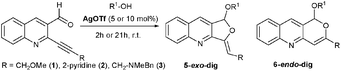
Silver-catalyzed tandem acetalization and cycloisomerization reactions were found to lead to various furoquinolines, and a nitrogen effect was noticed for AgOTf reactivity, since the cyclization mode switched from 6-endo-dig to 5-exo-dig; from these observations silver imidazolate polymer is proposed as a stable silver catalyst.
Of the coinage metals (Cu, Ag and Au), silver has received less attention as a catalyst,1 since it has been mostly used in stoichiometric quantities in oxidation reactions, as an halogen scavenger, or for anion metathesis reactions (counterion exchange). Our main focus is directed towards silver-catalyzed cycloisomerization reactions.2 However, based on Marshall’s work3 on allenyl ketones/aldehydes, only a few groups have contributed to the development of silver-catalyzed cycloisomerization reactions involving carbonyl groups and alkynyl moieties.4
We recently reported a versatile access to pyranoquinoline and furoquinoline cores4d and found that the nature of the silver salt used (AgOTfvs. Ag2O) could force the regioselectivity (6-endo-dig vs. 5-exo-dig) of the cycloisomerization reaction. During further experiments intended to study the role of traces of bases or acids in the reaction outcome, we found an interesting nitrogen effect (Table 1): the presence of a nitrogen group in the R or R1 substituents or in the reaction media (as an additive) had no impact on Ag2O catalysis, whereas it changed the behaviour of AgOTf dramatically. Indeed, compound 1, bearing a methoxymethyl substituent on the alkynyl group (entry 1, Table 1), gave exclusively selective 6-endo-dig cyclization, whereas the 5-exo-dig cyclization mode occurred preferentially for compound 2 (pyridinyl group, entry 2 (Table 1), 5-exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6-endo 85
6-endo 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) or compound 3 (–CH2–NMeBn, entry 3 (Table 1), 100
15) or compound 3 (–CH2–NMeBn, entry 3 (Table 1), 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0). From compound 1 (entry 4, Table 1), the use of an aminoalcohol instead of methanol (as solvent and nucleophile) afforded opposite cycloisomerization selectivity (5-exo-dig, 100%) to entry 1 (6-endo-dig, 100%). Moreover, reacting compound 1 in the presence of various additives provided interesting information (Table 1). Several amine derivatives (1 equiv.) were added to the reaction mixture – one of these, quinoline (entry 6, Table 1), did not have any effect on 6-endo-dig cycloisomerization selectivity compared to entry 1 (Table 1), which was not surprising, since our starting materials all bear this heterocyclic nucleus. But additives in entries 7 and 8 (Table 1) were used as mimics of compounds 2 and 3, since the pyridine or triethylamine is closely related to the alkynyl substituent in compounds 2 and 3. Although the conversion was moderate in the case of triethylamine (55%), both reactions led preferentially to the 5-exo-dig product (5-exo
0). From compound 1 (entry 4, Table 1), the use of an aminoalcohol instead of methanol (as solvent and nucleophile) afforded opposite cycloisomerization selectivity (5-exo-dig, 100%) to entry 1 (6-endo-dig, 100%). Moreover, reacting compound 1 in the presence of various additives provided interesting information (Table 1). Several amine derivatives (1 equiv.) were added to the reaction mixture – one of these, quinoline (entry 6, Table 1), did not have any effect on 6-endo-dig cycloisomerization selectivity compared to entry 1 (Table 1), which was not surprising, since our starting materials all bear this heterocyclic nucleus. But additives in entries 7 and 8 (Table 1) were used as mimics of compounds 2 and 3, since the pyridine or triethylamine is closely related to the alkynyl substituent in compounds 2 and 3. Although the conversion was moderate in the case of triethylamine (55%), both reactions led preferentially to the 5-exo-dig product (5-exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6-endo ratio, 75
6-endo ratio, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 for pyridine and 85
25 for pyridine and 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 for triethylamine).
15 for triethylamine).
| Entry | R | R1–OH | Additive | pKac | Conversion (%)d | Selectivityd (5-exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6-endo) 6-endo) |
|---|---|---|---|---|---|---|
| a Entries 1–4: the reaction was carried out for 2 h at room temperature (r.t.) in MeOH (500 equiv.) or Et2N(CH2)2OH (150 equiv.). b Entries 1 and 5–11: the reaction was carried out for 21 h with 1 (0.05 M) in dichoroethane (DCE), with MeOH (1.2 equiv.), at r.t., with AgOTf (10 mol%) and additive (1 equiv.). c Determined in water for deprotonation of conjugated acid5 (values in parentheses were determined in acetonitrile6). d Determined by 1H NMR analysis. e DABCO = 1,4-diazabicyclo[2.2.2]octane; DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; TTBP = 2,4,6-tri-tert-butylpyrimidine. f Instead of AgOTf catalysis, this reaction was performed with 10 mol% of triflic acid (TfOH). | ||||||
| 1 | 1 | MeOH | — | — | 100 (2 h or 21 h) | 0 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 2 | 2 | MeOH | — | — | 100 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 3 | 3 | MeOH | — | — | 100 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | 1 | Et2N(CH2)2OH | — | — | 100 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 5 | 1 | MeOH | DABCO e | 8.82 | 0 | — |
| 6 | 1 | MeOH | Quinoline | 4.92 | 95 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 7 | 1 | MeOH | Pyridine | 5.21 (12.53) | 100 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 8 | 1 | MeOH | Et3N | 10.75 (18.82) | 55 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 9 | 1 | MeOH | DBU e | (24.34) | 10 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 10 | 1 | MeOH | TTBP e | — | 100 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 11 | 1 | MeOH | TfOH f | — | 5 (2 h), 50 (77 h) | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
Attempts at cyclization in the presence of DABCO (entry 5, Table 1) didn't afford any reaction. According to previously reported results,7 this could be due to insoluble polymeric [Agn-DABCOm]n, which would inhibit silver reactivity. Under the same reaction conditions DBU as an additive led to very low conversion (10%, entry 9, Table 1) but in this case some of the catalytic system formed8 could account for the reversal of selectivity (5-exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6-endo ratio, 90
6-endo ratio, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Since traces of triflic acid (TfOH)9 could also explain the transformation studied here, the reaction was tested with and without a proton scavenger (TTBP,10 entries 1 vs. 10, Table 1). We were pleased to note by 1H NMR analysis that the only product formed in both cases was the 6-endo-dig pyranoquinoline derivative, ruling out any possibility of Brønsted acid catalysis (TTBP is too hindered for complexing silver). Additional experiments where AgOTf was replaced by 10 mol% TfOH (entry 11, Table 1), led only to 5% conversion in favor of 6-endo-dig compound (vs. 100% conversion for AgOTf in 2 h). A 50% conversion was reached after 77 h with TfOH (10 mol%). TTBP and TfOH results confirm the critical need for the silver cation and therefore exclude the hypothesis of Brønsted acid catalysis.
10). Since traces of triflic acid (TfOH)9 could also explain the transformation studied here, the reaction was tested with and without a proton scavenger (TTBP,10 entries 1 vs. 10, Table 1). We were pleased to note by 1H NMR analysis that the only product formed in both cases was the 6-endo-dig pyranoquinoline derivative, ruling out any possibility of Brønsted acid catalysis (TTBP is too hindered for complexing silver). Additional experiments where AgOTf was replaced by 10 mol% TfOH (entry 11, Table 1), led only to 5% conversion in favor of 6-endo-dig compound (vs. 100% conversion for AgOTf in 2 h). A 50% conversion was reached after 77 h with TfOH (10 mol%). TTBP and TfOH results confirm the critical need for the silver cation and therefore exclude the hypothesis of Brønsted acid catalysis.
The steric difference around the nitrogen lone pair between quinoline and pyridine does not explain the huge selectivity scrambling; rather, the strength of the complexation seems to be the best explanation.11 Another way to rationalize these results would be ranking by the additive amine pKa values. Further comparison of pKa values (entries 7–9, Table 1) show that the greater the pKa is, the better selectivity the reaction in favour of the 5-exo-dig product.
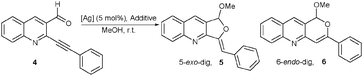
Inspired by the nitrogen effect described above, and also knowing that the same selectivity (5-exo-dig) was seen with silver salts having a counterion pKa greater than 10 (Ag2O, AgO, Ag2CO3),4d we envisioned a new “Ag–nitrogen” catalyst. A known silver imidazolate polymer ([Ag(Im)]n) had the necessary requirements (pKa = 14.52 for imidazolate compared with pKa = 6.92 for imidazole).12 This was isolated in 1964 by Bauman and Wang13 and fully characterized only thirty years later.14 Since then, this polymer has been reported to be useful for its antimicrobial activities,15 as a latent heat-activatable crosslinking catalyst for epoxy resins/coating, and as a stoichiometric promoter in glycosidation reactions.16 But, to our knowledge, no further catalytic properties have been reported. This polymer is stable to light and moisture, making it very useful, since the main drawback mostly encountered with silver catalysts are their high sensitivity to light and/or moisture. Moreover, a mixture of [Ag(Im)]n with various quantities of PPh3 forms soluble complexes that have been characterized,14b,c but that have found no use in catalysis. Therefore, we studied the catalytic properties of [Ag(Im)]n in parallel with Ag2O (Table 2). Ag2O-catalyzed tandem acetalization and cycloisomerization reactions with compound 44d in methanol (entry 1, Table 2) gave furoquinoline 5 accompanied by traces of 6-endo-dig compound 6 (structure confirmed by 2D 15N–1H NMR and also X-ray analysis; data not shown).
| Entry | Catalyst | Additiveb | Time | Conversion (%)c | Selectivityc (5-exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6-endo) 6-endo) |
|---|---|---|---|---|---|
| a The reactions were carried out at 0.05 M in MeOH, at room temperature. b 5 mol%. c Determined by 1H NMR analysis. | |||||
| 1 | Ag2O | — | 30 min | 100 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trace trace |
| 2 | [Ag(Im)]n | — | 40 h | 100 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 3 | [Ag(Im)]n | PPh3 | 10 min | 100 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | [Ag(Im)]n | P(OPh)3 | 10 min | 100 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 5 | — | PPh3 | 48 h | 0 | — |
| 6 | AgNO3 | — | 12 h | 66 | trace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
Under [Ag(Im)]n heterogeneous catalysis, a longer reaction time was required (40 h (entry 2, Table 2), compared with 30 min for Ag2O (entry 1, Table 2) but the reaction was cleaner and selective for the 5-exo-dig compound 5. Then, PPh3 or P(OPh)3 were used as additives in the reaction with [Ag(Im)]n (entries 3 and 4, Table 2), which dramatically shortened the reaction time (only 10 min in both cases) and led to furoquinoline 5 as the only product. These additives helped to generate a soluble catalyst and therefore, under homogeneous conditions, the reaction proceeded faster. A reaction with PPh3 and no silver catalyst (entry 5, Table 2) gave no results. More interestingly, using AgNO3 (the starting material for [Ag(Im)]n synthesis), the major compound formed was the 6-endo-dig derivative 6 (entry 6, Table 1), proving that [Ag(Im)]n was not contaminated with residual AgNO3.
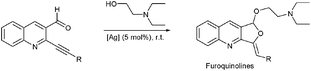
Having the optimized conditions in hand, we examined the reactivity of AgOTf, Ag2O and [Ag(Im)]n/PPh3 on a set of functionalized quinolines (Table 3). In order to compare furoquinoline synthesis by these different silver catalysts, the reaction was performed in 2-(diethylamino)ethanol.
| Entry | Product | [Ag] (time, yield)b |
|---|---|---|
| a Reaction with quinoline substrate (0.05 M) in 2-(diethylamino)ethanol at r.t., 0.2 M for [Ag(Im)]n/PPh3. b Isolated yields. c 1H NMR analysis. | ||
| 1 |
|
AgOTf (12 h, 40%) |
| Ag2O (1 h, 95%) | ||
| [Ag(Im)]n/PPh3 (12 h, 44%) | ||
| 2 |
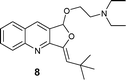
|
AgOTf (19 h, 23% conv.c) |
| Ag2O (3 h, 99%) | ||
| [Ag(Im)]n/PPh3 (18 h, 29%) | ||
| 3 |
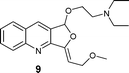
|
AgOTf (5 h, 74%) |
| Ag2O (1.5 h, quant.) | ||
| [Ag(Im)]n/PPh3 (0.5 h, 99%) | ||
| 4 |
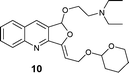
|
AgOTf (6 h, 40%) |
| Ag2O (4 h, 86%) | ||
| [Ag(Im)]n/PPh3 (12 h, 50%) | ||
| 5 |
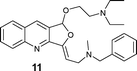
|
AgOTf (12 h, 20%) |
| Ag2O (4 h, 86%) | ||
| [Ag(Im)]n/PPh3 (12 h, 92%) | ||
| 6 |
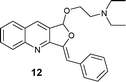
|
AgOTf (12 h, 51%) |
| Ag2O (3 h, 93%) | ||
| [Ag(Im)]n/PPh3 (0.5 h, quant.) | ||
| 7 |
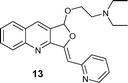
|
AgOTf (2 h, 92%) |
| Ag2O (2 h, 95%) | ||
| [Ag(Im)]n/PPh3 (1.5 h, 71%) | ||
| 8 |
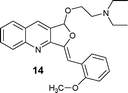
|
AgOTf (12 h, 70%) |
| Ag2O (3 h, 90%) | ||
| [Ag(Im)]n/PPh3 (24 h, trace) |
AgOTf catalysis usually needed a longer reaction time (2–12 h) compared to Ag2O (1–4 h), but [Ag(Im)]n/PPh3 showed a broader range (0.5–18 h; see Table 3). The range of yields is pretty wide for AgOTf (20–92%) and [Ag(Im)]n/PPh3 (29%–quantitative), but the mean yield is more informative – 93% for Ag2O, 70% for [Ag(Im)]/PPh3 and 51% for AgOTf. This analysis shows us that [Ag(Im)]n/PPh3 is overall the most promising, although the reactivity is not as high as for Ag2O. On the other hand, handling [Ag(Im)]n (and even the reaction medium, [Ag(Im)]n/PPh3) is more practical regarding moisture, light and storage. With [Ag(Im)]n/PPh3, furoquinolines 7 and 8 bearing alkyl substituents (entries 1–2, Table 3) were obtained in fair yields (44 and 29%), but results were worse with the o-methoxyphenyl derivative 14, which was detected only in trace amounts (entry 8, Table 3). Steric hindrance could explain this behaviour, since having an extra methylene unit such as in furoquinolines 9–11 increases all the yields (99%, 50% and 92%, respectively).
With [Ag(Im)]n/PPh3, furoquinolines 12 and 13 were efficiently synthesized (yields being quantitative and 71%, and reaction times 0.5 and 1.5 h, respectively).
For a mechanistic point of view, AgNO3 and AgOTf (without additives) are well known for interacting with alkynesvia π-complexes, and the reactions proceed via a pyrylium intermediate4a,17a,b leading to the 6-endo-dig product. Based on these results and previous work,4d we propose a different mechanism pathway for the other silver catalysts (Scheme 1). The reactivity of [Ag(Im)]n/PPh3, AgOTf/amine and Ag2O would favor direct coordination to the oxygen atom of the carbonyl function (intermediate A); the silver salt acts as a Lewis acid,17c,d promoting the formation of acetalB, which on cyclization would give complex C (anti addition)4d and after [Ag] release would yield the 5-exo-dig product.
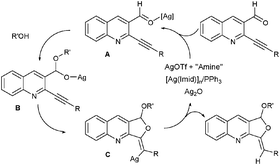 | ||
| Scheme 1 Plausible mechanism. | ||
In conclusion, we have shed light on the interesting behaviour of AgOTf with some amine additives – namely, an inversion of the cycloisomerization regioselectivity, from 6-endo-dig to 5-exo-dig. Driven by this observation, we have shifted [Ag(Im)]n from its use in material sciences to organometallic catalysis. Indeed, this polymer is particularly attractive, since it is resistant to air exposure, moisture and light, thus distinguishing it from other silver derivatives. The use of triphenylphosphine for the homogeneous reaction is of interest and will be investigated for an enantioselective version.
We would like to thank the French Ministry of Research, INCa, CNRS, and the “Cluster Recherche Chimie de la Région Rhône-Alpes”. Thanks also to D. Bouchu, N. Henriques and C. Duchamp for mass spectrometry.
Notes and references
- (a) B. H. Lipshutz and Y. Yamamoto, Chem. Rev., 2008, 108, 2793 CrossRef CAS; (b) M. Naodovic and H. Yamamoto, Chem. Rev., 2008, 108, 3132 CrossRef CAS; (c) J.-M. Weibel, A. Blanc and P. Pale, Chem. Rev., 2008, 108, 3149 CrossRef CAS; (d) M. Álvarez-Corral, M. Muñoz-Dorado and I. Rodríguez-Garcia, Chem. Rev., 2008, 108, 3174 CrossRef CAS; (e) Y. Yamamoto, Chem. Rev., 2008, 108, 3199–3222 CrossRef CAS; (f) H. V. R. Dias and C. J. Lovely, Chem. Rev., 2008, 108, 3223 CrossRef CAS; (g) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS.
- P. Belmont and E. Parker, Eur. J. Org. Chem., 2009, 6075 CrossRef CAS.
- J. A. Marshall and E. D. Robinson, J. Org. Chem., 1990, 55, 3450 CrossRef CAS.
- (a) J. Zhu, A. R. Germain and J. A. Porco, Jr., Angew. Chem., Int. Ed., 2004, 43, 1239 CrossRef CAS; (b) T. Yao, X. Zhang and R. C. Larock, J. Am. Chem. Soc., 2004, 126, 11164 CrossRef CAS; (c) N. T. Patil, N. K. Pahadi and Y. Yamamoto, J. Org. Chem., 2005, 70, 10096 CrossRef CAS; (d) T. Godet, C. Vaxelaire, C. Michel, A. Milet and P. Belmont, Chem.–Eur. J., 2007, 13, 5632 CrossRef CAS; (e) I. Cikotiene, R. Buksnaitiene, S. Rudys, M. Morkunas and D. Motiejaitis, Tetrahedron, 2010, 66, 251 CrossRef CAS; (f) X. Yu, Q. Ding, W. Wang and J. Wu, Tetrahedron Lett., 2008, 49, 4390 CrossRef CAS.
- M. B. Smith and J. March, March's Advanced Organic Chemistry Reactions, Mechanisms and Structure, 5th edn, 2000 Search PubMed.
- I. Kaljurand, I. Kütt, L. Sooväli, T. Rodima, V. Mäemets, I. Leito and I. A. Koppel, J. Org. Chem., 2005, 70, 1019 CrossRef CAS.
- (a) H. Krishna, S. S. Krishnamurthy and M. Nethaji, Inorg. Chim. Acta, 2009, 362, 38 CrossRef CAS; (b) Y. Qu, L. Su and J. Wu, J. Chem. Crystallogr., 2007, 37, 579 CrossRef CAS; (c) J. T. Sampanthar and J. J. Vittal, Cryst. Eng., 2000, 3, 117 CrossRef CAS.
- (a) C. Qi, L. Huang and H. Jiang, Synthesis, 2010, 1433 CAS; (b) W. Yamada, Y. Sugawara, H. M. Cheng, T. Ikeno and T. Yamada, Eur. J. Org. Chem., 2007, 2604 CrossRef CAS; (c) Y. Sugawara, W. Yamada, S. Yoshida, H. M. Cheng, T. Ikeno and T. Yamada, J. Am. Chem. Soc., 2007, 129, 12902 CrossRef CAS.
- (a) A. Li, P. J. Kindelin and D. A. Klumpp, Org. Lett., 2006, 8, 1233 CrossRef CAS; (b) A. S. K. Hashmi, Catal. Today, 2007, 122, 211 CrossRef CAS; (c) J. U. Rhee and M. J. Krische, Org. Lett., 2005, 7, 2493 CrossRef CAS.
- T. Schwier, A. W. Sromek, D. M. L. Yap, D. Chernyak and V. Gevorgyan, J. Am. Chem. Soc., 2007, 129, 9868 CrossRef CAS.
- (a) N. K. Mills and A. H. White, J. Chem. Soc., Dalton Trans., 1984, 229 RSC; (b) S. Del Piero, R. Fedele, A. Melchior, R. Portanova, M. Tolazzi and E. Zangrando, Inorg. Chem., 2007, 46, 4683 CrossRef CAS; (c) R. P. Feazell, C. E. Carson and K. K. Klausmeyer, Inorg. Chem., 2006, 45, 935 CrossRef CAS.
- T. Eicher, S. Haputmann and H. Suschitzky, The Chemistry of Heterocycles: Structure, Reactions, Syntheses and Applications, 2003 Search PubMed.
- J. E. Bauman, Jr. and J. C. Wang, Inorg. Chem., 1964, 3, 368 CrossRef.
- (a) N. Masciocchi, M. Moret, P. Cairati, A. Sironi, G. Attilio Ardizzoia and G. La Monica, J. Chem. Soc., Dalton Trans., 1995, 1671 RSC; (b) K. Nomiya, K. Tsuda, Y. Tanabe and H. Nagano, J. Inorg. Biochem., 1998, 69, 9 CrossRef CAS; (c) G. A. Ardizzoia, S. Brenna, F. Castelli, S. Galli, G. LaMonica, N. Masciocchi and A. Maspero, Polyhedron, 2004, 23, 3063 CrossRef CAS.
- K. Nomiya, K. Tsuda, T. Sudoh and M. Oda, J. Inorg. Biochem., 1997, 68, 39 CrossRef CAS.
- (a) P. J. Garegg and B. Samuelsson, Carbohydr. Res., 1980, 84, C1 CrossRef CAS; (b) D. R. Rae, e-EROS – Encycl. Reagents Org. Synth., 2001 Search PubMed.
- (a) N. Asao, H. Aikawa and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 7458 CrossRef CAS; (b) N. Asao, K. Takahashi, S. Lee, T. Kasahara and Y. Yamamoto, J. Am. Chem. Soc., 2002, 124, 12650 CrossRef CAS; (c) Y. Yamamoto, J. Org. Chem., 2007, 72, 7817 CrossRef CAS; (d) N. Asao, T. Nogami, K. Takahashi and Y. Yamamoto, J. Am. Chem. Soc., 2002, 124, 764 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and analytical data. See DOI: 10.1039/c0cc02623a |
| ‡ This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| This journal is © The Royal Society of Chemistry 2011 |