Direct arylations of electron-deficient (hetero)arenes with aryl or alkenyl tosylates and mesylates†‡
Lutz
Ackermann
* and
Sabine
Fenner
Institut fuer Organische und Biomolekulare Chemie, Georg-August-Universitaet, Tammannstrasse 2, 37077 Goettingen, Germany. E-mail: Lutz.Ackermann@chemie.uni-goettingen.de; Fax: +49 551 396777
First published on 20th September 2010
Abstract
A palladium catalyst derived from the ligand X-Phos enabled generally applicable direct arylations of electron-deficient heteroarenes and arenes with aryl and alkenyl tosylates or mesylates.
Biaryls are indispensable structural motifs in inter alia bioactive small molecules or functional materials. Traditionally, their syntheses strongly rely on cross-coupling reactions with organometallic or main group-element nucleophiles,1,2 which continue to be particularly challenging with electron-deficient nucleophiles.3,4 However, recent progress is represented by the development of transition-metal-catalyzed direct arylations, which resulted in more sustainable methods for the preparation of biaryl scaffolds.5,6 These methods enable a streamlining of organic synthesis by avoiding substrate pre-activation through the use of simple (hetero)arenes, which predominantly serve as surrogates for difficult to access organometallic reagents.
Catalyzed direct arylations through C–H bond cleavages have been thoroughly explored with aryl halides or aryl triflates as electrophilic coupling partners.5 Contrarily, aryl tosylates are generally more attractive electrophiles, since they are moisture-stable, inexpensive and easily prepared from readily available phenols.7,8 Unfortunately, their remarkable stabilities render these electrophiles significantly less reactive, and thus highly challenging in catalytic arylation reactions. As a consequence, direct arylations with aryl tosylates are as of yet restricted to ruthenium-catalyzed functionalizations of arenes displaying Lewis-basic directing groups,9 or palladium-catalyzed C–H functionalizations on electron-rich azoles.10 On the contrary, we wish to report herein on the development of first direct arylations of electron-deficient (hetero)arenes11 with difficult to activate aryl tosylates. Notably, the optimized catalytic system also proved amenable to the first general use of more atom-economical aryl mesylates as electrophiles, and allowed for direct functionalizations of electron-deficient arenes.
Our studies commenced with applying reaction conditions previously established for C–H functionalizations of electron-rich azoles10 to the conversion of electron-deficient N-oxide 1a with aryl tosylates 2 (Scheme 1). Unfortunately, only an unsatisfactory low conversion of heteroarene 1a was observed here. Subsequent detailed optimization studies (see ESI‡) highlighted that superior reaction conditions involved the use of X-Phos (4) as the ligand, a t-BuOH/toluene solvent mixture and CsF as the base.12 Further, it is noteworthy that the addition of cocatalytic amounts of pivalic acid did not have a beneficial effect.
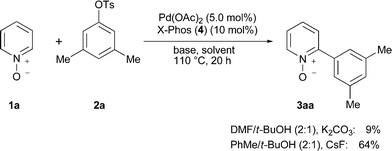 | ||
| Scheme 1 Summary of optimization studies on the direct arylation of heteroarene 1a with aryl tosylate 2a (see ESI‡; X-Phos (4) = 2-(dicyclohexylphosphino)-2′,4′,6′-triisopropylbiphenyl). | ||
With optimized reaction conditions in hand, we probed the scope of direct arylations of pyridine N-oxides 1 with representative moisture-stable aryl tosylates 2 as electrophiles (Scheme 2). Notably, both electron-deficient as well as electron-rich, hence electronically deactivated aryl tosylates 2 provided the desired products 3, as did ortho-substituted aryl or heteroaryl tosylates. Additionally, the diastereoselective formation of alkenes proved viable, as showcased with the synthesis of N-oxide 3aj. All reactions featured a high chemoselectivity in that the mono-arylated products 3 were exclusively formed. Furthermore, intramolecular competition experiments indicated a dependence of regioselectivity on C–H bond acidity, which led to the selective formation of N-oxides 3ai and 3aj as the sole products.
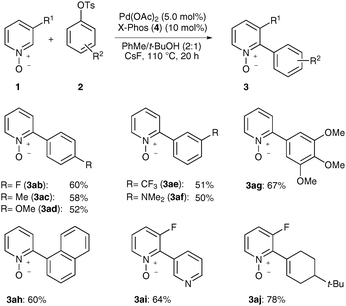 | ||
| Scheme 2 Direct arylations of electron-deficient pyridine N-oxides 1. | ||
Subsequently, we were pleased to find that diazine N-oxides 1 were also suitable substrates for direct arylations with aryl tosylates 2 under the optimized reaction conditions (Scheme 3). Hence, products 3ak–3ap and 3aq–3at derived from pyridazine or pyrazine N-oxides 1, respectively, were obtained from diversely-substituted aryl tosylates 2. Moreover, mono-arylated quinoline and quinoxaline N-oxides 3au–3av and 3aw–3ba, respectively, could be selectively prepared likewise. In addition to aryl tosylates, an alkenylic electrophile was exploited for a selective access to alkene 3ba.
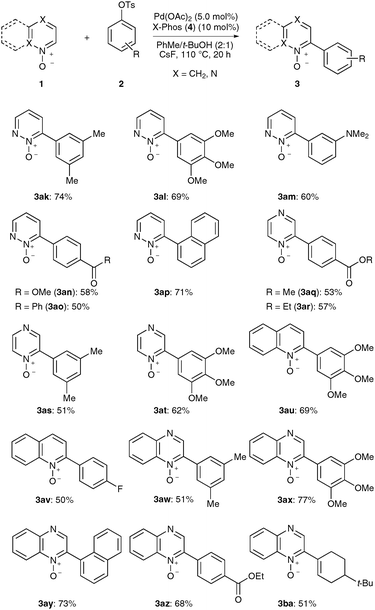 | ||
| Scheme 3 Scope of direct arylations with electron-deficient N-oxides 1. | ||
Aryl mesylates 5 have significantly lower molecular weights than the corresponding tosylates 2, and thus improve the atom-economy of direct arylations. Thus far, only two examples of direct arylations with aryl mesylates were reported, notably employing electron-deficient, hence activated electrophiles 5.10 Therefore, we were pleased to observe that various aryl mesylates 5 could be successfully used for direct arylations of pyridine N-oxide 1b, including electron-rich and/or ortho-substituted derivatives 5 (Scheme 4).
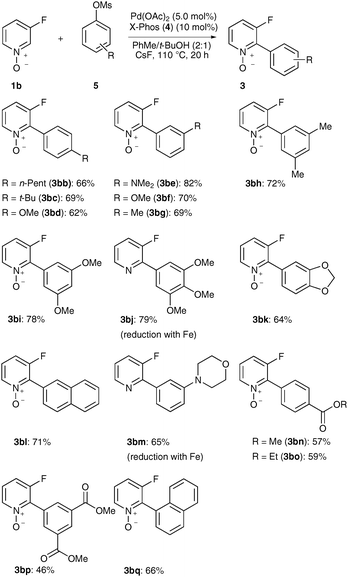 | ||
| Scheme 4 Direct arylations of N-oxide 1b with aryl mesylates 5. | ||
Finally, the optimized catalytic system also allowed for direct arylations of electron-deficient arenes13 with deactivated aryl tosylates, as illustrated for C–H bond functionalizations on arene 6 (Scheme 5).
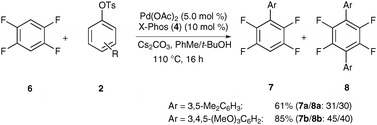 | ||
| Scheme 5 Direct arylations of arene 6 with electron-rich aryl tosylates 2. | ||
In conclusion, we have developed a catalytic system for unprecedented direct arylations of electron-deficient heteroarenes with moisture-stable aryl tosylates. Importantly, the optimized palladium catalyst also enabled first general direct arylations with atom-economical aryl mesylates, and was found applicable to direct functionalizations of electron-deficient arenes.
Notes and references
-
Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2004 Search PubMed
.
-
J. Tsuji, Palladium Reagents and Catalysts, Wiley, Chichester, 2004 Search PubMed
.
- For representative examples of traditional cross-coupling reactions with 2-pyridyl organometallics, see:
(a) Suzuki–Miyaura: D. M. Knapp, E. P. Gillis and M. D. Burke, J. Am. Chem. Soc., 2009, 131, 6961–6963 Search PubMed
; (b) L. Ackermann and H. K. Potukuchi, Synlett, 2009, 2852–2856 CrossRef CAS
; (c) K. L. Billingsley and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 4695–4698 CrossRef CAS
; (d) Kumada–Corriu: L. Ackermann, H. K. Potukuchi, A. R. Kapdi and C. Schulzke, Chem.–Eur. J., 2010, 16, 3300–3303 Search PubMed
; (e) Stille: R. Wittenberg, J. Srogl, M. Egi and L. S. Liebeskind, Org. Lett., 2003, 5, 3033–3035 Search PubMed
; (f) Negishi: B. M. Coleridge, C. S. Bello, D. H. Ellenberger and A. Leitner, Tetrahedron Lett., 2010, 51, 357–359 Search PubMed
, and references cited therein.
- L.-C. Campeau and K. Fagnou, Chem. Soc. Rev., 2007, 36, 1058–1068 RSC
.
- Select recent reviews:
(a) L. Ackermann, Chem. Commun., 2010, 46, 4866–4877 RSC
; (b) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Commun., 2010, 46, 677–685 RSC
; (c) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS
; (d) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS
; (e) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074–1086 CrossRef CAS
; (f) P. Thansandote and M. Lautens, Chem.–Eur. J., 2009, 15, 5874–5883 CrossRef CAS
; (g) F. Bellina and R. Rossi, Tetrahedron, 2009, 65, 10269–10310 CrossRef CAS
; (h) J. C. Lewis, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2008, 41, 1013–1025 CrossRef CAS
; (i) T. Satoh and M. Miura, Chem. Lett., 2007, 200–205 CrossRef CAS
; (j) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173–1193 RSC
; (k) S. Pascual, P. de Mendoza and A. M. Echavarren, Org. Biomol. Chem., 2007, 5, 2727–2734 RSC
; (l) L. Ackermann, Synlett, 2007, 507–526 CrossRef CAS
; (m) L.-C. Campeau, D. R. Stuart and K. Fagnou, Aldrichimica Acta, 2007, 40, 35–41
.
-
Modern Arylation Methods, ed. L. Ackermann, Wiley-VCH, Weinheim, 2009 Search PubMed
.
- For representative examples of conventional palladium-catalyzed coupling reactions between aryl tosylates and pre-activated (hetero)arenes, see:
(a) Suzuki–Miyaura couplings: C. M. So, C. Po Lau and F. Y. Kwong, Angew. Chem., Int. Ed., 2008, 47, 8059–8063 Search PubMed
; (b) H. N. Nguyen, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 11818–11819 CrossRef CAS
; (c) Negishi couplings: J. Zhou and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 12527–12530 Search PubMed
; (d) Kumada–Corriu couplings: L. Ackermann and A. Althammer, Org. Lett., 2006, 8, 3457–3460 Search PubMed
; (e) A. H. Roy and J. F. Hartwig, J. Am. Chem. Soc., 2003, 125, 8704–8705 CrossRef CAS
; (f) Hiyama couplings: L. Zhang and J. Wu, J. Am. Chem. Soc., 2008, 130, 12250–12251 Search PubMed
, and references cited therein.
- Direct arylations and alkenylations with sulfamates: L. Ackermann, S. Barfüsser and J. Pospech, Org. Lett., 2010, 12, 724–726 Search PubMed
.
-
(a) L. Ackermann, A. Althammer and R. Born, Angew. Chem., Int. Ed., 2006, 45, 2619–2622 CrossRef CAS
; (b) L. Ackermann, R. Vicente and A. Althammer, Org. Lett., 2008, 10, 2299–2302 CrossRef CAS
; (c) L. Ackermann and R. Vicente, Top. Curr. Chem., 2010, 292, 211–229
; (d) ruthenium-catalyzed directed arylations with phenols: L. Ackermann and M. Mulzer, Org. Lett., 2008, 10, 5043–5045 Search PubMed
.
- L. Ackermann, A. Althammer and S. Fenner, Angew. Chem., Int. Ed., 2009, 48, 201–204 CrossRef CAS
.
- For examples of direct arylations of pyridine N-oxides with aryl halides or triflates, see:
(a) L.-C. Campeau, D. R. Stuart, J.-P. Leclerc, M. Bertrand-Laperle, E. Villemure, H.-Y. Sun, S. Lasserre, N. Guimond, M. Lecavallier and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 3291–3306 CrossRef
; (b) D. J. Schipper, M. El-Salfiti, C. J. Whipp and K. Fagnou, Tetrahedron, 2009, 65, 4977–4983 CrossRef CAS
; (c) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254–9256 CrossRef CAS
; (d) L.-C. Campeau, D. J. Schipper and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3266–3267 CrossRef CAS
; (e) H.-Q. Do and O. Daugulis, J. Am. Chem. Soc., 2007, 129, 12404–12405 CrossRef CAS
; (f) L.-C. Campeau, S. Rousseaux and K. Fagnou, J. Am. Chem. Soc., 2005, 127, 18020–18021 CrossRef CAS
; (g) K. Fagnou, Top. Curr. Chem., 2010, 292, 35–56 CAS
; (h) see also: H. Andersson, T. S.-L. Banchelin, S. Das, R. Olsson and F. Almqvist, Chem. Commun., 2010, 46, 3384–3386 Search PubMed
; (i) A. Larivee, J. J. Mousseau and A. B. Charette, J. Am. Chem. Soc., 2008, 130, 52–54 CrossRef CAS
; (j) K. S. Kanyiva, Y. Nakao and T. Hiyama, Angew. Chem., Int. Ed., 2007, 46, 8872–8874 CrossRef CAS
.
- Under otherwise identical reaction conditions, a lower catalyst loading or reduced reaction temperatures provided less satisfactory yields.
- Representative examples of direct arylations of oligofluoroarenes with aryl halides:
(a) O. Rene and K. Fagnou, Org. Lett., 2010, 12, 2116–2119 CrossRef CAS
; (b) H.-Q. Do, R. M. K. Khan and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 15185–15192 CrossRef CAS
; (c) M. Lafrance, C. N. Rowley, T. K. Woo and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 8754–8756 CrossRef
.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Optimization of reaction conditions, experimental procedures and characterization data. See DOI: 10.1039/c0cc02360d |
| This journal is © The Royal Society of Chemistry 2011 |
