Surface-mediated release of a synthetic small-molecule modulator of bacterial quorum sensing: Gradual release enhances activity†
Anthony S.
Breitbach‡
a,
Adam H.
Broderick‡
b,
Christopher M.
Jewell
b,
Suvai
Gunasekaran
b,
Qi
Lin
a,
David M.
Lynn
*ab and
Helen E.
Blackwell
*a
aDepartment of Chemistry, University of Wisconsin-Madison, 1101 University Ave., Madison, WI 53706, USA. E-mail: blackwell@chem.wisc.edu
bDepartment of Chemical and Biological Engineering, University of Wisconsin-Madison, 1415 Engineering Drive, Madison, WI 53706, USA. E-mail: dlynn@engr.wisc.edu
First published on 9th September 2010
Abstract
We demonstrate an approach to the surface-mediated release of a synthetic N-acylated L-homoserine lactone (AHL) modulator of bacterial quorum sensing (QS). AHL released gradually from thin films of poly(lactide-co-glycolide) (PLG) is shown to activate QS in the model symbiont Vibrio fischeri at levels that exceed those promoted by direct solution-based administration.
Many types of bacteria communicate to assess their local population densities in a phenomenon known as “quorum sensing” (QS).1 This signaling system is based on the intercellular exchange of small-molecule signals (or autoinducers). Several of the most notorious human pathogens, including P. aeruginosa and S. aureus, use QS to organize into structured impermeable communities called “biofilms” and activate virulence pathways at high cell densities that are the basis for acute and chronic infections.1d As a result, QS presents an important and relevant target for the development of new anti-virulence strategies.2
Gram-negative bacteria have the best-understood QS systems, which are largely based on N-acylated L-homoserine lactone (AHL) autoinducers and their associated LuxI-type synthase enzymes and LuxR-type receptors.1c,eAHL:LuxR-type receptor binding is essential for the QS system to activate, and thus represents a central target for the interception of QS networks.3 As part of a broader program aimed at elucidating the chemical mechanisms of QS in bacteria, we have recently identified a series of non-native AHLs capable of either strongly inhibiting or activating LuxR-type receptors in a range of bacterial pathogens and symbionts.4 These compounds are readily synthesized and provide potent antagonists and agonists of QS that can be used to understand mechanisms of bacterial communication. In addition, these AHL-derived antagonists serve as lead scaffolds for the development of new anti-virulence agents.2,3c
One challenge to the application of these new QS inhibitors as anti-virulence agents lies in developing methods for the administration of these compounds in ways that can be tailored in a range of different therapeutic contexts (e.g., systemic vs. localized delivery, rapid vs. sustained release, and addressing issues associated with the stability of these molecules in physiological media). Bacterial colonization and the formation of biofilms on the surfaces of indwelling medical devices, for example, represent two primary points of entry for bacteria into the body.5 Approaches aimed at inhibiting or attenuating QS in bacteria locally (i.e., at or near the surfaces of these objects) presents challenges that differ substantially from strategies based on the systemic delivery of these molecules. The work reported here takes a first step toward addressing several of these challenges through the design of thin polymer films that provide time-dependent control over the surface-mediated release of a non-native, AHL-derived QS modulator. We demonstrate that this materials-based approach can be used to control and activate a QS phenotype in a model bacterial system, and that it has potential advantages relative to methods for the direct (i.e., solution-based) administration of AHLs used in several past studies.3a Although many different approaches have been reported for the design of materials that control the release of antibiotics and other bacteriocidal agents,6 approaches to the release of agents that disrupt bacterial communication directly have not, to our knowledge, been reported.
 | ||
| Fig. 1 Structures of AHL 1 (264.23 g mol−1) and its hydrolysis product. | ||
We selected the synthetic QS modulator N-(3-nitrophenylacetanoyl)-L-homoserine lactone (AHL 1; Fig. 1) for use in our initial studies for several reasons: (i) past work in our group has shown that this non-native AHL is a potent modulator of QS in bacteria, most notably as an inhibitor in the pathogen P. aeruginosa and as a “super-activator” in the bioluminescent symbiont V. fischeri,4 and (ii) the nitrophenyl substituent on this molecule provides a convenient method for monitoring concentrations of AHL in solution. We selected poly(lactide-co-glycolide) (PLG) as a matrix for the encapsulation and surface-mediated release of AHL 1. PLG is both biocompatible and biodegradable, and it has a well-documented history of use in drug delivery and other biomedical applications.6a,7 In addition, the environments within matrices of PLG have been demonstrated to be acidic (owing, in part, to the presence of carboxylic acid groups that arise from backbone ester hydrolysis).8 In this respect, PLG can serve to stabilize the structures of molecules that are base-sensitive or that may otherwise hydrolyze or degrade upon prolonged exposure to aqueous media.8c We note, in this context, that AHLs contain a hydrolyzable lactone moiety (Fig. 1), and that past studies by our group and others have demonstrated that the hydrolysis of these lactone groups (in both native and non-native AHLs; half-lives from ∼12 to 48 h) in aqueous media leads to ring-opened products that are essentially inactive as QS modulators.4a,9 The use of PLG as a matrix for the release of AHLs could therefore lead to materials that both stabilize and prolong the release of these agents, and thereby lead to surfaces and coatings that modulate QS more effectively than the direct administration of these compounds in solution.
To explore the feasibility of this approach, we performed a series of experiments to characterize the encapsulation and release of AHL 1 from thin solvent-cast films of PLG fabricated directly in the wells of 96-well microtiter plates (see ESI† for full details). This approach resulted in uniform and transparent thin films of polymer with loadings of AHL 1 that could be controlled reproducibly. To characterize the release of AHL 1 from these films under physiologically relevant conditions, we incubated AHL-loaded films in an M9-type aqueous buffer (pH = 7.5) at 37 °C. The concentration of AHL released into solution was monitored over time by characterizing the absorbance of the buffer solutions at 267 nm.
Fig. 2 shows representative release profiles of two films fabricated to have initial loadings of either 9 or 36 μg of AHL 1. These results demonstrate that AHL 1 is released relatively rapidly (∼80% is released over 4.5 d) and that the amount of AHL released can be controlled by the amount incorporated during fabrication. We comment in this context that, in general, the release of small molecules from PLG can be made to occur over a broad range of times (e.g., from hours or days to weeks or months) by manipulation of polymer structure and other factors (film thickness, method of fabrication, etc.).6a,7 The relatively fast release shown in Fig. 2 suggests that release occurs by a mechanism that does not require substantial degradation or physical film erosion. Although it should be possible to design films that permit more extended release, the timescales of the release profiles shown in Fig. 2 are relevant in the context of certain potential applications (e.g., preventing biofilm growth on short-term indwelling devices) and were suitable for all subsequent studies.
 | ||
| Fig. 2 Plot of release vs. time for two PLG thin films containing AHL 1 (with initial loadings of either 9 μg or 36 μg) incubated in M9 buffer (pH 7.5) at 37 °C. Dashed lines indicate the initial loading of AHL 1 in each film. Each data point represents the average for 4 replicate wells; error bars are STE. | ||
We performed a series of cell-based experiments to characterize the activity of AHL 1 released from the films described above. We used V. fischeri as a model for these experiments because this organism uses QS to control bioluminescence at high population densities and thus provides a straightforward means of characterizing changes in QS. We selected a V. fischeri mutant strain (ES114; Δ-luxI) that lacks a functional AHL synthase (as a result, the exogenous addition of an appropriate agonist is required to activate QS). As mentioned above, AHL 1 behaves as a highly potent QS agonist in this strain, with an EC50 value of ∼0.2 μM (10-fold more potent than V. fischeri's native autoinducer).4aCell-based experiments were performed by collecting aliquots of AHL released from films during release experiments, diluting these samples in series into separate 96-well plates, and then adding suspensions of V. fischeri. For these cell-based experiments, films containing AHL 1 were incubated in LBS growth medium, a medium that is similar to M9 buffer but also contains additional nutrients and salts required to support V. fischerigrowth (see ESI† for details).
After an appropriate period of growth, the bioluminescence of V. fischeri in each well was measured, normalized to cell density, and plotted as percent of the authentic AHL 1 positive control vs. concentration to generate sigmoidal dose curves and determine EC50 values for released AHL 1 at each time point. We note here that the absorbance spectrum of LBS media at 267 nm prevented direct characterization of concentrations of the AHL released from the films in these cell-based experiments. As a result, we used concentrations of released AHL measured using otherwise identical films incubated in M9 media (as described above) to generate the dose–response curves shown in Fig. 3.
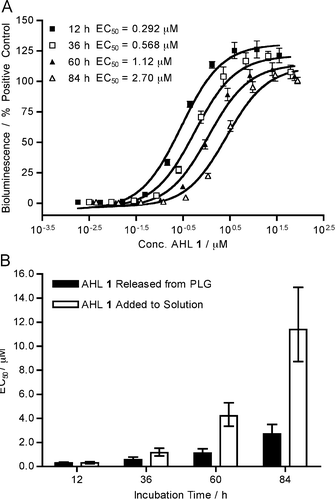 | ||
Fig. 3 (A) Dose–response curves for aliquots of AHL 1 released from PLG films incubated in buffer (LBS; pH 7.5, 37 °C) at selected time points and added to V. fischeri (ES114) (see text). Initial film loading of AHL 1 = 36 μg. QS activation measured via luminescence output per well. Concentration of AHL 1 determined from replicate wells of PLG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AHL 1 films in M9 buffer (see Fig. 2). Each data point is an average of 4 replicate wells; error bar is STE. (B) Comparison of the change in EC50 value for AHL 1vs. time for AHL 1 released from PLG films (loading of 1 = 36 μg) or direct treatment with a bolus of 36 μg AHL 1 (see text). Error bars are 95% CI. AHL 1 films in M9 buffer (see Fig. 2). Each data point is an average of 4 replicate wells; error bar is STE. (B) Comparison of the change in EC50 value for AHL 1vs. time for AHL 1 released from PLG films (loading of 1 = 36 μg) or direct treatment with a bolus of 36 μg AHL 1 (see text). Error bars are 95% CI. | ||
Fig. 3A shows representative dose curves and corresponding EC50 values for V. fischeri using films with an initial loading of 36 μg of AHL 1 (i.e., identical to the films shown in Fig. 2, closed squares). Inspection of these data reveals that AHL released from these films retains its activity as a QS agonist at each time point sampled over the ∼4 d course of this experiment. Further inspection also reveals the dose curves to shift to higher concentrations as a function of time [shown in Fig. 3A and summarized in Fig. 3B (black bars)]. This shift to higher concentrations over time was also observed for control solutions of AHL 1 (36 μg per well; see Fig. 3B (white bars) for a comparison) incubated under otherwise identical conditions (we note, however, that these two shifts in EC50 values occur to two different extents; we return to this observation again in the discussion below).
As described above, both naturally occurring and synthetic AHLs undergo hydrolysis in aqueous media to yield ring-opened structures that are QS-inactive (e.g., Fig. 1). On the basis of this knowledge, we interpret the time-dependent shifts in EC50 values observed in Fig. 3B to arise, at least in part, from the partial hydrolysis of AHL 1 during these experiments. Such hydrolysis would, over time, result in a decrease in the amount of active compound present in solution and lead to apparent EC50 values that increase over time (as shown in Fig. 3A). Support for this view is provided by the results of additional solution-based experiments that confirm that the hydrolysis of AHL 1 leads to a product that is indeed QS-inactive in V. fischeri (see ESI† for details).
Finally, a comparison of the data in Fig. 3B reveals that the time-dependent shift to higher EC50 values occurs more rapidly for solutions of AHL 1 (white bars) than it does for experiments using compound that was released gradually from polymer films (black bars). These results demonstrate that gradual, surface-mediated release of AHL 1 yields solutions of agonist that are more active for longer periods of time (compared to the activity of an equivalent amount of AHL incubated in solution) and hint that the polymer used to fabricate these films could play a protective role. Additional characterization will be required to understand the origin of increased activity observed in these experiments more completely. However, our current results are consistent with the broader view that acidic environments in water-swollen PLG matrices can stabilize the structures of base-sensitive drugs8c and thereby prolong the effectiveness of active agents in ways that extend beyond control over rates of release.
In summary, our results demonstrated a polymer-based approach to the release of a synthetic AHL. We demonstrate that this compound is released in a form that is active and able to modulate (turn on) QS in the marine symbiont V. fischeri, a bacterial model used widely for fundamental studies of QS. Our results also show that this polymer-based approach can be used to prolong the activities of AHLs relative to one-time treatments with AHL in solution. To the best of our knowledge, this study presents the first demonstration of the controlled release of a non-native, AHL-derived QS modulator from a polymer matrix. The results of this study could provide a basis for the design of coatings that intercept bacterial communication in ways that are important in both fundamental and applied contexts (e.g., surfaces that prevent the formation of biofilms).
Support to H.E.B. was provided by the NIH (AI063326), the Greater Milwaukee Foundation, the Burroughs Welcome Fund, and Johnson & Johnson. Support to D.M.L. was provided by the Univ. of Wisconsin and the UW Vilas Trust. A.S.B. was funded in part by an NIH Chemistry Biology Interface Training Grant (NIGMS T32 GM008505). A.H.B. is a NSF Graduate Research Fellow. H.E.B. and D.M.L. are Alfred P. Sloan Research Fellows.
Notes and references
- (a) A. Camilli and B. L. Bassler, Science, 2006, 311, 1113–1116 CrossRef CAS; (b) C. M. Waters and B. L. Bassler, Annu. Rev. Cell Dev. Biol., 2005, 21, 319–346 CrossRef; (c) C. Fuqua and E. P. Greenberg, Nat. Rev. Mol. Cell Biol., 2002, 3, 685–695 CrossRef CAS; (d) R. S. Smith and B. H. Iglewski, Curr. Opin. Microbiol., 2003, 6, 56–60 CrossRef CAS; (e) C. Fuqua, M. R. Parsek and E. P. Greenberg, Annu. Rev. Genet., 2001, 35, 439–468 CrossRef CAS.
- (a) D. A. Rasko and V. Sperandio, Nat. Rev. Drug Discovery, 2010, 9, 117–128 CrossRef CAS; (b) L. Cegelski, G. R. Marshall, G. R. Eldridge and S. J. Hultgren, Nat. Rev. Microbiol., 2008, 6, 17–27 Search PubMed.
- (a) G. D. Geske, J. C. O'Neill and H. E. Blackwell, Chem. Soc. Rev., 2008, 37, 1432–1447 RSC; (b) M. Welch, H. Mikkelsen, J. E. Swatton, D. Smith, G. L. Thomas, F. G. Glansdorp and D. R. Spring, Mol. BioSyst., 2005, 1, 196–202 RSC; (c) T. B. Rasmussen and M. Givskov, Microbiology, 2006, 152, 895–904 CrossRef CAS.
- (a) G. D. Geske, J. C. O'Neill and H. E. Blackwell, ACS Chem. Biol., 2007, 2, 315–319 CrossRef CAS; (b) G. D. Geske, J. C. O'Neill, D. M. Miller, M. E. Mattmann and H. E. Blackwell, J. Am. Chem. Soc., 2007, 129, 13613–13625 CrossRef CAS; (c) G. D. Geske, J. C. O'Neill, D. M. Miller, R. J. Wezeman, M. E. Mattmann, Q. Lin and H. E. Blackwell, ChemBioChem, 2008, 9, 389–400 CrossRef CAS.
- (a) L. Passerini, K. Lam, J. W. Costerton and E. G. King, Crit. Care Med., 1992, 20, 665–673 CrossRef CAS; (b) R. Mittal, S. Sharma, S. Chhibber, S. Aggarwal, V. Gupta and K. Harjai, FEMS Immunol. Med. Microbiol., 2010, 58, 237–243 CrossRef CAS.
- (a) P. Wu and D. W. Grainger, Biomaterials, 2006, 27, 2450–2467 CrossRef CAS; (b) E. M. Hetrick and M. H. Schoenfisch, Chem. Soc. Rev., 2006, 35, 780–789 RSC.
- (a) J. Panyam, Adv. Drug Delivery Rev., 2003, 55, 329–347 CrossRef CAS; (b) M. Shive and J. Anderson, Adv. Drug Delivery Rev., 1997, 28, 5–24 CrossRef CAS; (c) G. Chen, T. Ushida and T. Tateishi, Macromol. Biosci., 2002, 2, 67–77 CrossRef CAS.
- (a) G. Zhu, S. R. Mallery and S. P. Schwendeman, Nat. Biotechnol., 2000, 18, 52–57 CrossRef CAS; (b) K. Fu, D. W. Pack, A. M. Klibanov and R. Langer, Pharm. Res., 2000, 17, 100–106 CrossRef CAS; (c) A. Shenderova, T. G. Burke and S. P. Schwendeman, Pharm. Res., 1999, 16, 241–248 CrossRef CAS.
- (a) F. G. Glansdorp, G. L. Thomas, J. J. K. Lee, J. M. Dutton, G. P. C. Salmond, M. Welch and D. R. Spring, Org. Biomol. Chem., 2004, 2, 3329–3336 RSC; (b) E. A. Yates, B. Philipp, C. Buckley, S. Atkinson, S. R. Chhabra, R. E. Sockett, M. Goldner, Y. Dessaux, M. Camara, H. Smith and P. Williams, Infect. Immun., 2002, 70, 5635–5646 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Full details for film fabrication, compound quantification, and bacteriological assays. See DOI: 10.1039/c0cc02316g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2011 |
