α-Aroyloxyaldehydes: scope and limitations as alternatives to α-haloaldehydes for NHC-catalysed redox transformations†‡
Kenneth B.
Ling
and
Andrew D.
Smith
*
EaStCHEM, School of Chemistry, University of St Andrews, North Haugh, St Andrews, KY16 9ST, UK. E-mail: ads10@st.andrews.ac.uk
First published on 23rd August 2010
Abstract
α-Aroyloxyaldehydes are readily prepared bench stable synthetic intermediates. Their ability to act as α-haloaldehyde surrogates for NHC-promoted redox esterifications and in [4+2] cycloadditions is described.
N-Heterocyclic carbenes (NHCs) have been used to promote a diverse range of organocatalytic transformations in recent years.1 Among these advances, the ability of NHCs to mediate oxidative esterification and amidation reactions of aldehydes has been demonstrated.2 Strategies in this area typically use either an NHC in combination with an oxidant,3 or proceed through a redox process using an α-functionalised aldehyde.4 Representative substrates for such redox processes include enals,5 α-haloaldehydes6 and epoxyaldehydes,7 which all undergo facile NHC-promoted esterifications, but require an additive such as HOBt, imidazole or 1,2,4-triazole to achieve amidations.8 Bode and co-workers have recently promoted α′-hydroxyenones as attractive surrogates for enals in a variety of NHC-promoted reactions9 including amidations,10 due to their simple preparation, crystallinity and long-term stability. Given our interest in NHC-mediated reactions,11 coupled with the potential synthetic versatility of these α-functionalised aldehyde substrates, we took inspiration from this work, and aimed to develop alternative bench stable substrates to α-haloaldehydes.12 Herein the development, scope and limitations of α-aroyloxyaldehydes, readily prepared from commercially available starting materials, as alternative precursors for NHC-promoted redox transformations is detailed (Fig. 1).
 | ||
| Fig. 1 Synthetic precursors for NHC-promoted redox reactions. | ||
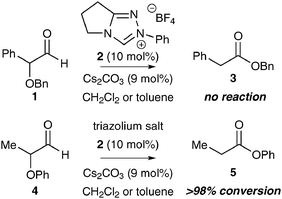
Two features were considered in the design of alternative substrates for NHC-promoted redox processes. Firstly, the leaving group ability of the α-functional group, and secondly, the synthetic availability and stability of these substrates. Model studies considered the internal redox reactions of α-benzyloxy and α-phenoxyaldehydes 1 and 4. While α-benzyloxyaldehyde 1 proved inert to NHC-mediated catalysis, under identical conditions facile and quantitative rearrangement of α-phenoxyaldehyde 4 to phenyl ester 5 was observed (Scheme 1). Scheidt and co-workers have described similar observations,13 consistent with the leaving group ability of the α-functional group being important in these transformations. Although these results were instructive, the lack of a general synthetic route to prepare bespoke α-substituted α-phenoxyaldehydes meant that alternative α-functionalised aldehydes were considered.
 | ||
| Scheme 1 α-Benzyloxy and α-phenoxyaldehydes in redox esterifications. | ||
α-Aroyloxyaldehydes were next investigated, as the leaving group ability of the α-functional group could be readily varied through appropriate substitution of the aroyl unit. Furthermore, two simple synthetic routes to these compounds from commercially available starting materials could be employed. Based upon Tomkinson's methodology for the α-oxidation of carbonyls,14 a series of α-aroyloxyaldehydes was prepared. Alternatively, α-aroyloxyaldehydes are readily synthesised via addition of vinyl magnesium bromide to the desired aldehyde or ketone, O-benzoylation and ozonolysis (Scheme 2). In our hands these aldehydes are typically stable solids that can be stored without special precaution.15
 | ||
| Scheme 2 Synthetic strategies for the synthesis of α-aroyloxyaldehydes. | ||
The ability of the parent α-benzoyloxyaldehyde 6 to undergo redox esterification with benzyl alcohol as an external nucleophile was tested to probe the reactivity of this substrate class (Table 1). In toluene, using Cs2CO3 as the base, N-mesityl precatalyst 10 gave improved reactivity in comparison to N-phenyl analogue 2, although imidazole was required as an additive to achieve acceptable product conversion (entries 1–4). Using excess NEt3 led to acceptable product conversion using N-mesityl precatalyst 10 without the need for an additive, while N-Ph precatalyst 2 proved unreactive under these conditions (entries 5–7). Further optimisation probed the effect of incorporating electron withdrawing NO2 substituents within the benzoyl group. Under standardised conditions, redox catalysis using 2-nitrobenzoyloxyaldehyde 7 proved sluggish, although improved reactivity was observed with 3- and 4-nitrobenzoyloxyaldehydes (entries 8–10 and 12), and using THF as solvent (entry 11). With 4-nitrobenzoyloxyaldehyde 9, Cs2CO3 could be used to good effect, but required imidazole as an additive to reach quantitative product conversion.
| Entry | Aldehyde | Salt (mol%) | Base (eq.) | Product conversiona |
|---|---|---|---|---|
| a All reaction conversions and product distributions were judged by 1H NMR spectroscopic analysis of the crude reaction product. b Using 1.1 eq. of imidazole as an additive. c Using THF (0.1 M) as solvent. | ||||
| 1 | 6 | 2 (20) | Cs2CO3 (0.18) | 42% |
| 2 | 6 | 10 (20) | Cs2CO3 (0.18) | 57% |
| 3b | 6 | 2 (20) | Cs2CO3 (0.18) | 64% |
| 4b | 6 | 10 (20) | Cs2CO3 (0.18) | 81% |
| 5 | 6 | 2 (20) | NEt3 (1.5) | 0% |
| 6 | 6 | 10 (10) | NEt3 (1.5) | 48% |
| 7 | 6 | 10 (20) | NEt3 (1.5) | 80% |
| 8 | 7 | 10 (10) | NEt3 (1.5) | 20% |
| 9 | 8 | 10 (10) | NEt3 (1.5) | 79% |
| 10 | 9 | 10 (10) | NEt3 (1.5) | 58% |
| 11c | 9 | 10 (10) | NEt3 (1.5) | 87% |
| 12 | 9 | 10 (20) | NEt3 (1.5) | >98% |
| 13b | 9 | 10 (20) | Cs2CO3 (0.18) | >98% |
The scope of this NHC-promoted redox process was next evaluated. Using benzyl alcohol, 4-nitrobenzoyloxyaldehydes 9, 12 and 13 containing both linear and β-branched α-alkyl substituents were readily transformed to their corresponding benzyl esters in good yield (Fig. 2). Further studies showed that methanol, ethanol, allyl alcohol, and furfuryl alcohol were all suitable coupling partners, giving the corresponding esters in good yields, although cyclohexanol gave poor product conversion and only a modest isolated yield of ester 19.
 | ||
| Fig. 2 NHC-promoted redox reactions. | ||
Limitations of this process were also identified; notably, α-phenyl substituted aldehyde 22 underwent preferential base-catalysed rearrangement to generate 23 as the exclusive reaction product,16 while tertiary α-aroyloxyaldehyde 24 was inert to redox esterification (Scheme 3).
 | ||
| Scheme 3 Limitations of NHC-promoted redox reactions. | ||
To extend the utility of this protocol, further investigation showed that phenol proved a competent nucleophile for redox esterification, giving the phenyl ester 25.17 This methodology allowed a simple redox amidation protocol to be established, as addition of benzylamine to the crude reaction product gave amide 26 in 65% overall yield from aldehyde 9 (Scheme 4).
 | ||
| Scheme 4 Redox amidation with benzylamine. | ||
Further preliminary and unoptimised results show that 4-nitrobenzoyloxyaldehydes can be used to generate azolium enolates in situ. Aldehyde 12 participates readily in an NHC-promoted formal [4+2] cycloaddition18 with β,γ-unsaturated α-ketoester 27, generating dihydropyranone 28 in 54% yield (69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 dr)19 (Scheme 5).
31 dr)19 (Scheme 5).
![NHC promoted [4+2] cycloaddition.](/image/article/2011/CC/c0cc02456b/c0cc02456b-s5.gif) | ||
| Scheme 5 NHC promoted [4+2] cycloaddition. | ||
Consistent with related proposals,4–8 our current mechanistic hypothesis for these transformations requires initial formation of a Breslow-type intermediate 29 from the reaction of the NHC and the α-aroyloxyaldehyde, with elimination of the carboxylate generating enol 30. Tautomerisation of enol 30 leads to acylazolium 32 that can be acylated directly by alcohols; deprotonation of enol 30 leads to azolium enolate 31, either of which can undergo [4+2] cycloaddition, with both pathways regenerating the NHC (Fig. 3).
 | ||
| Fig. 3 Mechanistic proposal for NHC-promoted reactions of α-aroyloxyaldehydes. | ||
To conclude, 4-nitrobenzoyloxyaldehydes are bench-stable, long-lived precursors for NHC-promoted redox esterifications and cycloaddition reactions. Ongoing studies within this laboratory will demonstrate alternative uses of NHCs and 4-nitrobenzoyloxyaldehydes in asymmetric catalysis.
The authors acknowledge the Royal Society for a URF (ADS), the EPSRC (KBL) and the EPSRC National Mass Spectrometry Service Centre (Swansea).
Notes and references
- For reviews see: (a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS; (b) N. Marion, S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988–3000 CrossRef CAS.
- K. Zeitler, Angew. Chem., Int. Ed., 2005, 44, 7506–7510 CrossRef CAS.
- For MnO2-mediated oxidations of hydroxyazoliums, see: (a) B. E. Maki and K. A. Scheidt, Org. Lett., 2008, 10, 4331–4334 CrossRef CAS; (b) B. E. Maki, A. Chan, E. M. Phillips and K. A. Scheidt, Org. Lett., 2007, 9, 371–374 CrossRef CAS; (c) B. E. Maki, A. Chan, E. M. Phillips and K. A. Scheidt, Tetrahedron, 2009, 65, 3102–3109 CrossRef CAS. For alternative strategies see: (d) H. Inoue and K. Higashiura, J. Chem. Soc., Chem. Commun., 1980, 549–550 RSC; (e) S. Shinkai, T. Yamashita, Y. Kusano and O. Manabe, J. Org. Chem., 1980, 45, 4947–4952 CrossRef CAS; (f) J. Castells, F. Pujol, H. Llitjos and M. Moreno-MaPas, Tetrahedron, 1982, 38, 337–346 CrossRef CAS; (g) S. W. Tam, L. Jimenez and F. Diederich, J. Am. Chem. Soc., 1992, 114, 1503–1505 CrossRef CAS; (h) A. Miyashita, Y. Suzuki, I. Nagasaki, C. Ishiguro, K. I. Iwamoto and T. Higashino, Chem. Pharm. Bull., 1997, 45, 1254–1258 CAS; (i) C. Noonan, L. Baragwanath and S. J. Connon, Tetrahedron Lett., 2008, 49, 4003–4006 CrossRef CAS; (j) J. Guin, S. De Sarkar, S. Grimme and A. Studer, Angew. Chem., Int. Ed., 2008, 47, 8727–8730 CrossRef CAS; (k) S. De Sarkar, S. Grimme and A. Studer, J. Am. Chem. Soc., 2010, 132, 1190–1191 CrossRef CAS.
- A range of NHC promoted redox protocols have been demonstrated. (a) For the conversion of ynals to α,β-unsaturated esters see: K. Zeitler, Org. Lett., 2006, 8, 637–640 Search PubMed; (b) For the ring expansion of formyl β-lactams see G.-Q. Li, Y. Li, L.-X. Dai and S.-L. You, Org. Lett., 2007, 9, 3519–3521 Search PubMed or (c) B. Alcaide, P. Almendros, G. Cabrero and M. P. Ruiz, Chem. Commun., 2007, 4788–4790 RSC; (d) For the ring expansion of oxacycloalkane-2-carboxaldehydes see L. Wang, K. Thai and M. Gravel, Org. Lett., 2009, 11, 891–893 Search PubMed; (e) For the ring expansion of 2-acyl-1-formylcyclopropanes see G.-Q. Li, L.-X. Dai and S.-L. You, Org. Lett., 2009, 11, 1623–1625 Search PubMed; (f) For a redox catalysed lactonisation see K. Zeitler and C. A. Rose, J. Org. Chem., 2009, 74, 1759–1762 Search PubMed; (g) For an interesting kinetic resolution process involving a redox transformation see G.-Q. Li, Y. Li, L.-X. Dai and S.-L. You, Adv. Synth. Catal., 2008, 350, 1258–1262 Search PubMed.
- (a) S. S. Sohn, E. L. Rosen and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 14370–14371 CrossRef CAS; (b) S. S. Sohn and J. W. Bode, Org. Lett., 2005, 7, 3873–3876 CrossRef CAS; (c) A. Chan and K. A. Scheidt, Org. Lett., 2005, 7, 905–908 CrossRef CAS.
- (a) N. T. Reynolds, J. Read de Alaniz and T. Rovis, J. Am. Chem. Soc., 2004, 126, 9518–9519 CrossRef CAS; (b) N. T. Reynolds and T. Rovis, J. Am. Chem. Soc., 2005, 127, 16406–16407 CrossRef.
- K. Y.-K. Chow and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 8126–8127 CrossRef CAS.
- (a) H. U. Vora and T. Rovis, J. Am. Chem. Soc., 2007, 129, 13796–13797 CrossRef CAS; (b) J. W. Bode and S. S. Sohn, J. Am. Chem. Soc., 2007, 129, 13798–13799 CrossRef CAS.
- P.-C. Chiang, M. Rommel and J. W. Bode, J. Am. Chem. Soc., 2009, 131, 8714–8718 CrossRef CAS.
- P.-C. Chiang, Y. Kim and J. W. Bode, Chem. Commun., 2009, 4566–4568 RSC.
- (a) J. E. Thomson, K. Rix and A. D. Smith, Org. Lett., 2006, 8, 3785–3788 CrossRef CAS; (b) J. E. Thomson, C. D. Campbell, C. Concellón, N. Duguet, K. Rix, A. M. Z. Slawin and A. D. Smith, J. Org. Chem., 2008, 73, 2784–2791 CrossRef CAS; (c) C. D. Campbell, N. Duguet, K. A. Gallagher, J. E. Thomson, A. G. Lindsay, A. C. O'Donoghue and A. D. Smith, Chem. Commun., 2008, 3528–3530 RSC; (d) N. Duguet, C. D. Campbell, A. M. Z. Slawin and A. D. Smith, Org. Biomol. Chem., 2008, 6, 1108–1113 RSC; (e) J. E. Thomson, A. F. Kyle, C. Concellón, K. A. Gallagher, P. Lenden, L. C. Morrill, A. J. Miller, C. Joannesse, A. M. Z. Slawin and A. D. Smith, Synthesis, 2008, 2805–2818 CAS; (f) C. Concellón, N. Duguet and A. D. Smith, Adv. Synth. Catal., 2009, 351, 3001–3009 CrossRef CAS; (g) N. Duguet, A. Donaldson, S. Leckie, J. Douglas, P. Shapland, G. Churchill, A. M. Z. Slawin and A. D. Smith, Tetrahedron: Asymmetry, 2010, 21, 582–600 CrossRef CAS; (h) N. Duguet, A. Donaldson, S. Leckie, P. Shapland, A. M. Z. Slawin and A. D. Smith, Tetrahedron: Asymmetry, 2010, 21, 601–616 CrossRef CAS.
- For asymmetric NHC-catalysed [4+2] reactions with haloaldehyde bisulfite salts see: M. He, B. J. Beahm and J. W. Bode, Org. Lett., 2008, 10, 3817–3820 Search PubMed.
- E. M. Phillips, M. Wadamoto, H. S. Roth, A. W. Ott and K. A. Scheidt, Org. Lett., 2009, 11, 105–108 CrossRef CAS; Y. Kawanaka, E. M. Phillips and K. A. Scheidt, J. Am. Chem. Soc., 2009, 131, 18028–18029 CrossRef CAS.
- C. S. Beshara, A. Hall, R. L. Jenkins, T. C. Jones, R. T. Parry, S. P. Thomas and N. C. O. Tomkinson, Chem. Commun., 2005, 1478–1480 RSC; C. S. Beshara, A. Hall, R. L. Jenkins, K. L. Jones, T. C. Jones, N. M. Killeen, P. H. Taylor, S. P. Thomas and N. C. O. Tomkinson, Org. Lett., 2005, 7, 5729–5732 CrossRef CAS; T. C. Jones and N. C. O. Tomkinson, Org. Synth., 2007, 84, 233–241 CAS.
- The ESI‡ contains full procedures for the preparation of α-aroyloxyaldehydes; these aldehydes have been stored for up to 3 months without decomposition.
- Reaction in the absence of triazolium salt 10 gave full conversion to 23.
- Phenyl ester 25 can be isolated in 40% yield by chromatography.
- For asymmetric NHC-catalysed [4+2] reactions using α-haloaldehydes see: M. He, G. J. Uc and J. W. Bode, J. Am. Chem. Soc., 2006, 128, 15088–15089 Search PubMed . For an asymmetric aza-diene [4+2] reaction using enals see: M. He, J. R. Struble and J. W. Bode, J. Am. Chem. Soc., 2006, 128, 8418–8420 CrossRef CAS.
- The relative configuration within 28 was assigned by 1H NMR spectroscopic analysis in comparison to an analogous p-tolyl derivative; see ref. 17.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Spectroscopic details for all compounds. See DOI: 10.1039/c0cc02456b |
| This journal is © The Royal Society of Chemistry 2011 |

